Abstract
Esophageal cancer ranks among the ten most common cancers worldwide. Despite the adoption of neoadjuvant concurrent chemoradiotherapy (nCCRT) followed by surgery as the standard treatment approach in recent years, the local recurrence rate remains high. In this study, we employed RNA-seq to investigate distinctive gene expression profiles in esophageal squamous cell carcinoma (ESCC) with or without recurrence following a standard treatment course. Our findings indicate that recurrent ESCC exhibits heightened keratinizing and epidermis development activity compared to non-recurrent ESCC. We identified TP63 as a potential candidate for distinguishing clinical outcomes. Furthermore, immunohistochemistry confirmed the trend of TP63 overexpression in ESCC recurrence. Patients with elevated TP63 expression had poorer overall survival and lower 3-year recurrence-free survival. This study underscores the potential of TP63 as a biomarker for detecting cancer recurrence and suggests its role in guiding future treatment options.
1. Introduction
Esophageal cancer ranks amongst the ten most common cancers in the world, with high incidence in developing countries. It is one of the most aggressive tumors and its growth is relatively rapid. The main histological subtype of esophageal cancer is esophageal adenocarcinoma (EAC) in Western areas [1,2], whereas 90% of esophageal cancer is esophageal squamous cell carcinoma (ESCC) in Taiwan. ESCC is mainly located in the upper to middle esophagus and is derived from the epidermis. Its risk factors are highly correlated with unfavorable habits, including a low intake of fruits and vegetables, smoking, excessive alcohol consumption, and betel nut chewing [3,4].
While several treatments, such as surgical resection of esophageal tumor, chemotherapy, and radiation therapy, have been applied to improve survival rate in patients with esophageal cancer, the efficacy of a single treatment remains poor [5]. Currently, multimodality treatment is predominantly employed, and with this approach, surgical resection remains the most effective treatment choice for patients with resectable esophageal cancer [6]. However, the most common cause of treatment failure in ESCC patients after surgery is recurrence [7]. Over the past few decades, neoadjuvant concurrent chemoradiotherapy (nCCRT) followed by surgery has been proven to prolong the survival of patients with resectable advanced esophageal cancer [8,9] and become the standard therapy in advanced esophageal cancer [10]. Nevertheless, the recurrence rate still ranges from 40 to 60%, and the prognosis of patients with recurrence is poor [11,12]. Although some clinical and pathological information may be related to tumor recurrence, the prediction is still unsatisfactory. Therefore, seeking early prognosis biomarkers associated with early esophageal cancer recurrence is important and instrumental to help decision making for effective treatment strategies.
A growing amount of research indicates that several genes such as CLDN4 [13], RUNX3 [14] and E-cadherin [15] could be potential ESCC prognostic biomarkers. Furthermore, CLDN4 [13] and AGR2 [16] could be a CCRT response indicator. However, none of them could be used to detect the potential early recurrence of ESCC after nCCRT followed by surgery. Genomic studies show that TP63, SOX2, and KLF5 consist of the core regulatory circuit that controls epigenetic and transcription patterns in ESCC [17]. TP63 is a transcription factor from the p53 family, and the TP63 mutation is much more commonly detected and has elevated expression in ESCC [18]. It also regulates the growth of esophageal squamous carcinoma cells through many other pathways, such as the β-catenin/c-Myc signaling pathway and the AKT signaling pathway [19,20]. However, few studies have focused on the relationship between TP63 and the ESCC early recurrence.
In this study, we leverage high-throughput next-generation sequencing to identify predictable prognostic biomarkers of early recurrence in esophageal squamous cell carcinoma. We demonstrate that the expression of TP63 is significantly correlated with early tumor recurrence in ESCC.
2. Materials and Methods
2.1. Patients and Specimen Collection
In this study, 15 patients with esophageal squamous cell carcinoma who underwent surgical resection after neoadjuvant CCRT were enrolled. They were classified based on their recurrence status during the follow-up period into non-recurrence and recurrence groups. Criteria of no recurrence included the following: (1) tumor resolution on CT; (2) grossly no tumor on endoscopic examination, and biopsy at the residual scar tissue or original tumor sites yielded a negative result for malignancy; (3) no documented distant metastases by PET-CT examinations. Frozen specimens were obtained from the BioBank of Taichung Veterans General Hospital (TCVGH) and utilized for RNA sequencing. Additionally, immunohistochemistry was employed to validate the tissue expression of the target gene. Tissues from an additional 50 patients were collected from the TCVGH Biobank, and clinicopathological information, including age, sex, histological subtype, and treatment response, was obtained from delinked medical records. All samples used in this study from March 2008 to January 2021 were approved by the TCVGH Institutional Review Board. The IRB number for RNA-seq is CE17279A and the IRB number for IHC is CF21046A.
2.2. RNA Sequencing and Gene Expression Analysis
The total RNA was extracted from frozen esophageal squamous cell carcinoma tissues using the RNeasy Mini Kit (Qiagen, Venlo, Limburg, The Netherlands), following the manufacturer’s instructions. RNA libraries were prepared using the TruSeq Stranded mRNA library Prep kit for cDNA reverse transcription and library construction (Illumina, SanDiego, CA, USA). Subsequently, the libraries were sequenced using HiSeq 2500 sequencers (Illumina, San Diego, CA, USA). To analyze the sequencing data, sequence reads were aligned to the human reference genome GRCh38 using the HISAT2 aligner tool (version 2.2.1) [21]. Read counts were calculated using featureCounts (version 2.0.1) [22], and differential gene expression profiles identified using the R package DESeq2 (R version 4.0.2; DEseq2 version no. 1.40.2) [23]. A gene was considered significant if its log2 fold change was >1 or <1, and the DESeq-adjusted p-value was <0.05. A principal component analysis (PCA) was also performed by the function “plotPCA” in R package DESeq2. The first two PCs were used to plot the results.
For gene set enrichment analysis (GSEA), the p-value metrics of all genes were converted to log form, and the positive or negative sign was added based on the fold change estimate obtained from DESeq2. The genes were then pre-ranked by the transformed value before being input into GSEA software (version 3.0) (PMID: 16199517, PMID: 12808457). Gene sets of the gene ontology biological process (GO:BP) with sizes above 15 and under 500 were used in the analysis.
2.3. Immunohistochemistry
To detect the expression of p63 using IHC analysis, ESCC tissues were formalin-fixed and paraffin-embedded. Following tissue sectioning, deparaffinization and rehydration were achieved using xylene and different concentrations of ethanol. Antigen retrieval was performed with citrate-hydrochloric acid. Non-specific protein blocking involved the use of 10% goat serum. The anti-p63 antibody (ab735, Abcam, Cambridge, UK) was then applied at a 1:50 dilution, followed by treatment with an HRP-conjugated secondary antibody. Subsequently, diaminobenzidine was used for coloration, and counterstaining was conducted with hematoxylin. Two experienced pathologists from VGHTC independently evaluated the staining intensity of TP63 in tumor cells. Staining intensity is scored on a scale of 0–2, with 0 indicating no expression, 1 indicating mid expression, and 2 indicating strong expression.
2.4. Statistical Analysis
The two-tailed Student’s t-test was used for analyzing the differences between two continuous variables and chi-square test for categorical variables. A p-value < 0.05 was considered to be significant. All data analyses were conducted using R (version 4.0.2).
3. Results
3.1. Characteristics of the Study Population
Fifteen patients were divided into two groups according to the recurrence status of ESCC. Eight (53.33%) patients experienced a recurrence within 47 to 182 days (mean, 141.5 ± 43.5 days) and seven patients did not experience a recurrence. All of these patients were male, and the average age of the patients was 56.60 ± 6.68 years. The pathologic stage was classified according to the tumor–node–metastasis (TNM) classification (TNM Classification of Malignant Tumours, 8th edition) [24]. Most recurrence developed in stage III and IV. The average age (p = 0.84, t-test) and the cancer stage distribution (p = 0.2, chi-squared) between recurrent and non-recurrent groups were similar (Table 1).

Table 1.
Characteristics of the enrolled ESCC patients.
3.2. RNA Expression Analysis of ESCC Recurrence Genes
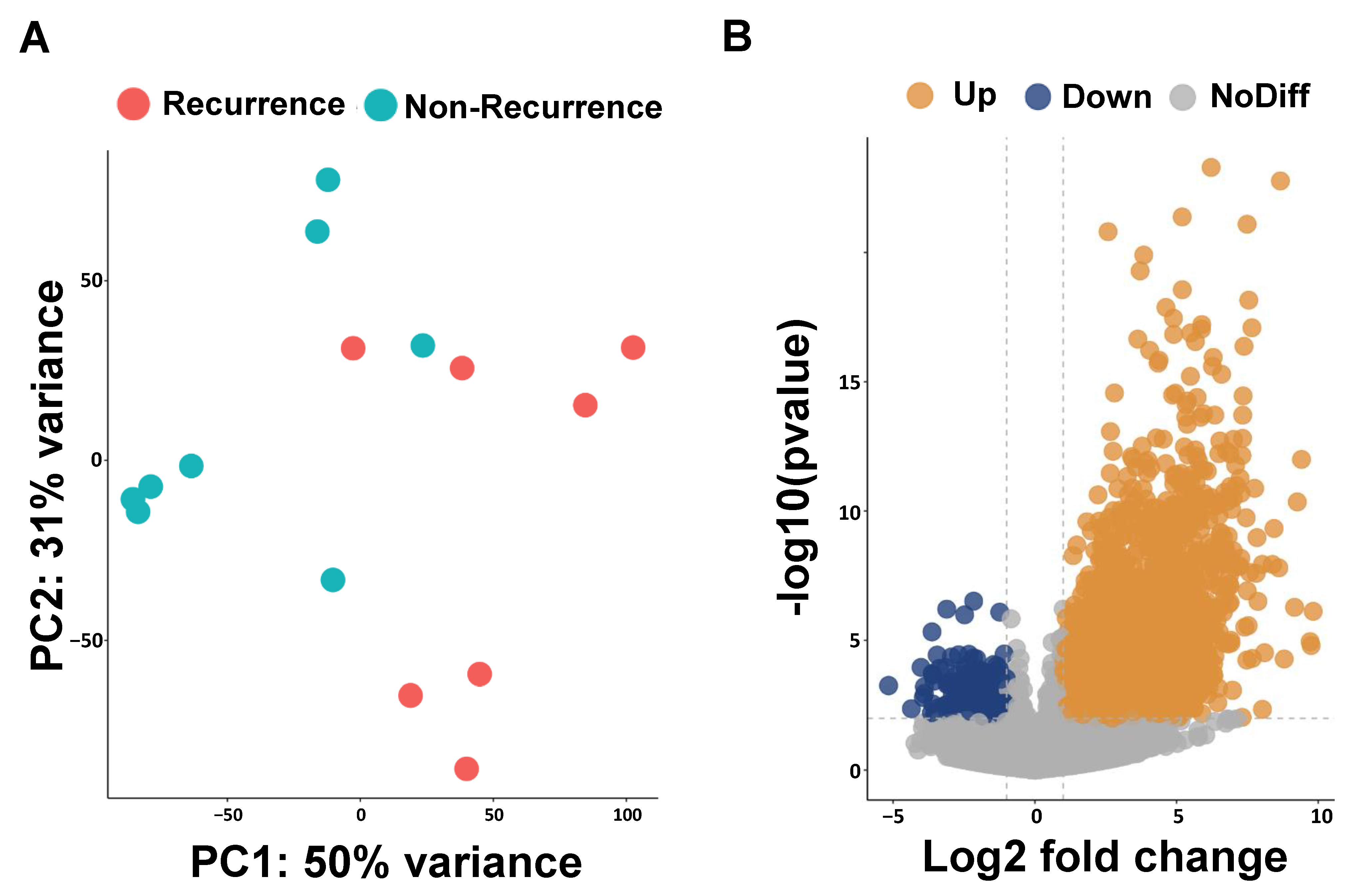
We performed RNA sequencing analysis to identify the possible prognosis biomarkers in ESCC-recurrence patients. The results obtained for the principal component analysis (PCA) are described in Figure 1A. The PC1 and PC2 values form two separate clusters between patients with and without a recurrence of ESCC; PC1 captures 50% of the overall variance and PC2 accounts for 31%. To evaluate the transcriptomic difference between the non-recurrence and recurrence groups, we identified 1132 up-regulated and 162 down-regulated differentially expressed genes (DEGs) using DESeq2 (Figure 1B). The top 20 up-regulated differential expression genes are presented in Table 2.
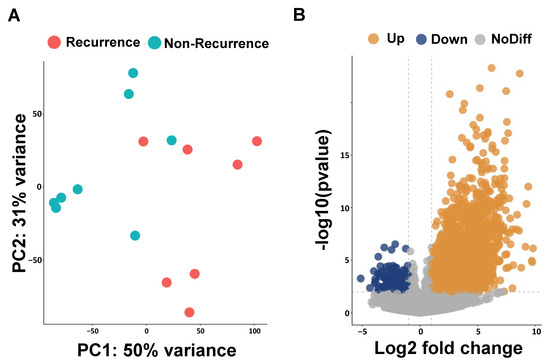
Figure 1.
Principal component analysis (PCA) and volcano plots of differentially expressed genes (DEGs). (A) PCA plot reveals two clusters of esophageal cancer patients with and without recurrence. (B) Volcano plot shows DEGs of esophageal cancer patients with and without recurrence.

Table 2.
Top 20 up-regulated differentially expressed genes.
3.3. Keratinocyte Proliferation Was More Activated in Non-Recurrent ESCC
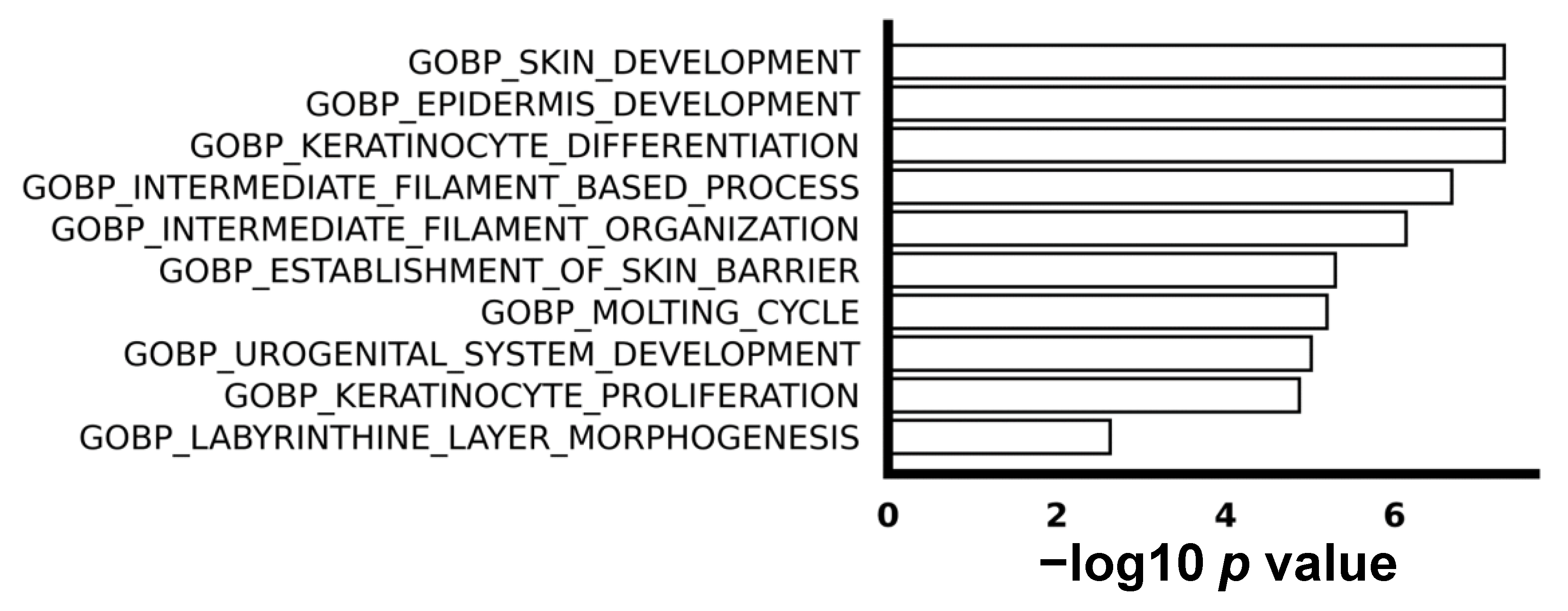
We utilized gene set enrichment analysis (GSEA) to explore the difference in biological process between ESCC with or without recurrence. The top 10 enriched up-regulated gene sets were associated with processes such as keratinization, proliferation, and epidermis development, as visually depicted in Figure 2. This implies a higher level of abnormal cell proliferation and differentiation in recurrent ESCC compared to non-recurrent ESCC. It is noteworthy that, despite the identification of some down-regulated genes in the comparison between non-recurrent and recurrent ESCC, no enriched down-regulated gene sets were discerned, emphasizing the specificity of the observed molecular signatures.
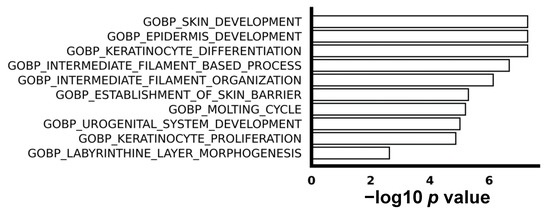
Figure 2.
The top 10 enriched biological processes in gene oncology. GSEA shows top 10 pathways of biological processes.
3.4. High TP63 Expression Is Related to Early ESCC Recurrence and Poor Prognosis
From the top 20 differentially expressed genes and the activated keratinization proliferation and epidermis development biological processes in recurrent ESCC, we identified TP63 as a putative biomarker to predict the clinical outcome. Through immunohistochemical (IHC) staining, we evaluated TP63 expression in 50 ESCC tissues, half of which had recurrent (25 patients) and the other half non-recurrent (25 patients) clinical outcomes. The clinical pathological characteristics of these 50 ESCC patients are shown in Table S1. These 25 patients with recurrence experienced recurrence within 25 to 235 days (average: 135.2 ± 53.5 days). All patients had an average age of 54.52 ± 7.71 years, including 48 males and 2 females.
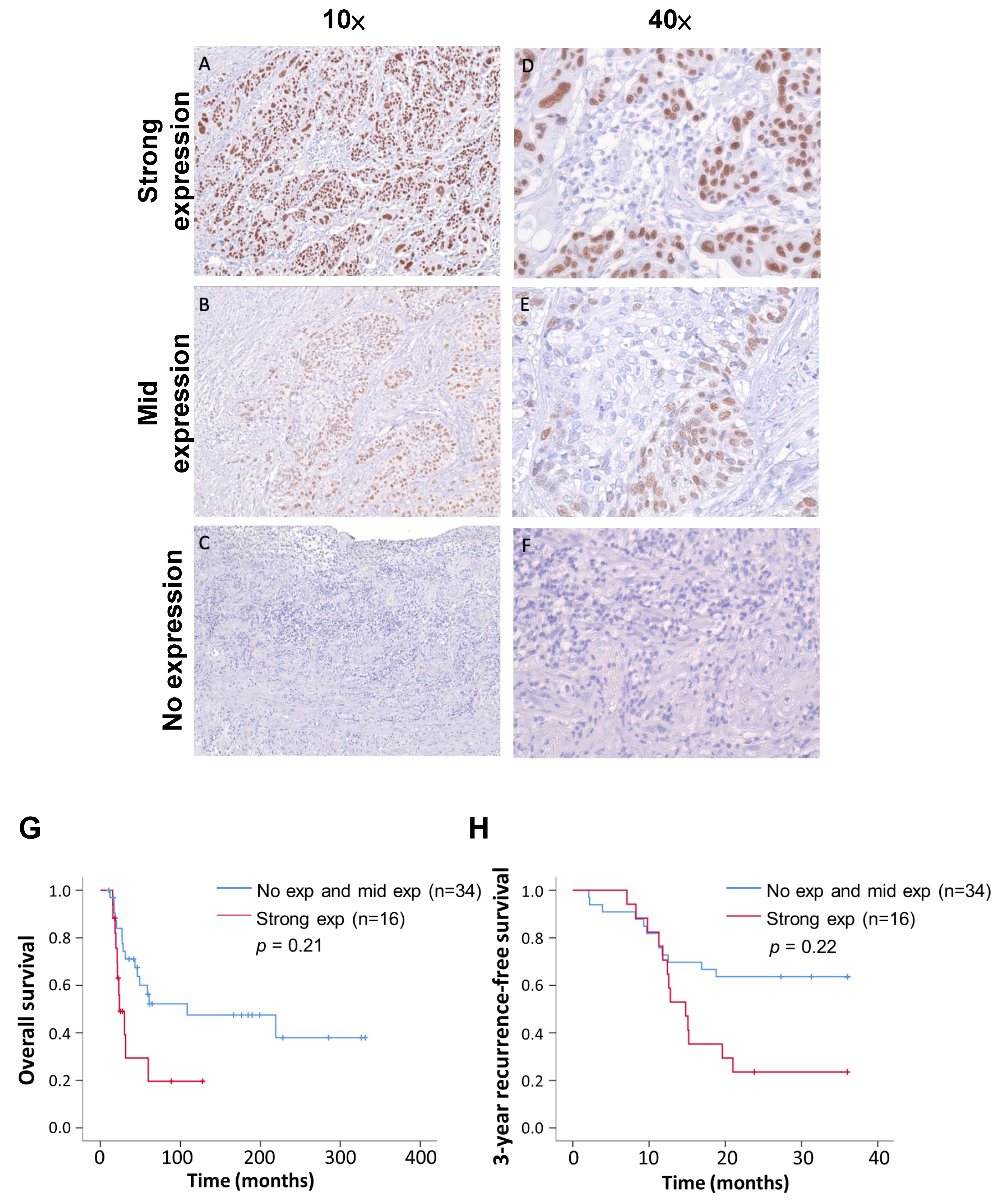
We classified the IHC staining results into three levels: strong expression (>80%), mid expression (<80%), and no expression (Figure 3). The tissue sections were all judged by professional pathologists, and the parts stained with TP63 antibody were all tumor cells. The distribution of the TP63 expression levels in recurrent and non-recurrent ESCC was significantly different (Table 3, p value < 0.01; chi-square test). Following surgery, the survival and recurrence status of patients were continuously monitored, and survival curves were plotted. In comparison to the group combining no expression and mid expression of TP63, patients with strong TP63 expression exhibited a poorer prognosis (p = 0.21) (Figure 3G). Furthermore, the 3-year recurrence-free survival rate in the strong-TP63-expression group was notably lower than that in the low-expression group (p = 0.22) (Figure 3H). Summarizing the above results, we confirm that TP63 is more commonly expressed in recurrent ESCC patients, and those with high TP63 expression have a lower postoperative survival rate.
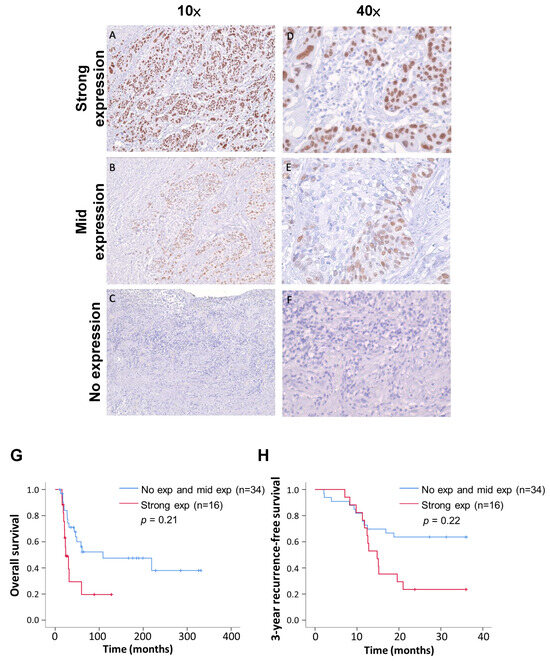
Figure 3.
Immunohistochemistry indicates three TP63 expression levels; also shown are Kaplan–Meier overall survival and recurrence-free survival curves of 50 patients. (A,D) ESCC tissue showed strong TP63 expression at different magnification. (B,E) ESCC tissue showed medium TP63 expression at different magnification. (C,F) ESCC tissue with no TP63 expression. (G) Analysis of postoperative overall survival rates for different TP63 manifestations using the Kaplan–Meier estimator. (H) Comparative analysis of recurrence-free survival rates during three-year follow-up of different TP63 expressions.

Table 3.
The chi-square test results indicate a significant difference in the expression distribution of TP63 from IHC between recurrent and non-recurrent patients of ESCC.
4. Discussion
In this study, we explored the differential gene expression between ESCC patients with or without recurrence after neoadjuvant CCRT followed by surgical resection. We found significant gene expression differences between the two clinical outcomes, which enriched some biological processes such as the abnormal growth and development of the epidermis in ESCC-recurrent patients. Furthermore, we demonstrated that TP63 protein expression tends to appear in patients who will suffer subsequent ESCC recurrence.
In clinical practice, the recurrence of esophageal cancer remains challenging to avoid and is often disheartening. A clinical trial examined 170 patients with stage I and stage II esophageal cancer. In this study, even with early-stage esophageal cancer receiving CCRT followed by surgery, the recurrence rate was still 31% [25]. There was already evidence suggesting that certain factors, such as tumor differentiation, resection margin, nodal status, and the response to neoadjuvant CCRT, may be associated with the recurrence of esophageal cancer [26,27]. However, the prediction of esophageal cancer recurrence remains inadequate. The use of biomarkers may provide a resolution. The discovery of the relationship between tumor recurrence and TP63 not only provides us with more information, but through further research into its correlations, it may help identify additional mechanisms contributing to tumor recurrence. Combining it with clinical data may potentially enhance the accuracy of predicting tumor recurrence.
We found several top up-regulated genes with high gene expression levels, such as KRT1, KRT17, ALDH3A1, S100A7A, and TP63 in recurrent ESCC (Table 2). Previous studies showed that oral squamous cell carcinoma with low ALDH3A1 expression was significantly worse than its high-ALDH3A1-expression counterpart [28], and its overexpression could drive cancer stem cell expansion and impair immune surveillance [29]. Furthermore, ALDH3A1 was identified as a novel downstream target of ESCC core regulatory circuitry [17]. On the other hand, keratins are used as diagnostic biomarkers in multiple cancers. A recent study found that a higher expression of KRT1 and KRT17 is associated with low overall survival in melanoma [30]. KRT17 could also activate AKT signaling and induce EMT in ESCC [31]. An abnormal expression of S100A7 has been observed in various cancers, including up-regulation in the tissues and blood of ESCC patients. Through the action of the S100A7/JAB1 axis, it facilitates the migration and proliferation of ESCC cells. Additionally, S100A7 has been found to promote the infiltration of M2 macrophages and the process of angiogenesis [32]. TP63 has a double role, functioning in either an oncogene or a tumor suppressor gene in different situations [33]. It could regulate tumor cell proliferation via the AKT signaling pathway and stimulate tumor cell invasion and metastasis in ESCC [19,20]. Therefore, we identified several differentially expressed genes associated with poor prognosis in tumors from ESCC patients who experienced recurrence after CCRT. These distinct genes may represent crucial factors contributing to the recurrence, highlighting their potential significance in understanding the underlying mechanisms of recurrent ESCC.
The cell fate of the epidermis depends on the balance of inputs from pro-survival and pro-differentiation cascades [34]. Therefore, aberrant epidermis development leads to epidermal cancer development. In this study, we found recurrent ESCC had excessive keratinization and epidermis development activity compared to non-recurrent ESCC. However, other studies indicate that there are different clinical outcomes that appear in skin cancers. For example, there were no significant differences in postoperative recurrence between keratinization and non-keratinization subtypes in lung squamous cell carcinoma [35]. Furthermore, more recurrences were discovered in patients with oral squamous cell carcinoma with low keratinization [36]. As previous studies have shown, TP63 is crucial to the development of the epidermis [37] and essential for the epithelial stem cell’s proliferative potential [38]. Therefore, the abnormal TP63 expression might cause aberrant epidermis development. In our findings, the abnormal TP63 expression tended to be discovered in recurrent ESCC after neoadjuvant CCRT treatment, which implies the TP63 has the potential to promote ESCC recurrence. However, the mechanism that causes the different responses to aberrant keratinization between these epidermal-derived cancers still needs more studies.
A cancer that is deemed recurrent usually reappears after a period in which the cancer cannot be detected. Therefore, a cancer stem cell may hide and harbor in the microenvironment until recurrence happens. TP63 expression is regulated through multiple complex pathways, such as Notch, Hedgehog, WNT, FGFR2, and EGFR [39]. The elevated expression of TP63 in recurrent ESCC corresponds to the final output of misregulation of these pathways. Previous research also found that TP63 cooperated with other transcription factors such as NF-κB to influence the tumor microenvironment (TME) [40]. The microenvironment might be changed under the regulation mechanism of ESCC and cause possible chemoresistance and subsequent cancer recurrence.
There are still several limitations in our study. First, it was a small-scale retrospective case–control study. The use of a case–control design may have led to selection bias, especially with the limited number of samples. This study aimed to confirm the correlation between recurrence and biomarkers, and we were concerned that a cohort study approach might result in too few recurrence cases to effectively address our research question. Therefore, we chose the case–control method for numerical matching. In the future, we also hope to further validate our findings by expanding the sample size, evolving into a prospective study, or utilizing multi-center data to enhance the representativeness and generalizability of our results. Second, our study only enrolled ESCC; further research is needed to confirm whether this applies to EAC. Third, with the development and widespread adoption of novel therapies, such as immunotherapy, it remains necessary to gather more data to confirm their applicability in the future.
In summary, our study revealed the gene expression difference between different clinical outcomes of ESCC. Most importantly, we identified TP63 as a biomarker for predicting early recurrence in ESCC. Therefore, our findings could provide a new aspect for therapeutic strategy making in the future.
5. Conclusions
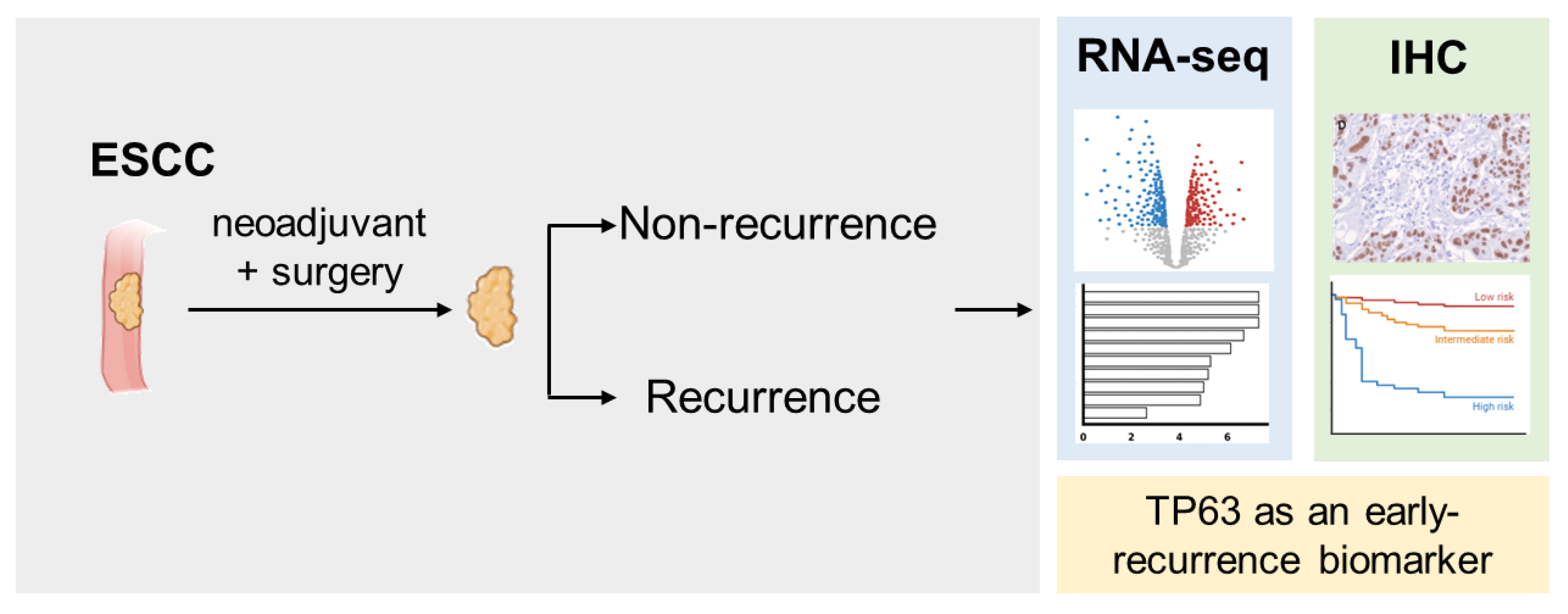
This study explores the differences in gene expression between groups of patients with ESCC who received neoadjuvant concurrent chemoradiotherapy combined with surgery. Our findings show that the TP63 gene and protein expression levels are significantly higher in the recurrence group, accompanied by increased keratinizing and epidermis development activity. A higher expression of TP63 is associated with lower overall survival rates and a three-year recurrence-free survival. We hope that TP63 can serve as a biomarker for predicting early recurrence in ESCC and provide a new direction for future treatment strategies (Figure 4).
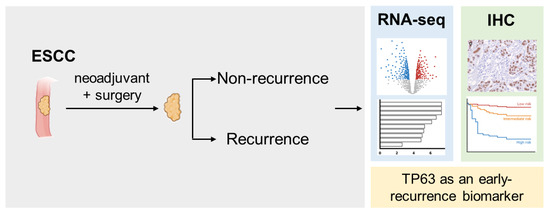
Figure 4.
Graphic summary of the current study. This study found that TP63 expression is significantly increased in recurrent ESCC patients treated with nCCRT and surgery, as demonstrated by RNA-seq and IHC. Higher TP63 levels are associated with lower overall survival rates and 3-year recurrence-free survival, indicating TP63 as a potential biomarker for early recurrence prediction in ESCC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12051101/s1, Table S1: Characteristics of the enrolled 50 ESCC patients form IHC.
Author Contributions
C.-H.L.: conceptualization, investigation and writing—original draft preparation; P.-L.C.: data curation, formal analysis and methodology; C.-Y.C.: data curation and methodology; Y.-T.K.: formal analysis, validation and visualization; L.-W.L.: data curation and investigation; T.-H.H.: conceptualization, supervision, funding acquisition, resources; C.-P.H.: funding acquisition, supervision and writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants MOST 110-2314-B-303-027 and MOST 111-2314-B-303-035 from the National Science and Technology Council, Taiwan, and grant VGHUST112-G2-3-1 from the Veterans General Hospital and Taiwan University System (VGHUST) Joint Research Program. We extend our gratitude to the Biobank of Taichung Veterans General Hospital for providing the specimens.
Institutional Review Board Statement
Approval for this study was granted by the Institutional Review Board of Taichung Veterans General Hospital under protocol number CE17279A and CF21046A.
Informed Consent Statement
In this study, all samples from participating patients (including frozen specimens and tissue sections) were obtained from the Biobank of Taichung Veterans General Hospital (TVGH). Before these samples were incorporated into the Biobank, written informed consent was obtained from each participant, agreeing that their tissue and blood samples could be used for future medical research purposes. When submitting the project to the IRB, since the Biobank had already de-identified patient data, the application could proceed under a waiver of informed consent. All protocols of this study were approved by the IRB of TCVGH. The use of specimens was also approved by the Ethics Governance Council (EGC) of the biobank.
Data Availability Statement
The results and the data analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have declared no competing interests.
References
- Devesa, S.S.; Blot, W.J.; Fraumeni, J.F., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998, 83, 2049–2053. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, I.C.; Chen, Y.H.; Chen, C.C.; Chuang, C.Y.; Lin, C.H. The influence of socioeconomic status on esophageal cancer in Taiwan: A population-based study. J. Pers. Med. 2022, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Chow, W.H.; Abnet, C.C.; Dawsey, S.M. Environmental Causes of Esophageal Cancer. Gastroenterol. Clin. N. Am. 2009, 38, 27–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.W.; Lin, M.C.; Huang, P.M.; Wang, C.P.; Chen, T.C.; Chen, C.N.; Tsai, M.H.; Cheng, J.C.; Chuang, E.Y.; Hsieh, M.S.; et al. Risk Factors and Genetic Biomarkers of Multiple Primary Cancers in Esophageal Cancer Patients. Front. Oncol. 2020, 10, 585621. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.D.; Cassano, A.D.; Neifeld, J.P. Neoadjuvant therapy for esophageal cancer. World J. Gastrointest. Oncol. 2014, 6, 403–406. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, H.; Li, J. Pattern of recurrence and prognostic factors in patients with pT1-3 N0 esophageal squamous cell carcinoma after surgery: Analysis of a single center experience. J. Cardiothorac. Surg. 2019, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Merkow, R.P.; Bilimoria, K.Y.; McCarter, M.D.; Chow, W.B.; Ko, C.Y.; Bentrem, D.J. Use of multimodality neoadjuvant therapy for esophageal cancer in the United States: Assessment of 987 Hospitals. Ann. Surg. Oncol. 2012, 19, 357–364. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, C.C.; Chien, C.R. Neoadjuvant vs. definitive concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma patients. World J. Surg. Oncol. 2018, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Huang, P.M.; Chu, P.Y.; Chen, P.M.; Lin, M.W.; Kuo, S.W.; Lee, J.M. Predictors of Survival in Esophageal Squamous Cell Carcinoma with Pathologic Major Response after Neoadjuvant Chemoradiation Therapy and Surgery: The Impact of Chemotherapy Protocols. BioMed Res. Int. 2016, 2016, 6423297. [Google Scholar] [CrossRef]
- Schuring, N.; Stam, W.T.; Plat, V.D.; Kalff, M.C.; Hulshof, M.; van Laarhoven, H.W.M.; Derks, S.; van der Peet, D.L.; van Berge Henegouwen, M.I.; Daams, F.; et al. Patterns of recurrent disease after neoadjuvant chemoradiotherapy and esophageal cancer surgery with curative intent in a tertiary referral center. Eur. J. Surg. Oncol. 2023, 49, 106947. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, C.; Makino, H.; Takagawa, R.; Yamamoto, N.; Nagano, Y.; Fujii, S.; Kosaka, T.; Ono, H.A.; Otsuka, Y.; Akiyama, H.; et al. Surgical outcomes in esophageal cancer patients with tumor recurrence after curative esophagectomy. J. Gastrointest. Surg. 2008, 12, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, H.Y.; Liu, Y.P.; Kuo, P.F.; Wang, W.C.; Lin, F.C.; Chang, W.L.; Sheu, B.S.; Wang, Y.C.; Hung, W.C.; et al. High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919875324. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; Huang, X.; Chen, L. Analysis of the RUNX3 gene methylation in serum DNA from esophagus squamous cell carcinoma, gastric and colorectal adenocarcinoma patients. Hepatogastroenterology 2011, 58, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, B.B.; Han, J.; Cho, E.Y.; Shim, Y.M.; Park, J.; Kim, D.H. CpG island hypermethylation of E-cadherin (CDH1) and integrin α4 is associated with recurrence of early stage esophageal squamous cell carcinoma. Int. J. Cancer 2008, 123, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chuang, H.N.; Hsiao, T.H.; Kumar, V.B.; Hsu, C.H.; Huang, C.Y.; Lee, L.W.; Mao, C.L.; Ko, J.L.; Hsu, C.P. AGR2 expression as a predictive biomarker for therapy response in esophageal squamous cell carcinoma. PLoS ONE 2022, 17, e0276990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Jiang, Y.; Li, C.Q.; Zhang, Y.; Dakle, P.; Kaur, H.; Deng, J.W.; Lin, R.Y.; Han, L.; Xie, J.J.; et al. TP63, SOX2, and KLF5 Establish a Core Regulatory Circuitry That Controls Epigenetic and Transcription Patterns in Esophageal Squamous Cell Carcinoma Cell Lines. Gastroenterology 2020, 159, 1311–1327.e1319. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; George, A.L.; Sakakibara, N.; Mahmood, K.; Ponnamperuma, R.M.; King, K.E.; Weinberg, W.C. Molecular Mechanisms of p63-Mediated Squamous Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 3590. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Lee, K.B.; Park, M.H.; Lee, J.S.; Kim, S.M. p63 regulates growth of esophageal squamous carcinoma cells via the Akt signaling pathway. Int. J. Oncol. 2014, 44, 2153–2159. [Google Scholar] [CrossRef][Green Version]
- Lee, K.B.; Ye, S.; Park, M.H.; Park, B.H.; Lee, J.S.; Kim, S.M. P63-Mediated activation of the β-catenin/c-Myc signaling pathway stimulates esophageal squamous carcinoma cell invasion and metastasis. Cancer Lett. 2014, 353, 124–132. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Robb, W.B.; Messager, M.; Dahan, L.; Mornex, F.; Maillard, E.; D’Journo, X.B.; Triboulet, J.P.; Bedenne, L.; Seitz, J.F.; Mariette, C.; et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br. J. Surg. 2016, 103, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Sihag, S.; Nobel, T.; Hsu, M.; Tan, K.S.; Bains, M.; Jones, D.R.; Molena, D. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J. Thorac. Cardiovasc. Surg. 2019, 157, 1249–1259.e1245. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Urooj, N.; Syed, A.A.; Khattak, S.; Kazmi, A.; Ashraf, M.I.; Batool, S. Prognostic Factors for Recurrence in Esophageal Cancer Patients Treated With Neoadjuvant Therapy and Surgery: A Single-institution Analysis. Cureus 2020, 12, e8108. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; He, Y.; Yang, Y.; Li, S.; An, W.; Li, Z.; Wang, X.; Han, Z.; Qin, L. ALDH3A1 acts as a prognostic biomarker and inhibits the epithelial mesenchymal transition of oral squamous cell carcinoma through IL-6/STAT3 signaling pathway. J. Cancer 2020, 11, 2621–2631. [Google Scholar] [CrossRef]
- Terzuoli, E.; Bellan, C.; Aversa, S.; Ciccone, V.; Morbidelli, L.; Giachetti, A.; Donnini, S.; Ziche, M. ALDH3A1 Overexpression in Melanoma and Lung Tumors Drives Cancer Stem Cell Expansion, Impairing Immune Surveillance through Enhanced PD-L1 Output. Cancers 2019, 11, 1963. [Google Scholar] [CrossRef]
- Han, W.; Hu, C.; Fan, Z.J.; Shen, G.L. Transcript levels of keratin 1/5/6/14/15/16/17 as potential prognostic indicators in melanoma patients. Sci. Rep. 2021, 11, 1023. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, S.; Ye, S.; Shen, Z.; Gao, L.; Han, Z.; Zhang, P.; Luo, F.; Chen, S.; Kang, M. Keratin 17 activates AKT signalling and induces epithelial-mesenchymal transition in oesophageal squamous cell carcinoma. J. Proteom. 2020, 211, 103557. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zheng, S.; Liu, C.; Wang, X.; Zhang, G.; Wang, F.; Wang, S.; Huang, J.; Mao, S.; Lei, Y.; et al. S100A7 as a potential diagnostic and prognostic biomarker of esophageal squamous cell carcinoma promotes M2 macrophage infiltration and angiogenesis. Clin. Transl. Med. 2021, 11, e459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, Y.; Fan, S.; Li, Y.; Xiao, Z.X.; Li, C. A double dealing tale of p63: An oncogene or a tumor suppressor. Cell. Mol. Life Sci. 2018, 75, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Efimova, T.; Dashti, S.R.; Balasubramanian, S.; Deucher, A.; Crish, J.F.; Sturniolo, M.; Bone, F. Keratinocyte survival, differentiation, and death: Many roads lead to mitogen-activated protein kinase. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2002; Volume 7. [Google Scholar]
- Chen, R.; Yang, X.; Ding, Z.; Zhu, L.; Lu, S.; Yu, Y. Lung squamous cell carcinoma: A postoperative recurrence analysis of keratinizing and nonkeratinizing subtypes. Eur. J. Surg. Oncol. 2019, 45, P838–P844. [Google Scholar] [CrossRef] [PubMed]
- Wolfer, S.; Elstner, S.; Schultze-Mosgau, S. Degree of Keratinization Is an Independent Prognostic Factor in Oral Squamous Cell Carcinoma. J. Oral. Maxillofac. Surg. 2018, 76, P444–P454. [Google Scholar] [CrossRef]
- Vanbokhoven, H.; Melino, G.; Candi, E.; Declercq, W. P63, a story of mice and men. J. Investig. Dermatol. 2011, 131, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Giovannini, S.; Wang, T.; Fang, J.; Li, P.; Shao, C.; Wang, Y.; TOR Centre; Shi, Y.; Candi, E.; et al. p63: A crucial player in epithelial stemness regulation. Oncogene 2023, 42, 3371–3384. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Prywes, R. Pathway regulation of p63, a director of epithelial cell fate. Front. Endocrinol. 2015, 6, 143040. [Google Scholar] [CrossRef] [PubMed]
- King, K.E.; George, A.L.; Sakakibara, N.; Mahmood, K.; Moses, M.A.; Weinberg, W.C. Intersection of the p63 and NF-κB pathways in epithelial homeostasis and disease. Mol. Carcinog. 2019, 58, 1571–1580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).