Abstract
Androgen receptor (AR) is a transcription factor expressed in various normal tissues and is a therapeutic target for prostate and possibly other cancers. A TMA containing 18,234 samples from 141 different tumor types/subtypes and 608 samples of 76 different normal tissue types was analyzed by immunohistochemistry. AR positivity was found in 116 tumor types including 66 tumor types (46.8%) with ≥1 strongly positive tumor. Moderate/strong AR positivity was detected in testicular sex cord-stromal tumors (93.3–100%) and neoplasms of the prostate (79.3–98.7%), breast (25.0–75.5%), other gynecological tumors (0.9–100%), kidney (5.0–44.1%), and urinary bladder (5.4–24.2%). Low AR staining was associated with advanced tumor stage (pTa versus pT2-4; p < 0.0001) in urothelial carcinoma; advanced pT (p < 0.0001), high tumor grade (p < 0.0001), nodal metastasis (p < 0.0001), and reduced survival (p = 0.0024) in invasive breast carcinoma; high pT (p < 0.0001) and grade (p < 0.0001) in clear cell renal cell carcinoma (RCC); and high pT (p = 0.0055) as well as high grade (p < 0.05) in papillary RCC. AR staining was unrelated to histopathological/clinical features in 157 endometrial carcinomas and in 221 ovarian carcinomas. Our data suggest a limited role of AR immunohistochemistry for tumor distinction and a prognostic role in breast and clear cell RCC and highlight tumor entities that might benefit from AR-targeted therapy.
1. Introduction
The androgen receptor (AR) belongs to the steroid receptor subfamily of nuclear receptors [1], is a ligand-dependent nuclear transcription factor, and is encoded by the AR gene located on the short arm of the X chromosome (Xq11-12). AR binds androgens and these can exert their actions in a DNA binding-dependent manner to regulate target gene transcription [2] or in a non-DNA-binding dependent manner to initiate rapid, cellular events such as the phosphorylation of second messenger signaling cascades [3]. AR is widely expressed in various tissues with the highest levels reported for reproductive tissues (testes, prostate, ovaries, uterus) and has a diverse range of physiological functions, such as the development and maintenance of the reproductive, musculoskeletal, cardiovascular, immune, neural, and hemopoietic systems [4].
As expected from its wide expression in normal tissue (proteinatlas.org, accessed on 28 December 2023), various different tumor types can express AR [5]. AR has been extensively investigated in “classic” hormone-dependent cancers such as prostate and breast cancer. However, data on AR immunostaining in tumors are highly variable, especially in supposedly hormone-independent cancers. For example, the reported AR positivity ranges 13–54% in urothelial carcinoma [6,7,8,9], 54–100% in salivary duct carcinoma [10,11,12,13,14], 22–78% in basal cell carcinoma of the skin [15,16,17,18], and 13–67% in oral squamous cell carcinoma [19,20,21,22]. The main relevance of AR expression in cancer comes from its role as a therapeutic target. Various drugs targeting the AR in several different ways are routinely used in prostate cancer patients (summarized in [23,24]). Successful targeting of the AR has also been reported in bladder cancer [25], salivary gland carcinoma [26], and breast cancer [27], where the AR is supposed to interact with estrogen receptor signaling [28]. A summary of published data on AR expression in human tumors is given in Figure 1.
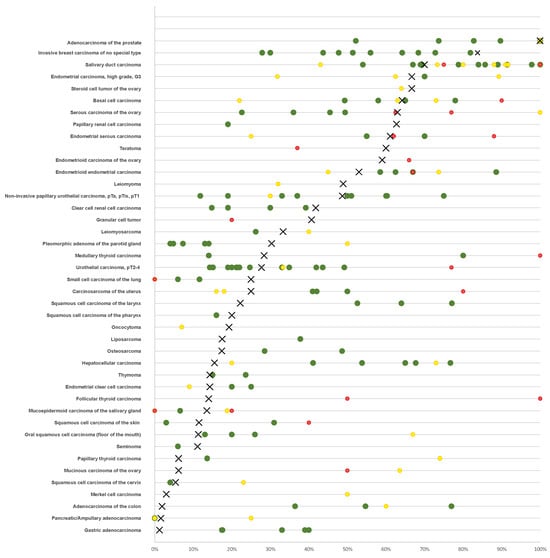
Figure 1.
Comparison with previous AR literature. An “X” indicates the fraction of AR positive cancers in the present study, dots indicate the reported frequencies from the literature for comparison: red dots mark studies with <10 analyzed tumors, yellow dots mark studies with >10–25 analyzed tumors, and green dots mark studies with >25 analyzed tumors.
Considering that AR immunostaining has a diagnostic, potential prognostic, and predictive role in other tumors apart from prostate cancer, a comprehensive and highly standardized study analyzing a large number of tumors from different tumor entities is needed. Therefore, AR expression was analyzed in more than 18,000 tumor tissue samples from 141 different tumor types and subtypes as well as 76 different non-neoplastic tissue types by immunohistochemistry (IHC) in a tissue microarray (TMA) format in this study.
2. Material and Methods
Tissue Microarrays (TMAs). Two sets of TMAs were used in this study. The first was a normal tissue TMA that was constructed from 8 samples from 8 different donors for each of the 76 different normal tissue types. The second was a set of cancer TMAs that were made from 18,234 primary tumors from 141 tumor types and subtypes, which were distributed across 62 TMA blocks containing between 85 and 613 tissue spots per block. Histopathological data including grade, pT, and pN status were available from 1073 urothelial carcinomas, 1680 breast carcinomas, 182 endometrioid endometrial cancers, 1224 clear cell renal cell carcinomas, and 369 serous ovarian carcinomas.
For the tumor TMAs, a database with clinical follow-up data was available. It included data from 254 patients (median follow-up time 14 months, range 1–77 months) with urothelial carcinoma, from 717 patients (median follow-up time 50 months, range 1–88 months) with invasive breast carcinoma (NST), and from 531 patients (median follow-up time 40 months, 1–250 months) with clear cell renal cell carcinoma. A detailed description of the composition of the normal tissue TMA and the cancer TMAs is given in the results section. All samples were taken from the tissue archive of the Institute of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and the Department of Pathology, Academic Hospital Fuerth, Germany. All tissues had been fixed in 4% buffered formalin prior to paraffin embedding. A single 0.6 mm tissue spot per tumor was used for TMA construction. The use of leftover tissue samples from diagnostic tissues for TMA construction and of anonymized patient data for statistical analysis is in accordance with local laws (HmbKHG, §12) and has been approved by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry (IHC). All 63 TMA sections were freshly prepared and immunostained at the same day in one experiment. After paraffin removal, slides were rehydrated through a graded alcohol series and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 7.8 buffer. Blocking of endogenous peroxidase with Dako Peroxidase Blocking Solution™ (Agilent Technologies Inc., Santa Clara, CA, USA; #S2023) was performed for 10 min. Primary antibody specific against AR (recombinant rabbit monoclonal, MSVA-367R; MS Validated Antibodies GmbH, Hamburg, Germany; #2145-376R) was applied at 37 °C for 60 min at a dilution of 1:450. The EnVision Kit™ (Agilent Technologies Inc., Santa Clara, CA, USA; #K5007) was used to detect bound antibody, and sections were counterstained with haemalaun. For the purpose of antibody validation, immunohistochemical staining of the normal tissue TMA was performed with a different anti-AR antibody (monoclonal rabbit, EPR1535(2), Abcam, Cambridge, UK; #133273) at a dilution of 1:300 and an otherwise identical protocol. One pathologist (SM) scored all tumors. For tumor tissues, the percentage of AR positive tumor cells was estimated and the staining intensity was semi-quantitatively recorded (0, 1+, 2+, 3+). The staining results were converted into four groups for statistical analysis: negative: no detectable staining; weak: staining intensity of 1+ in ≤70% or staining intensity of 2+ in ≤30% of tumor cells; moderate: staining intensity of 1+ in >70%, staining intensity of 2+ in >30% but in ≤70%, or staining intensity of 3+ in ≤30% of tumor cells; strong: staining intensity of 2+ in >70% or staining intensity of 3+ in >30% of tumor cells.
Statistics. Statistical calculations were performed with JMP 16 software (SAS Institute Inc., Cary, NC, USA). Contingency tables and the chi2-test were performed to search for associations between different AR staining levels, tumor phenotype, and patient gender. Survival curves for different levels of AR immunostaining in breast cancer samples were calculated according to Kaplan–Meier. The Log-Rank test was applied to detect significant differences between groups. A p-value of ≤0.05 was defined as significant.
3. Results
3.1. Technical Issues
A total of 14,408 (79.0%) of the arrayed tumors were interpretable for AR staining. Reasons for non-interpretable samples included unequivocal tumor cells or loss of the tissue spot during technical procedures. For each normal tissue type, a sufficient number of samples was evaluable.
3.2. AR Immunostaining in Normal Tissues
AR immunostaining was seen in a broad range of cell types and in most organs. AR staining was particularly strong in epithelial and stromal cells of the prostate and the seminal vesicle, tall columnar cells of the epididymis, Sertoli and Leydig cells of the testis, and in a subset of epithelial cells of the fallopian tube. A moderate nuclear AR staining was observed in sebaceous glands, stromal and epithelial cells of the endocervix, stroma cells of the endometrium, granulosa, theca interna, and stromal cells of the ovary, hepatocytes, a subset of glandular cells of the breast, basal cells of the respiratory epithelium, myometrium, and in skeletal muscle cells. In squamous epithelium, a weak to moderate staining of a fraction of cells occurred, predominantly in the lower half of the epithelium. A weak AR immunostaining also occurred in some cells of proximal tubuli and glomeruli in some kidney samples, gallbladder epithelium, excretory and intercalated ducts as well as some islet cells of the pancreas, glandular cells of salivary glands, gastric glands, thyroid glandular cells, urothelium, decidua cells of the pregnant uterus, smooth muscle cells, and in a fraction of cells in the aortic wall. AR immunostaining was completely absent in the adrenal gland, parathyroid, hematopoietic and lymphoid tissues, lung, hypophysis, and the brain. Representative images of AR staining are shown in Figure 2. All these findings were observed by both MSVA-367R and EPR1535(2) (Supplementary Figure S1).

Figure 2.
AR immunostaining in normal tissues. (A) Positive AR immunostaining of epithelial cells, basal cells, and stromal cells in the prostate gland. (B) Positive AR immunostaining of Sertoli cells and Leydig cells in testis. (C) Positive AR immunostaining of epithelial and stromal cells in epididymis. (D) Positive AR immunostaining of secretory cells in the fallopian tube. (E) Positive AR immunostaining of ovarian stromal cells (F) Positive AR immunostaining of stromal cells in proliferative endometrium. (G) Positive AR immunostaining of hepatocytes. (H) Positive AR immunostaining in a sebaceous gland of the skin.
3.3. AR Immunostaining in Neoplastic Tissues
AR immunostaining was found in 30.8% of 14,408 cases, including 37.3% with weak, 16.1% with moderate, and 46.6% with strong positivity. A total of 116 (82.3%) of 141 tumor categories included at least one AR positive case and 66 (46.8%) contained at least one tumor with strong AR staining (Table 1; a graphical summary is given in Supplementary Figure S2). Representative images of AR positive tumors are shown in Figure 3. A ranking of tumor categories according to the rate of AR positivity is given in Figure 4. A particularly high percentage of tumors with moderate to strong AR immunostaining was detected in sex cord-stromal tumors of the testis (93.3–100%), adenocarcinoma of the prostate (79.3–98.7%), breast neoplasms (25.0–75.5%), other gynecological tumors (0.9–100%), renal cell carcinomas (5.0–44.1%), and in urothelial carcinomas (5.4–24.2%).

Table 1.
AR immunostaining in tumors. Int. = interpretable, Neg. = negative, Mod. = moderate, Str. = strong.
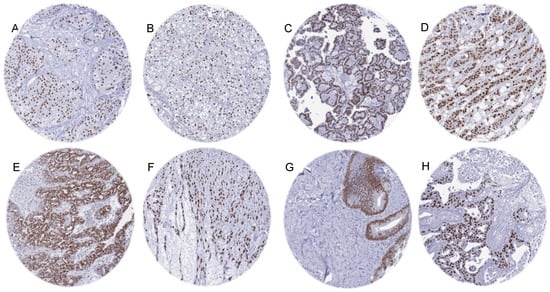
Figure 3.
Moderate to strong AR immunostaining in “non-genital” tumors. (A) Urothelial carcinoma of the bladder. (B) Clear cell renal cell carcinoma. (C) Papillary renal cell carcinoma. (D) Invasive breast carcinoma of no special type (NST). (E) High-grade serous carcinoma of the ovary. (F) Uterine leiomyosarcoma. (G) Adenocarcinoma of the colon. (H) Salivary duct carcinoma.

Figure 4.
Ranking order of AR immunostaining in cancers. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots).
3.4. AR Immunostaining, Tumor Phenotype, and Prognosis
Low AR staining was significantly associated with adverse histopathological and clinical features in several tumor types (Table 2). Low AR was linked to advanced tumor stage (pTa versus pT2-4; p < 0.0001) in urothelial carcinoma. In invasive breast carcinoma (NST), low AR staining was associated with high tumor stage (p < 0.0001), high tumor grade (p < 0.0001), lymph node metastasis (p < 0.0001), and shorter overall survival (p = 0.0094, Supplementary Figure S3). In clear cell renal cell carcinoma (RCC), low AR staining was liked to high pT stage (p < 0.0001) and high tumor grade (p < 0.0001). In papillary RCC, low AR staining was related to high pT stage (p = 0.0055) as well as high ISUP (p = 0.0179) and Fuhrman grade (p = 0.0018). AR staining was unrelated to histopathological features and clinical features in 157 endometroid endometrial carcinomas and in 221 serous ovarian carcinomas.

Table 2.
AR immunostaining and prognosis in invasive breast carcinoma (NST), urothelial carcinoma, and clear cell carcinoma of the kidney. Neg. = negative, Mod. = moderate, Str. = strong.
3.5. AR Immunostaining, Gender and Age Distribution
Within non-mammary, non-gynecological, non-prostate, and non-testicular tumors, AR positivity was more common in tumors from male (25.9% of 4329) than from female patients (13.1% of 2868; p < 0.0001). This also held true within the separate cohorts of tumors of the urinary bladder and tumors of the kidney (p < 0.0001; Table 3). A subdivision of tumors from male patients in different age groups showed that AR positive tumors were significantly more common in older patients (p = 0.0044).

Table 3.
AR immunostaining in female and male patients (excluding tumors of the female genital tract, tumors of the male genital tract, and breast tumors).
4. Discussion
More than 2650 articles listed in PubMed® have described immunohistochemical evaluations of AR in cancer (PubMed® search (10/23: “androgen receptor cancer immunohisto*”). The articles generally agree on prostatic adenocarcinomas being the most commonly AR positive cancer entities but published data on AR expression in other tumor entities vary considerably. The high diversity of published data on AR expression in human tumors primarily reflects the range of different antibodies, laboratory protocols, interpretation criteria, and thresholds to define positive AR staining in these studies. Our highly standardized analysis of 18,234 tumors from 141 human tumor types and subtypes has resulted in a ranking order according to the prevalence of AR expression. This represents the key finding of our study. A compilation of data from previous studies (Figure 1) demonstrates that such information could not be extracted from the literature. Our data demonstrate that AR positivity is similarly frequent in testicular and ovarian sex-cord stroma tumors as in prostatic adenocarcinomas although the AR expression levels tend to be lower in sex-cord stroma tumors and that neoplasms of the breast, other carcinomas of the female genital tract, renal cell carcinomas, and urothelial carcinomas represent further tumor categories with frequent and often strong AR expression. Most importantly, our data also show that—often at lower frequency—AR expression can also be found in a broad variety of other tumor types. The occurrence of high-level AR expression in many different tumor entities is consistent with the RNA expression data summarized in the TCGA database (https://www.cancer.gov/tcga, accessed on 28 December 2023).
In diagnostic pathology, several applications of AR immunostaining have been proposed but are not strongly supported by our data. Based on the frequent high-level AR expression in prostatic adenocarcinoma, AR IHC is applied for the distinction of poorly differentiated prostatic cancer from urothelial carcinoma. For example, Downes et al. described intense AR positivity in 100% of prostatic cancers and negative or only weak staining in urothelial carcinomas and found that AR IHC was superior to prostate specific markers such as PSA and PSAP in discriminating poorly differentiated urothelial carcinoma from high grade prostate carcinoma [29]. However, considering that 27.7% of muscle-invasive urothelial carcinomas were AR positive (5.6% with strong AR staining) in our study, the diagnostic potential of AR appears to be limited in this setting. AR immunostaining was also proposed for the distinction of sebaceous from basal cell and squamous cell carcinoma of the skin [30]. Yet in our study, 64.2% of basal cell carcinomas and 11.5% of squamous cell carcinomas of the skin were AR positive. In breast cancer, AR IHC has been proposed as a tool for the detection of apocrine differentiation [31] and metastases from triple-negative cancers [32]. Given that in 54.7% of our breast carcinomas, NST showed strong AR positivity, AR IHC may not be optimal for verifying apocrine differentiation. Since only 36.8% of triple negative breast carcinomas showed AR positivity in this study and the overall specificity of AR for breast cancer was low, the use of AR IHC for verifying breast cancer origin of AR positive metastases is limited. With GATA3 and TRPS1, more specific breast cancer markers are available that showed 55.4% (GATA3, [33]) and 77.8% positivity (TRPS1, manuscript in revision) in triple-negative breast cancers in our tumor cohort.
The large number of tumors analyzed for several tumor categories enabled an analysis of the potential clinical significance of AR expression. In breast, urothelial, and renal cell carcinomas, detectable AR staining was associated with favorable histopathological and clinical features. For breast cancer, previous data are controversial. Some authors also reported a favorable prognostic role of AR expression [34,35], but others found no association between AR expression and prolonged disease-free survival [36]. The correlation between high AR expression and the non-invasive stage for urothelial carcinoma is consistent with results from several earlier studies [37,38,39]. The significant association between positive AR staining and favorable tumor features seen in our clear cell carcinomas of the kidney is also in line with previous studies [9,37,40,41,42]. Given the tendency towards adjuvant therapy in high-risk renal cell carcinomas and the corresponding need for risk assessment in these tumors, AR might deserve further evaluation as a potential clinically useful prognostic marker in these tumors.
Most of the importance of AR comes from its well-proven role as a highly suited therapeutic target for adenocarcinomas of the prostate [23]. Given the frequent and often high-level AR expression in many other tumor types, it is obvious to consider AR targeting therapies for these tumors. The successful use of anti-androgen drugs has been reported in various cancer types including carcinomas of the breast [43], salivary gland carcinoma [44,45], ovarian cancer [46], bladder cancer [47], and salivary duct carcinoma [10,48,49]. Clinical trials on the use of anti-androgen drugs in non-prostate cancers are currently underway (according to clinicaltrials.gov, accessed on 28 December 2023) in breast carcinoma (especially triple negative breast cancer) (n = 12), salivary gland carcinoma (n = 6), or bladder cancer (n = 2). Once a more widespread clinical utility of these drugs should become evident, our ranking order of tumors according to their AR positivity rates would define the tumor entities that can benefit most from such approaches and which would be best suited for further clinical trials.
Whether AR has a biological role in tumors with only minor immunohistochemical AR positivity remains unclear. Mechanistically, AR associates with heat shock proteins and cellular chaperones in the cytoplasm in its inactive form. Upon binding of androgens, AR dissociates from the complex, forms homodimers, and migrates into the nucleus. Such activated AR binds to androgen response elements together with co-regulators and leads to the transcriptional regulation of a large variety of target genes. However, the number and kind of AR-co-regulators and AR-responsive genes in different normal and cancerous tissues, particularly in cancers other than prostatic tumors, and the impact of the level of AR are still the subjects of current research [50,51,52]. The theory that low-level AR expression is non-random is supported by the significantly higher rate of positive AR immunostaining in “non-genital” tumors from male than from female patients. Since comprehensive understanding of the mechanisms controlling the expression of the receptor in different target tissues is lacking, it can only be hypothesized that this is due to gender-associated differences in the testosterone serum levels. Intuitively, one would assume that a cell would benefit more from AR upregulation in a testosterone-rich environment. It should be noted, however, that regulation of AR is cell- and tissue specific and androgens can have a negative as well as positive auto-regulation on AR expression [53]. A negative auto-regulation by dihydrotestosterone on AR expression [53] could potentially explain the lower percentage of AR-positive tumors in younger male patients.
The large scale of our study prompted us to place special emphasis on a thorough validation of our assay. We followed the proposal of the International Working Group for Antibody Validation (IWGAV). According to the IWGAV, validation of antibodies used for IHC on formalin-fixed tissues must include either a comparison of the findings obtained by two different independent antibodies or a comparison with expression data obtained by another independent method [54]. The rather ubiquitous expression of AR identified in our IHC analysis of normal tissues is largely consistent with RNA expression data from three public databases [55], which were compiled in the Human Protein Atlas [56,57]. but given the cell type specificity of AR expression, a comparison with a method based on disaggregated tissue is suboptimal for this protein. The critical evidence for the validity of our assay comes from confirmation that all AR positive cell types seen by MSVA-367R were also seen by the independent second antibody EPR1535(2). It is of note that the use of a very broad range of different tissues (76 different normal tissue categories) for antibody validation increases the likelihood of detecting undesired cross-reactivities because virtually all proteins occurring in normal cells of adult humans are subjected to the validation experiment.
There were some limitations to our study. The number of tumors analyzed for many common tumor types was not always as high as would have been desirable, and some rare tumor types were not even represented in our series of TMAs. In addition, clinical follow-up data were not available for all samples and tumor types. It would be interesting for future studies to investigate the clinical significance of AR expression in all these different tumor types that recurrently showed positive AR immunostaining.
5. Conclusions
Our analysis of 141 different tumor types for AR immunostaining provides a comprehensive overview on AR in human tumors and identifies AR expression in many “hormone-dependent” and “hormone-independent” tumor types. The data suggest a limited role of AR IHC for the distinction of tumors and a strong prognostic role in breast cancer and clear cell RCC and highlight numerous tumor entities that could benefit from androgen-targeting cancer drugs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12050957/s1, Figure S1. IHC validation by comparison of antibodies. The panels show a concordance of immunostaining results obtained by two independent AR antibodies (MSVA-367R, EPR1535(2)). Using MSVA-367R, a nuclear AR positivity of variable intensity was seen in sebaceous glands of the skin (A), stromal and epithelial cells of the endocervix (B), a subset of glandular cells of the breast (C), some glandular cells of salivary glands (D), few basal cells of the respiratory epithelium (E), a subset of epithelial cells of the gallbladder (F), and in some tubular cells in the kidney (G), while the adenohypophysis did not show unequivocal staining (H). Using clone EPR1535(2), a staining of identical cell types was seen in the skin (I), endocervix (K), breast (L), salivary gland (M), respiratory epithelium (N), gallbladder (O), and the kidney (P), while the adenohypophysis was again negative (Q). The images A-H and I-Q are from consecutive tissue sections. Figure S2. Graphical summary of AR immunostaining in tumors. Figure S3. Prognostic relevance of AR immunostaining in breast cancers of no special type (NST). “Low” indicates negative or weak AR immunostaining, “high” indicates moderate to strong staining.
Author Contributions
F.V., J.H., L.-M.T., M.K., R.S., G.S. and S.M.: contributed to conception, design, data collection, data analysis, and manuscript writing. C.B., C.F., A.M.L., F.B., T.K., A.H., F.J., E.B., S.S., A.H.M., T.S.C. and P.L.: participated in pathology data analysis, data interpretation, and collection of samples. R.S., M.K. and C.H.-M.: data analysis. S.M., R.S. and G.S.: study supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis have been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study (HmbKHG, §12).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We are grateful to Laura Behm, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Conflicts of Interest
Guido Sauter is a family member of the owner of MS Validated Antibodies GmbH, who provided the antibody MSVA-367R. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.J.; Wang, C.; Mizokami, A. Androgen receptor: An overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef]
- Eder, I.E.; Culig, Z.; Putz, T.; Nessler-Menardi, C.; Bartsch, G.; Klocker, H. Molecular biology of the androgen receptor: From molecular understanding to the clinic. Eur. Urol. 2001, 40, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Davey, R.A.; Zajac, J.D. Human androgen deficiency: Insights gained from androgen receptor knockout mouse models. Asian J. Androl. 2014, 16, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lee, S.O.; Yeh, S.; Chang, T.M. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014, 33, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Mir, C.; Shariat, S.F.; van der Kwast, T.H.; Ashfaq, R.; Lotan, Y.; Evans, A.; Skeldon, S.; Hanna, S.; Vajpeyi, R.; Kuk, C.; et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: A large multi-institutional study. BJU Int. 2011, 108, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.M.; Higgins, J.P.; Sangoi, A.R.; McKenney, J.K.; Troxell, M.L. Androgen receptor immunohistochemistry in genitourinary neoplasms. Int. Urol. Nephrol. 2015, 47, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Lo Vullo, S.; Giannatempo, P.; Raggi, D.; Perrone, F.; Nicolai, N.; Catanzaro, M.; Biasoni, D.; Torelli, T.; Piva, L.; et al. Association of Androgen Receptor Expression on Tumor Cells and PD-L1 Expression in Muscle-Invasive and Metastatic Urothelial Carcinoma: Insights for Clinical Research. Clin. Genitourin. Cancer 2018, 16, e403–e410. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.; Ugras, S.; Mongan, N.P.; Gudas, L.J.; You, X.; Tickoo, S.K.; Scherr, D.S. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 2004, 64, 383–388. [Google Scholar] [CrossRef]
- van Boxtel, W.; Uijen, M.J.M.; Verhaegh, G.W.; Willems, S.M.; Jonker, M.A.; Group, P.; Schalken, J.A.; van Engen-van Grunsven, I.C.H.; van Herpen, C.M.L. Prognostic value of PSMA, c-MET and E-cadherin in salivary duct carcinoma. Oral Oncol. 2020, 110, 105018. [Google Scholar] [CrossRef]
- Xu, B.; Dogan, S.; Haroon Al Rasheed, M.R.; Ghossein, R.; Katabi, N. Androgen receptor immunohistochemistry in salivary duct carcinoma: A retrospective study of 188 cases focusing on tumoral heterogeneity and temporal concordance. Hum. Pathol. 2019, 93, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.A.; Gauthier, M.A.; Blackburn, J.; Grady, J.P.; Kraitsek, S.; Hajdu, E.; Dettmer, M.S.; Dahlstrom, J.E.; Lee, C.S.; Luk, P.P.; et al. Molecular patterns in salivary duct carcinoma identify prognostic subgroups. Mod. Pathol. 2020, 33, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Villepelet, A.; Lefevre, M.; Verillaud, B.; Janot, F.; Garrel, R.; Vergez, S.; Bertolus, C.; Malard, O.; de Gabory, L.; Mauvais, O.; et al. Salivary duct carcinoma: Prospective multicenter study of 61 cases of the Reseau d’Expertise Francais des Cancers ORL Rares. Head Neck 2019, 41, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Gargano, S.M.; Senarathne, W.; Feldman, R.; Florento, E.; Stafford, P.; Swensen, J.; Vranic, S.; Gatalica, Z. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. 2019, 8, 7322–7329. [Google Scholar] [CrossRef] [PubMed]
- Bourlond, F.; Velter, C.; Cribier, B. Androgen receptor expression in epidermal and adnexal tumours. Ann. Dermatol. Venereol. 2021, 148, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Arits, A.H.; Van Marion, A.M.; Lohman, B.G.; Thissen, M.R.; Steijlen, P.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Differentiation between basal cell carcinoma and trichoepithelioma by immunohistochemical staining of the androgen receptor: An overview. Eur. J. Dermatol. 2011, 21, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Astarci, H.M.; Unsal, G.; Sengul, D.; Hucumenoglu, S.; Kocer, U.; Ustun, H. Significance of androgen receptor and CD10 expression in cutaneous basal cell carcinoma and trichoepithelioma. Oncol. Lett. 2015, 10, 3466–3470. [Google Scholar] [CrossRef]
- Izikson, L.; Bhan, A.; Zembowicz, A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am. J. Dermatopathol. 2005, 27, 91–95. [Google Scholar] [CrossRef]
- Adnan, Y.; Ali, S.M.A.; Awan, M.S.; Idress, R.; Awan, M.O.; Farooqui, H.A.; Kayani, H.A. Hormone receptors AR, ER, PR and growth factor receptor Her-2 expression in oral squamous cell carcinoma: Correlation with overall survival, disease-free survival and 10-year survival in a high-risk population. PLoS ONE 2022, 17, e0267300. [Google Scholar] [CrossRef]
- Tomasovic-Loncaric, C.; Fucic, A.; Andabak, A.; Andabak, M.; Ceppi, M.; Bruzzone, M.; Vrdoljak, D.; Vucicevic-Boras, V. Androgen Receptor as a Biomarker of Oral Squamous Cell Carcinoma Progression Risk. Anticancer Res. 2019, 39, 4285–4289. [Google Scholar] [CrossRef]
- Marocchio, L.S.; Giudice, F.; Correa, L.; Pinto Junior Ddos, S.; de Sousa, S.O. Oestrogens and androgen receptors in oral squamous cell carcinoma. Acta Odontol. Scand. 2013, 71, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.F.; Luo, F.J.; Chang, Y.L.; Huang, C.M.; Chiu, W.J.; Weng, C.F.; Hsu, Y.K.; Yuan, T.C. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells. Oral Dis. 2015, 21, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Hankey, W.; Fang, X.; Yuan, F. Second generation androgen receptor antagonists and challenges in prostate cancer treatment. Cell Death Dis. 2022, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Gupta, S. Androgen receptor in bladder cancer: A promising therapeutic target. Asian J. Urol. 2020, 7, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.N.; Lakritz, S.; Mandair, D.; Ulanja, M.B.; Bowles, D.W. A molecular guide to systemic therapy in salivary gland carcinoma. Head Neck 2023, 45, 1315–1326. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.R.; Torlakovic, E.E.; Aldaoud, N.; Zlotta, A.R.; Evans, A.J.; van der Kwast, T.H. Diagnostic utility of androgen receptor expression in discriminating poorly differentiated urothelial and prostate carcinoma. J. Clin. Pathol. 2013, 66, 779–786. [Google Scholar] [CrossRef]
- Asadi-Amoli, F.; Khoshnevis, F.; Haeri, H.; Jahanzad, I.; Pazira, R.; Shahsiah, R. Comparative examination of androgen receptor reactivity for differential diagnosis of sebaceous carcinoma from squamous cell and basal cell carcinoma. Am. J. Clin. Pathol. 2010, 134, 22–26. [Google Scholar] [CrossRef]
- Quinn, C.M.; D’Arcy, C.; Wells, C. Apocrine lesions of the breast. Virchows Arch. 2022, 480, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Tozbikian, G.H.; Zynger, D.L. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple-negative breast cancer. Hum. Pathol. 2019, 85, 221–227. [Google Scholar] [CrossRef]
- Reiswich, V.; Schmidt, C.E.; Lennartz, M.; Hoflmayer, D.; Hube-Magg, C.; Weidemann, S.; Fraune, C.; Buscheck, F.; Moller, K.; Bernreuther, C.; et al. GATA3 Expression in Human Tumors: A Tissue Microarray Study on 16,557 Tumors. Pathobiology 2023, 90, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Tsvetkova, V.; Griguolo, G.; Miglietta, F.; Mantiero, M.; Tasca, G.; Cumerlato, E.; Giorgi, C.A.; Giarratano, T.; Faggioni, G.; et al. Androgen Receptor Expression and Association With Distant Disease-Free Survival in Triple Negative Breast Cancer: Analysis of 263 Patients Treated With Standard Therapy for Stage I–III Disease. Front. Oncol. 2019, 9, 452. [Google Scholar] [CrossRef]
- Jahan, N.; Jones, C.; Rahman, R.L. Androgen receptor expression in breast cancer: Implications on prognosis and treatment, a brief review. Mol. Cell Endocrinol. 2021, 531, 111324. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, M.; Caramanti, M.; Biscotti, T.; Santinelli, A.; Pagliacci, A.; De Lisa, M.; Ballatore, Z.; Ridolfi, F.; Maccaroni, E.; Bracci, R.; et al. Androgen receptor expression in early triple-negative breast cancer: Clinical significance and prognostic associations. Cancers 2014, 6, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yao, J.L.; Chaux, A.; Zheng, Y.; Hsu, I.; Izumi, K.; Chang, C.; Messing, E.M.; Netto, G.J.; Yeh, S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012, 109, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Hussain, Z.; Janjua, T.K.; Hashmi, A.A.; Qureshi, S.S.; Tariq, M.U.; Faridi, N. Androgen Receptor: Evaluation and Correlation with Recurrence and Clinicopathological Parameters in Papillary Urothelial Carcinomas of the Urinary Bladder. Cureus 2020, 12, e6715. [Google Scholar] [CrossRef] [PubMed]
- Tuygun, C.; Kankaya, D.; Imamoglu, A.; Sertcelik, A.; Zengin, K.; Oktay, M.; Sertcelik, N. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: A comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol. Oncol. 2011, 29, 43–51. [Google Scholar] [CrossRef]
- Hata, S.; Ise, K.; Azmahani, A.; Konosu-Fukaya, S.; McNamara, K.M.; Fujishima, F.; Shimada, K.; Mitsuzuka, K.; Arai, Y.; Sasano, H.; et al. Expression of AR, 5alphaR1 and 5alphaR2 in bladder urothelial carcinoma and relationship to clinicopathological factors. Life Sci. 2017, 190, 15–20. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, L.; Li, L.; Dang, Q.; Song, W.; Yeh, S.; He, D.; Chang, C. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology 2014, 83, 510.e19–510.e24. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ge, Y.; Liu, X.; Wang, S.; Ye, Z.; Xu, H.; Chen, Z. The Association of Androgen Receptor Expression with Renal Cell Carcinoma Risk: A Systematic Review and Meta-Analysis. Pathol. Oncol. Res. 2020, 26, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Choupani, E.; Mahmoudi Gomari, M.; Zanganeh, S.; Nasseri, S.; Haji-Allahverdipoor, K.; Rostami, N.; Hernandez, Y.; Najafi, S.; Saraygord-Afshari, N.; Hosseini, A. Newly Developed Targeted Therapies Against the Androgen Receptor in Triple-Negative Breast Cancer: A Review. Pharmacol. Rev. 2023, 75, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Kiyota, N.; Tahara, M.; Hanai, N.; Asakage, T.; Matsuura, K.; Ota, I.; Saito, Y.; Sano, D.; Kodaira, T.; et al. Systemic therapy for salivary gland malignancy: Current status and future perspectives. Jpn. J. Clin. Oncol. 2022, 52, 293–302. [Google Scholar] [CrossRef]
- Yeoh, C.C.; Dabab, N.; Rigby, E.; Chhikara, R.; Akaev, I.; Gomez, R.S.; Fonseca, F.; Brennan, P.A.; Rahimi, S. Androgen receptor in salivary gland carcinoma: A review of an old marker as a possible new target. J. Oral Pathol. Med. 2018, 47, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Limaye, S.; Kumar, P.; Pragya, R.; Sambath, J.; Patil, D.; Srinivasan, A.; Apurva, S.; Srivastava, N.; Patil, S.; Patil, R.; et al. Addendum: A case report of androgen receptor inhibitor therapy in recurrent high-grade serous ovarian cancer. Oncotarget 2022, 13, 982. [Google Scholar] [CrossRef]
- Kourbanhoussen, K.; McMartin, C.; Lodde, M.; Zlotta, A.; Bryan, R.T.; Toren, P. Switching Cancers: A Systematic Review Assessing the Role of Androgen Suppressive Therapy in Bladder Cancer. Eur. Urol. Focus 2021, 7, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, W.; Locati, L.D.; van Engen-van Grunsven, A.C.H.; Bergamini, C.; Jonker, M.A.; Fiets, E.; Cavalieri, S.; Tooten, S.; Bos, E.; Quattrone, P.; et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor-positive salivary duct carcinoma. Eur. J. Cancer 2019, 110, 62–70. [Google Scholar] [CrossRef]
- Boon, E.; van Boxtel, W.; Buter, J.; Baatenburg de Jong, R.J.; van Es, R.J.J.; Bel, M.; Fiets, E.; Oosting, S.F.; Slingerland, M.; Hoeben, A.; et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands. Head Neck 2018, 40, 605–613. [Google Scholar] [CrossRef]
- Quintero, J.C.; Diaz, N.F.; Rodriguez-Dorantes, M.; Camacho-Arroyo, I. Cancer Stem Cells and Androgen Receptor Signaling: Partners in Disease Progression. Int. J. Mol. Sci. 2023, 24, 15085. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.P.; Quan, C.; Zu, X.; Ou, Z.; Tsai, Y.C.; Messing, E.; Yeh, S.; Chang, C. The androgen receptor in bladder cancer. Nat. Rev. Urol. 2023, 20, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, S.; Maltoni, R.; Pasculli, B.; Parrella, P.; Giudetti, A.M.; Vergara, D.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. Androgen receptor in breast cancer: The “5W” questions. Front. Endocrinol. 2022, 13, 977331. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.W.; Watt, K.; Hunter, I.; Lavery, D.N.; MacKenzie, A.; McEwan, I.J. Negative regulation of the androgen receptor gene through a primate-specific androgen response element present in the 5′ UTR. Horm. Cancer 2014, 5, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Bandrowski, A.; Carr, S.; Edwards, A.; Ellenberg, J.; Lundberg, E.; Rimm, D.L.; Rodriguez, H.; Hiltke, T.; Snyder, M.; et al. A proposal for validation of antibodies. Nat. Methods 2016, 13, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Bjorling, E.; Agaton, C.; Szigyarto, C.A.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).