Sex Differences in the Expression of Cardiac Remodeling and Inflammatory Cytokines in Patients with Obstructive Sleep Apnea and Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Sample Management
2.2. Study of Overnight PSG and Determination of SRBD Metrics
2.3. Definition of AF, HF with Preserved or Mildly Reduced EF, and Echocardiographic Parameters
2.4. HL-1 Cell Culture
2.5. Isolation, Purification, Quantification, and Incubation of Exosomes with HL-1 Cells
2.6. Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction Analysis (qRT-PCR) of mRNA Expression in HL-1 Cells
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Echocardiographic Data of Those OSA Patients with and without AF and Control Subjects
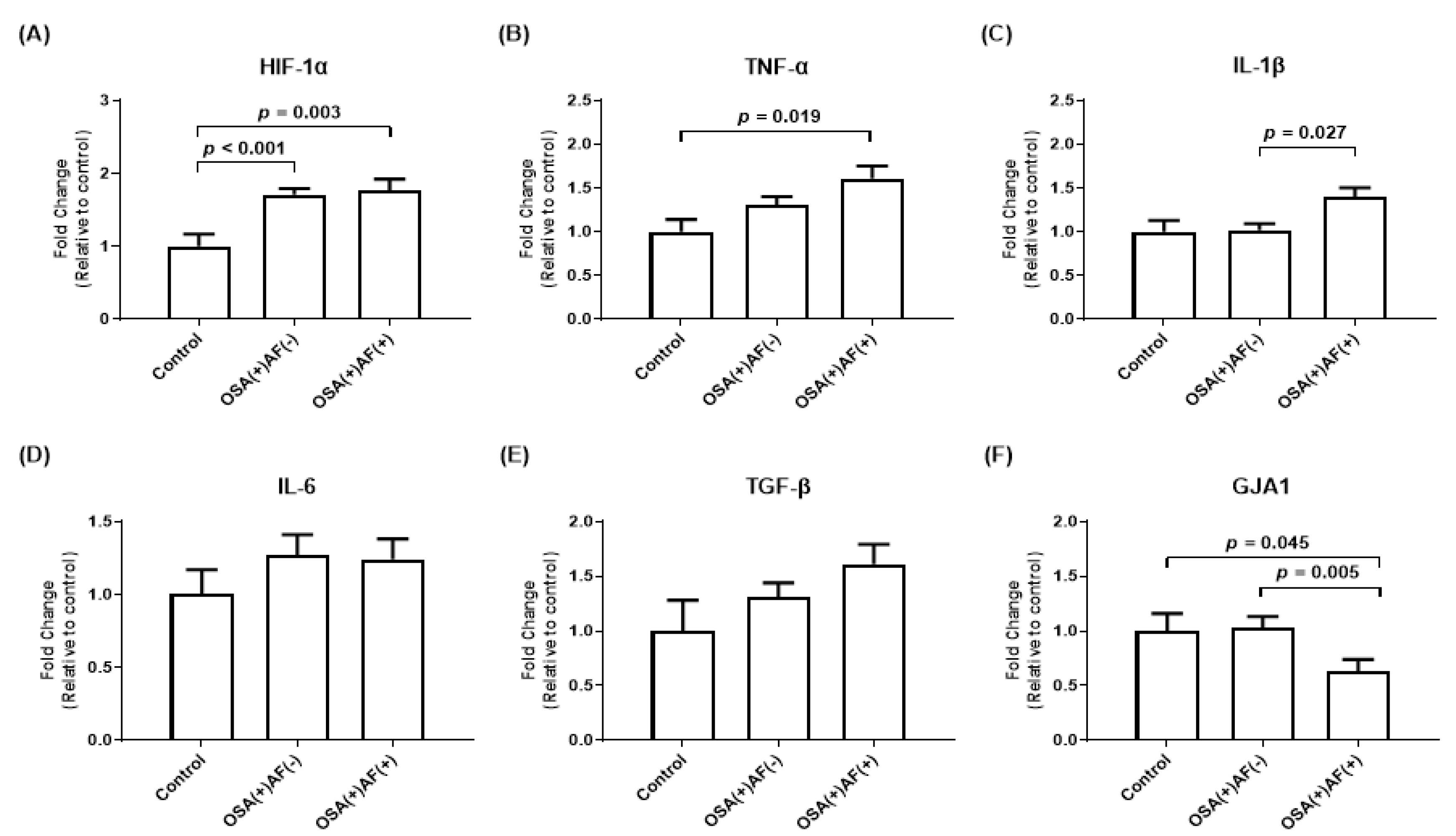
3.2. mRNA Gene Expression of HIF-1α and Inflammatory Cytokines in HL-1 Cells Treated with Exosomes Obtained from OSA Patients with and without AF and Control Subjects
3.3. The Differences in Baseline Characteristics and Echocardiographic Data among Those Male OSA Patients with and without AF and Control Subjects
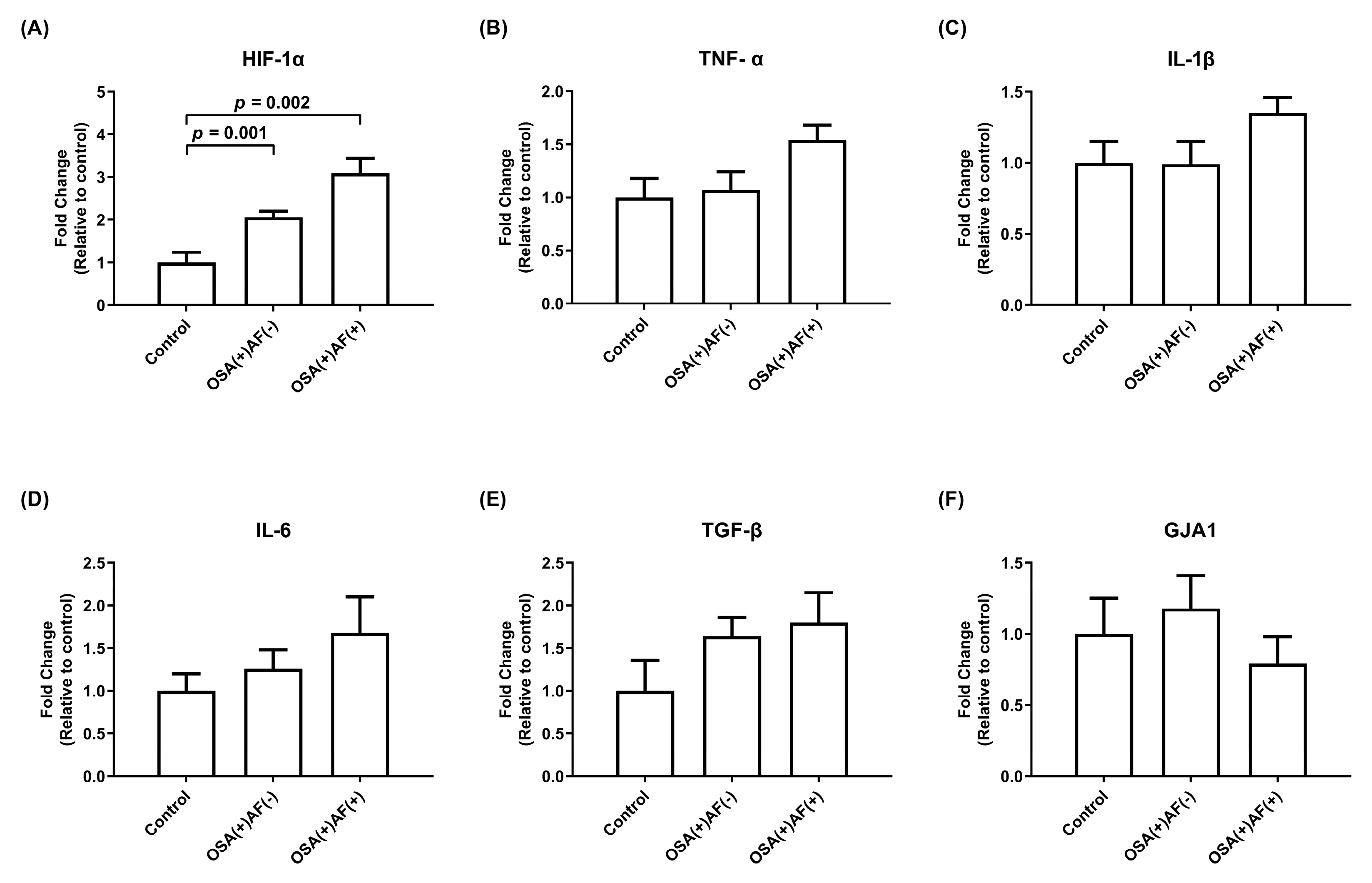
3.4. mRNA Gene Expression of HIF-1α and Inflammatory Cytokines in HL-1 Cells Treated with Exosomes Obtained from Those Male OSAS Patients with and without AF and Control Subjects
3.5. The Differences in Baseline Characteristics and Echocardiographic Data among Those Female OSA Patients with and without AF and Control Subjects
3.6. mRNA Gene Expression of HIF-1α and Inflammatory Cytokines in HL-1 Cells Treated with Exosomes Obtained from Female Significant OSAS Patients with and without AF and Control Subjects
3.7. The Relationship between Age, BMI, Echocardiographic Parameters (LA Size, LVEF, and E/e’ Ratio), Gene Expression of Biomarkers (IL-1β and GJA1), and AF Occurrence in OSA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/american College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Lip, G.Y.; Lane, D.A. Recent developments in understanding epidemiology and risk determinants of atrial fibrillation as a cause of stroke. Can. J. Cardiol. 2013, 29, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, I.H.; Roberts-Thomson, K.C.; Kistler, P.M.; Edwards, G.A.; Spence, S.; Sanders, P.; Kalman, J.M. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: Implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm. 2010, 7, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.K.; Kato, T.; Xiong, F.; Shi, Y.F.; Naud, P.; Maguy, A.; Mizuno, K.; Tardif, J.C.; Comtois, P.; Nattel, S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J. Am. Coll. Cardiol. 2014, 64, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Lo Martire, V.; Silvani, A.; Bastianini, S.; Berteotti, C.; Zoccoli, G. Effects of ambient temperature on sleep and cardiovascular regulation in mice: The role of hypocretin/orexin neurons. PLoS ONE 2012, 7, e47032. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Grimaldi, D.; Vandi, S.; Barletta, G.; Vetrugno, R.; Provini, F.; Pierangeli, G.; Berteotti, C.; Montagna, P.; Zoccoli, G.; et al. Sleep-dependent changes in the coupling between heart period and blood pressure in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1686–R1692. [Google Scholar] [CrossRef]
- Silvani, A.; Dampney, R.A. Central control of cardiovascular function during sleep. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1683–H1692. [Google Scholar] [CrossRef]
- Abboud, F.; Kumar, R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J. Clin. Investig. 2014, 124, 1454–1457. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Lebek, S.; Hegner, P.; Tafelmeier, M.; Rupprecht, L.; Schmid, C.; Maier, L.S.; Arzt, M.; Wagner, S. Female Patients With Sleep-Disordered Breathing Display More Frequently Heart Failure With Preserved Ejection Fraction. Front. Med. 2021, 8, 675987. [Google Scholar] [CrossRef]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Schla, H.F.I. Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Simon, D.N.; Steinberg, B.A.; Thomas, L.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; Hylek, E.; Kowey, P.R.; Reiffel, J.A.; et al. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results from the ORBIT-AF Registry. JAMA Cardiol. 2016, 1, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, B.; Nattel, S. MicroRNAs and atrial fibrillation: Mechanisms and translational potential. Nat. Rev. Cardiol. 2015, 12, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.E.; Deiss, S.; Dilling-Boer, D.; Didenko, M.; Eriksson, U.; Nedios, S.; Ng, F.S.; Roca Luque, I.; Sanchez Borque, P.; Vernooy, K.; et al. Mechanisms of sex differences in atrial fibrillation: Role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace 2019, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, Y.C.; Chang, Y.T.; Wang, H.T.; Liu, W.H.; Chong, S.Z.; Lin, P.T.; Hsu, P.Y.; Su, M.C.; Lin, M.C. GJA1 Expression and Left Atrial Remodeling in the Incidence of Atrial Fibrillation in Patients with Obstructive Sleep Apnea Syndrome. Biomedicines 2021, 9, 1463. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Su, M.C.; Liu, W.H.; Wang, C.C.; Lin, M.C.; Chen, M.C. Influence and predicting variables of obstructive sleep apnea on cardiac function and remodeling in patients without congestive heart failure. J. Clin. Sleep. Med. 2014, 10, 57–64. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, Y.C.; Wang, H.T.; Chang, Y.T.; Fang, Y.N.; Hsueh, S.; Liu, W.H.; Lin, P.T.; Hsu, P.Y.; Su, M.C.; et al. The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome. Life 2022, 12, 148. [Google Scholar] [CrossRef]
- Redline, S.; Budhiraja, R.; Kapur, V.; Marcus, C.L.; Mateika, J.H.; Mehra, R.; Parthasarthy, S.; Somers, V.K.; Strohl, K.P.; Sulit, L.G.; et al. The scoring of respiratory events in sleep: Reliability and validity. J. Clin. Sleep. Med. 2007, 3, 169–200. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wang, H.T.; Chen, H.C.; Chai, H.T.; Lee, Y.W.; Liu, W.H. Localization of right ventricular non-apical lead position: Comparison of three-dimensional echocardiography, computed tomography, and fluoroscopic imaging. J. Int. Med. Res. 2021, 49, 300060521996159. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Guo, B.F.; Chen, Y.L.; Tsai, T.H.; Fu, M.; Chua, S.; Chen, M.C. Right ventricular outflow tract pacing causes intraventricular dyssynchrony in patients with sick sinus syndrome: A real-time three-dimensional echocardiographic study. J. Am. Soc. Echocardiogr. 2010, 23, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Kosugi, S.; Ozaki, T.; Mishima, T.; Date, M.; Ueda, Y.; Uematsu, M.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Impact of Echocardiographic Congestion Grade in HFpEF with and without Atrial Fibrillation. JACC Asia 2022, 2, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Sickinger, S.; Frotschnig, S.; Grimm, M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta 2015, 1853, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Hsi, E.; Wang, T.M.; Lin, R.T.; Liao, Y.C.; Juo, S.H. MicroRNA let-7g possesses a therapeutic potential for peripheral artery disease. J. Cell. Mol. Med. 2017, 21, 519–529. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Claycomb, W.C. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem. Biophys. Res. Commun. 1999, 265, 382–386. [Google Scholar] [CrossRef]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kuo, T.B.J.; Chung, C.H.; Tzeng, N.S.; Lai, H.C.; Chien, W.C.; Yang, C.C.H. Age and sex differences on the association between anxiety disorders and obstructive sleep apnea: A nationwide case-control study in Taiwan. Psychiatry Clin. Neurosci. 2022, 76, 251–259. [Google Scholar] [CrossRef]
- Khamsai, S.; Junkrasien, C.; Limpawattana, P.; Chindaprasirt, J.; Senthong, V.; Boonsawat, W.; Sawanyawisuth, K. Prevalence and risk factors for persistent atrial fibrillation in obstructive sleep apnea. Sleep Sci. 2022, 15, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Gao, Y.; Chen, K.; Gao, Y.; Guo, J.; Shi, M.; Zou, X.; Xu, W.; Zhao, L.; et al. Prevalence and factors associated with atrial fibrillation in older patients with obstructive sleep apnea. BMC Geriatr. 2022, 22, 204. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Rosenberg, M.A.; Gottdiener, J.S.; Heckbert, S.R.; Mukamal, K.J. Echocardiographic diastolic parameters and risk of atrial fibrillation: The Cardiovascular Health Study. Eur. Heart J. 2012, 33, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.M.; Redfield, M.M.; Shen, W.K.; Gersh, B.J. Atrial fibrillation and ventricular dysfunction: A vicious electromechanical cycle. Circulation 2004, 109, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, H.; Ng, M.; Brooks, A.G.; Kuklik, P.; Stiles, M.K.; Lau, D.H.; Antic, N.; Thornton, A.; Saint, D.A.; McEvoy, D.; et al. Atrial remodeling in obstructive sleep apnea: Implications for atrial fibrillation. Heart Rhythm. 2012, 9, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Pepin, J.L.; Levy, P. Pathophysiology of cardiovascular risk in sleep apnea syndrome (SAS). Rev. Neurol. 2002, 158, 785–797. [Google Scholar]
- Kannel, W.B.; Benjamin, E.J. Current perceptions of the epidemiology of atrial fibrillation. Cardiol. Clin. 2009, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Stradling, J.R. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 2010, 7, 677–685. [Google Scholar] [CrossRef]
- Sinner, M.F.; Tucker, N.R.; Lunetta, K.L.; Ozaki, K.; Smith, J.G.; Trompet, S.; Bis, J.C.; Lin, H.; Chung, M.K.; Nielsen, J.B.; et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 2014, 130, 1225–1235. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Zhang, J.; Huang, X.; Ye, Z.; Zhao, Y. Association Between GJA1 rs13216675 T>C Polymorphism and Risk of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 585268. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Huang, W.; Zeng, Z.; He, Y.; Luo, B.; Liu, H.; Li, J.; Xu, J. Connexin 43 is involved in the sympathetic atrial fibrillation in canine and canine atrial myocytes. Anatol. J. Cardiol. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Severs, N.J. Pathophysiology of gap junctions in heart disease. J. Cardiovasc. Electrophysiol. 1994, 5, 462–475. [Google Scholar] [CrossRef]
- Saffitz, J.E.; Laing, J.G.; Yamada, K.A. Connexin expression and turnover: Implications for cardiac excitability. Circ. Res. 2000, 86, 723–728. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Buratti, L.; Rocchi, C.; Totaro, V.; Broggi, S.; Lattanzi, S.; Viticchi, G.; Falsetti, L.; Silvestrini, M. Sex-Related Differences in Polygraphic Parameters in a Population of Patients with Obstructive Sleep Apnea Syndrome. CNS Neurol. Disord. Drug Targets 2022, 21, 492–499. [Google Scholar] [CrossRef]
- Sheng, X.; Scherlag, B.J.; Yu, L.; Li, S.; Ali, R.; Zhang, Y.; Fu, G.; Nakagawa, H.; Jackman, W.M.; Lazzara, R.; et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J. Am. Coll. Cardiol. 2011, 57, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Brady, D.C.; Po, P.; Chuang, L.P.; Marcondes, L.; Kim, E.Y.; Keenan, B.T.; Guo, X.; Maislin, G.; Galante, R.J.; et al. Simulating obstructive sleep apnea patients’ oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J. Appl. Physiol. (1985) 2015, 118, 544–557. [Google Scholar] [CrossRef]
- Abadie, C.; Foucart, S.; Page, P.; Nadeau, R. Modulation of noradrenaline release from isolated human atrial appendages. J. Auton. Nerv. Syst. 1996, 61, 269–276. [Google Scholar] [CrossRef]
- Abadie, C.; Foucart, S.; Page, P.; Nadeau, R. Interleukin-1 beta and tumor necrosis factor-alpha inhibit the release of [3H]-noradrenaline from isolated human atrial appendages. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 355, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cobo, M.; Gingalewski, C.; Drujan, D.; De Maio, A. Downregulation of connexin 43 gene expression in rat heart during inflammation. The role of tumour necrosis factor. Cytokine 1999, 11, 216–224. [Google Scholar] [CrossRef] [PubMed]
- George, S.A.; Calhoun, P.J.; Gourdie, R.G.; Smyth, J.W.; Poelzing, S. TNFalpha Modulates Cardiac Conduction by Altering Electrical Coupling between Myocytes. Front. Physiol. 2017, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Ham, S.A.; Gozal, D. The effect of sex and age on the comorbidity burden of OSA: An observational analysis from a large nationwide US health claims database. Eur. Respir. J. 2016, 47, 1162–1169. [Google Scholar] [CrossRef]
- Lin, C.-H.; Liu, Y.-B.; Lin, L.-Y.; Huang, H.-C.; Ho, L.-T.; Wu, Y.-W.; Lai, L.-P.; Chen, W.-C.; Ho, Y.-L.; Yu, C.-C. Sex-based differences in obstructive sleep apnea and atrial fibrillation: Implication of atrial fibrillation burden. IJC Heart Vasc. 2024, 50, 101320. [Google Scholar] [CrossRef]

| Variable | OSA with AF (n = 23) | OSA without AF (n = 103) | Control (n = 28) | p-Value |
|---|---|---|---|---|
| Age (years) | 61.4 (9.7) b | 54.3 (10.8) a | 53.3 (9.9) a | 0.009 |
| Male sex | 19 (82.6) b | 78 (75.7) b | 13 (46.4) a | 0.004 |
| BMI (kgs/m2) | 27.0 (3.3) b | 26.3 (3.3) b | 24.4 (3.2) a | 0.010 |
| Alcohol consumption | 3 (13) | 19 (18.4) | 6 (21.4) | 0.737 |
| AF type | <0.001 | |||
| Paroxysmal AF | 13 (56.5) | 0 | 0 | |
| Persistent AF | 10 (43.5) | 0 | 0 | |
| HTN | 12 (52.2) | 37 (35.9) | 4 (25.0) | 0.147 |
| DM | 5 (21.7) | 8 (7.8) | 3 (10.7) | 0.160 |
| HFpEF or HFmrEF | 3 (13.0) | 0 | 0 | 0.003 |
| CAD | 2 (8.7) | 3 (2.9) | 1 (3.6) | 0.288 |
| Stroke | 2 (8.7) | 4 (3.9) | 0 | 0.213 |
| Vital signs at enrollment | ||||
| Systolic blood pressure | 135.9 ± 17.1 | 134.8 ± 14.4 | 133.4 ± 16.8 | 0.852 |
| Diastolic blood pressure | 79.1 ± 11.0 | 77.8 ± 10.0 | 75.5 ± 11.4 | 0.450 |
| Heart rate | 78.4 ± 11.9 | 80.9 ± 12.1 | 84.3 ± 14.5 | 0.237 |
| Medication | ||||
| ACEI/ARB | 9 (39.1) | 30 (29.1) | 5 (17.9) | 0.241 |
| Beta-blockers | 14 (60.9) | 25 (24.3) | 8 (28.6) | 0.003 |
| Calcium channel blockers | 13 (56.5%) | 23 (22.3) | 6 (21.4) | 0.003 |
| Diuretics | 2 (8.7) | 8 (7.8) | 3 (10.7) | 0.883 |
| Oral antidiabetic drugs | 3 (13) | 7 (6.8) | 3 (10.7) | 0.555 |
| Statins | 5 (21.7) | 35 (34) | 8 (28.6) | 0.491 |
| Antiarrhythmic drugs | 7 (30.4) | 0 | 0 | <0.001 |
| Aspirin | 2 (8.7) | 13 (12.6) | 4 (14.3) | 0.824 |
| P2Y12 inhibitors | 1 (4.3) | 7 (6.8) | 1 (3.6) | 0.769 |
| Direct oral anticoagulants | 12 (52.2) | 0 | 0 | <0.001 |
| PSG data | ||||
| ESS | 8.0 (4.0–11.0) | 9.0 (6.0–12.0) | 9.0 (7.0–12.0) | 0.398 |
| AHITST (/h) | 39.5 (26.0–63.5) b | 40.3 (24.8–57.0) b | 8.0 (4.5–11.2) a | <0.001 |
| AHIREM (/h) | 44.9 (37.7–59.1) b | 48.2 (32.0–63.2) b | 13.5 (9.8–21.0) a | <0.001 |
| AHINREM (/h) | 35.8 (23.3–63.9) b | 40.4 (21.9–56.9) b | 6.7 (3.6–9.4) a | <0.001 |
| ODITST (/h) | 30.4 (14.4–54.1) b | 26.2 (14.2–44.3) b | 4.7 (1.4–7.5) a | <0.001 |
| ODIREM (/h) | 37.9 (23.2–55.0) b | 41.0 (18.4–55.3) b | 9.6 (5.2–17.9) a | <0.001 |
| ODINREM (/h) | 31.6 (11.6–56.5) b | 23.3 (10.7–43.2) b | 3.9 (1.4–6.5) a | <0.001 |
| Lowest SpO2 (%) | 79.0 (62.0–88.0) b | 80.0 (73.0–85.0) b | 86 (85–90.7) a | <0.001 |
| Mean SpO2 (%) | 92.5 (91.5–94.6) b | 93.8 (92.2–94.9) b | 94.7 (93.3–95.9) a | 0.010 |
| SpO2 < 90% | 7.6 (4.2–22.0) b | 6.0 (2.2–16.7) b | 1.7 (0.2–7.0) a | 0.008 |
| Echocardiography | ||||
| Aorta (mm) | 33.3 (4.3) | 32.5 (3.7) | 31.6 (4.5) | 0.337 |
| LA (mm) | 40.3 (6.0) b | 35.9 (4.4) a | 34.2 (5.7) a | <0.001 |
| IVS (mm) | 11.8 (2.6) | 11.5 (1.7) | 11.0 (1.3) | 0.337 |
| LVPW (mm) | 9.5 (1.9) | 9.2 (1.5) | 9.1 (1.3) | 0.590 |
| LVEDV (mL) | 108.7 (41.3) | 110.8 (27.6) | 105.9 (23.0) | 0.787 |
| LVESV (mL) | 42.2 (26.7) | 34.5 (17.3) | 29.8 (7.6) | 0.072 |
| LVEF (%) | 63.9 (9.1) b | 69.2 (7.2) a | 71.4 (3.3) a | 0.001 |
| E/e’ | 10.4 (3.3) b | 8.3 (2.6) a | 9.0 (4.2) ab | 0.036 |
| Male (110) | ||||
|---|---|---|---|---|
| Variable | OSA with AF (n = 19) | OSA without AF (n = 78) | Control (n = 13) | p-Value |
| Age (years) | 60.5 (9.5) b | 51.8 (10.4) a | 50.0 (9.9) a | 0.003 |
| BMI (kgs/m2) | 26.7 (3.6) b | 26.6 (3.1) b | 23.6 (2.6) a | 0.005 |
| Alcohol consumption | 3 (15.8) | 18 (23.1) | 5 (38.5) | 0.326 |
| AF type | <0.001 | |||
| Paroxysmal AF | 11 (57.9) | 0 | 0 | |
| Persistent AF | 8 (42.1) | 0 | 0 | |
| HTN | 9 (47.4) | 27 (34.6) | 4 (30.8) | 0.549 |
| DM | 3 (15.8) | 5 (6.4) | 1 (7.7) | 0.335 |
| HFpEF or HFmrEF | 3 (15.8) | 0 | 0 | 0.006 |
| CAD | 2 (10.5) | 2 (2.6) | 0 | 0.203 |
| Stroke | 2 (10.5) | 4 (5.1) | 0 | 0.400 |
| Vital signs at enrollment | ||||
| Systolic blood pressure | 134.1 ± 16.1 | 135.6 ± 13.7 | 134.2 ± 19.4 | 0.889 |
| Diastolic blood pressure | 78.8 ± 10.8 | 79.1 ± 9.9 | 78.7 ± 11.9 | 0.988 |
| Heart rate | 77.9 ± 11.0 | 82.5 ± 11.6 | 85.1 ± 15.0 | 0.200 |
| Medication | ||||
| ACEI/ARB | 7 (36.8) | 20 (25.6) | 3 (23.1) | 0.578 |
| Beta-blockers | 12 (63.2) | 14 (17.9) | 3 (23.1) | <0.001 |
| Calcium channel blockers | 11 (57.9) | 16 (20.5) | 3 (23.1) | 0.004 |
| Diuretics | 1 (5.3) | 6 (7.7) | 1 (7.7) | 0.934 |
| Oral antidiabetic drugs | 2 (10.5) | 6 (7.7) | 2 (15.4) | 0.652 |
| Statins | 4 (21.1) | 21 (26.9) | 2 (15.4) | 0.621 |
| Antiarrhythmic drugs | 7 (36.8) | 0 | 0 | <0.001 |
| Aspirin | 2 (10.5) | 11 (14.1) | 2 (15.4) | 0.903 |
| P2Y12 inhibitors | 0 | 4 (5.1) | 0 | 0.427 |
| Direct oral anticoagulants | 7 (36.8) | 0 | 0 | <0.001 |
| PSG data | ||||
| ESS | 8.0 (4.0–11.0) | 9.0 (6.0–12.0) | 9.0 (5.0–11.0) | 0.480 |
| AHITST (/h) | 39.5 (26.0–63.0) b | 42.3 (25.7–58.3) b | 7.6 (3.4–10.6) a | <0.001 |
| AHIREM (/h) | 45.0 (37.7–58.5) b | 48.0 (31.0–62.9) b | 13.5 (5.7–19.8) a | <0.001 |
| AHINREM (/h) | 34.7 (23.3–63.9) b | 44.1 (23.2–58.4) b | 6.3 (3.2–8.7) a | <0.001 |
| ODITST (/h) | 29.9 (13.4–62.0) b | 27.5 (13.0–45.1) b | 4.6 (1.1–6.8) a | <0.001 |
| ODIREM (/h) | 39.1 (22.7–55.9) b | 41.1 (18.9–55.0) b | 7.3 (1.7–12.8) a | <0.001 |
| ODINREM (/h) | 31.2 (11.6–61.8) b | 29.3 (11.2–44.1) b | 4.5 (1.8–6.4) a | <0.001 |
| Lowest SpO2 (%) | 78.0 (62.5–87.0) b | 79.0 (73.0–84.0) b | 89 (85–91.5) a | <0.001 |
| Mean SpO2 (%) | 92.2 (91.0–94.9) b | 93.8 (92.3–95.0) ab | 94.6 (93.4–95.7) a | 0.035 |
| SpO2 < 90% | 11.6 (4.2–26.4) | 5.9 (2.0–18.5) | 1.30 (0.25–7.5) | 0.054 |
| Echocardiography | ||||
| Aorta (mm) | 34.0 (4.1) | 33.5 (3.5) | 33.3 (5.2) | 0.881 |
| LA (mm) | 40.0 (6.3) b | 36.0 (4.7) a | 36.4 (3.5) ab | 0.011 |
| IVS (mm) | 11.7 (2.8) | 11.6 (1.8) | 11.4 (0.8) | 0.904 |
| LVPW (mm) | 9.6 (2.1) | 9.3 (1.5) | 8.7 (0.6) | 0.446 |
| LVEDV (mL) | 112.4 (44.0) | 114.5 (27.8) | 119.6 (21.6) | 0.851 |
| LVESV (mL) | 43.7 (28.6) | 36.6 (19.0) | 33.2 (8.0) | 0.332 |
| LVEF (%) | 64.0 (7.8) b | 68.3 (7.8) ab | 72.3 (4.1) a | 0.027 |
| E/e’ | 10.3 (3.5) b | 7.7 (2.1) a | 6.6 (2.2) a | 0.001 |
| Female (44) | ||||
|---|---|---|---|---|
| Variable | OSA with AF (n = 4) | OSA without (n = 25) | Control (n = 15) | p-Value |
| Age (years) | 65.5 (11.1) | 62.0 (7.9) | 56.2 (9.2) | 0.069 |
| BMI (kgs/m2) | 28.3 (0.9) | 25.2 (3.8) | 25.4 (3.6) | 0.270 |
| Alcohol consumption | 0 | 1 (4) | 1 (6.7) | 0.834 |
| AF type | <0.001 | |||
| Paroxysmal AF | 2 (50) | 0 | 0 | |
| Persistent AF | 2 (50) | 0 | 0 | |
| HTN | 3 (75.0) | 10 (40.0) | 3 (20.0) | 0.092 |
| DM | 2 (50.0) | 3 (12.0) | 2 (13.3) | 0.179 |
| HFpEF or HFmrEF | 0 | 0 | 0 | - |
| CAD | 0 | 1 (4.0) | 1 (6.7) | 1.000 |
| Stroke | 0 | 0 | 0 | - |
| Vital signs at enrollment | ||||
| Systolic blood pressure | 144.5 ± 21.9 | 132.2 ± 16.3 | 132.8 ± 14.6 | 0.376 |
| Diastolic blood pressure | 80.5 ± 13.3 | 74.1 ± 9.2 | 72.6 ± 10.5 | 0.386 |
| Heart rate | 80.5 ± 17.6 | 75.9 ± 12.6 | 83.6 ± 14.4 | 0.243 |
| Medication | ||||
| ACEI/ARB | 2 (50) | 10 (40) | 2 (13.3) | 0.154 |
| Beta-blockers | 2 (50) | 11 (44) | 5 (33.3) | 0.744 |
| Calcium channel blockers | 2 (50) | 7 (28) | 3 (20) | 0.485 |
| Diuretics | 1 (25) | 2 (8) | 2 (13.3) | 0.584 |
| Oral antidiabetic drugs | 1 (25) | 1 (4) | 1 (6.7) | 0.302 |
| Statins | 1 (25) | 14 (56) | 6 (40) | 0.392 |
| Antiarrhythmic drugs | 0 | 0 | 0 | |
| Aspirin | 0 | 2 (8) | 2 (13.3) | 0.683 |
| P2Y12 inhibitors | 1 (25) | 3 (12) | 1 (6.7) | 0.584 |
| Direct oral anticoagulants | 1 (25) | 0 | 0 | 0.006 |
| PSG data | ||||
| ESS | 7.5 (1.7–11.0) | 9.0 (5.5–15.5) | 9.0 (8.0–12.0) | 0.541 |
| AHITST (/h) | 50.1 (23.5–64.9) b | 31.0 (22.2–47.4) b | 9.6 (5.8–11.8) a | <0.001 |
| AHIREM (/h) | 41.3 (38.4) ab | 51.8 (33.1–63.3) b | 14.4 (9.9–23.1) a | <0.001 |
| AHINREM (/h) | 49.6 (21.1–64.9) b | 24.6 (21.3–44.4) b | 8.9 (4.7–9.6) a | <0.001 |
| ODITST (/h) | 35.4 (18.4–49.5) b | 23.6 (14.7–34.0) b | 4.9 (1.7–8.6) a | <0.001 |
| ODIREM (/h) | 34.1 (25.3) ab | 40.6 (13.9–55.3)b | 12.8 (6.8–22.9) a | 0.006 |
| ODINREM (/h) | 36.0 (16.6–49.2) b | 20.8 (10.3–30.3) b | 3.3 (1.0–6.8) a | <0.001 |
| Lowest SpO2 (%) | 85.0 (79.0–88.0) b | 82.0 (74.2–86.7) ab | 86.0 (83.0–90.0) a | 0.021 |
| Mean SpO2 (%) | 93.3 (92.6–94.1) | 93.7 (92.0–94.7) | 95.0 (93.3–96.0) | 0.249 |
| SpO2 < 90% | 5.2 (2.9–6.3) | 6.0 (2.4–11.6) | 2.1 (0.1–5.8) | 0.162 |
| Echocardiography | ||||
| Aorta (mm) | 30.5 (4.7) | 29.8 (2.7) | 30.3 (3.7) | 0.874 |
| LA (mm) | 42.0 (4.9) b | 35.6 (3.6) ab | 32.5 (6.5) a | 0.005 |
| IVS (mm) | 12.0 (1.4) | 11.2 (1.4) | 10.6 (1.4) | 0.256 |
| LVPW (mm) | 9.2 (0.9) | 8.9 (1.4) | 9.3 (1.7) | 0.759 |
| LVEDV (mL) | 91.0 (19.7) | 101.0 (25.1) | 95.6 (18.7) | 0.636 |
| LVESV (mL) | 34.7 (15.6) | 28.8 (10.0) | 27.2 (6.5) | 0.423 |
| LVEF (%) | 63.7 (7.8) b | 71.6 (4.7) a | 70.8 (2.6) a | 0.012 |
| E/e’ | 10.9 (1.9) | 9.9 (3.2) | 10.2 (4.5) | 0.906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-T.; Wang, H.-T.; Chen, Y.-C.; Chang, Y.-T.; Lin, P.-T.; Hsu, P.-Y.; Lin, M.-C.; Chen, Y.-L. Sex Differences in the Expression of Cardiac Remodeling and Inflammatory Cytokines in Patients with Obstructive Sleep Apnea and Atrial Fibrillation. Biomedicines 2024, 12, 1160. https://doi.org/10.3390/biomedicines12061160
Shih C-T, Wang H-T, Chen Y-C, Chang Y-T, Lin P-T, Hsu P-Y, Lin M-C, Chen Y-L. Sex Differences in the Expression of Cardiac Remodeling and Inflammatory Cytokines in Patients with Obstructive Sleep Apnea and Atrial Fibrillation. Biomedicines. 2024; 12(6):1160. https://doi.org/10.3390/biomedicines12061160
Chicago/Turabian StyleShih, Chun-Ting, Hui-Ting Wang, Yung-Che Chen, Ya-Ting Chang, Pei-Ting Lin, Po-Yuan Hsu, Meng-Chih Lin, and Yung-Lung Chen. 2024. "Sex Differences in the Expression of Cardiac Remodeling and Inflammatory Cytokines in Patients with Obstructive Sleep Apnea and Atrial Fibrillation" Biomedicines 12, no. 6: 1160. https://doi.org/10.3390/biomedicines12061160
APA StyleShih, C.-T., Wang, H.-T., Chen, Y.-C., Chang, Y.-T., Lin, P.-T., Hsu, P.-Y., Lin, M.-C., & Chen, Y.-L. (2024). Sex Differences in the Expression of Cardiac Remodeling and Inflammatory Cytokines in Patients with Obstructive Sleep Apnea and Atrial Fibrillation. Biomedicines, 12(6), 1160. https://doi.org/10.3390/biomedicines12061160






