Generation of a Specific Fluorescence In Situ Hybridization Test for the Detection of Ovarian Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
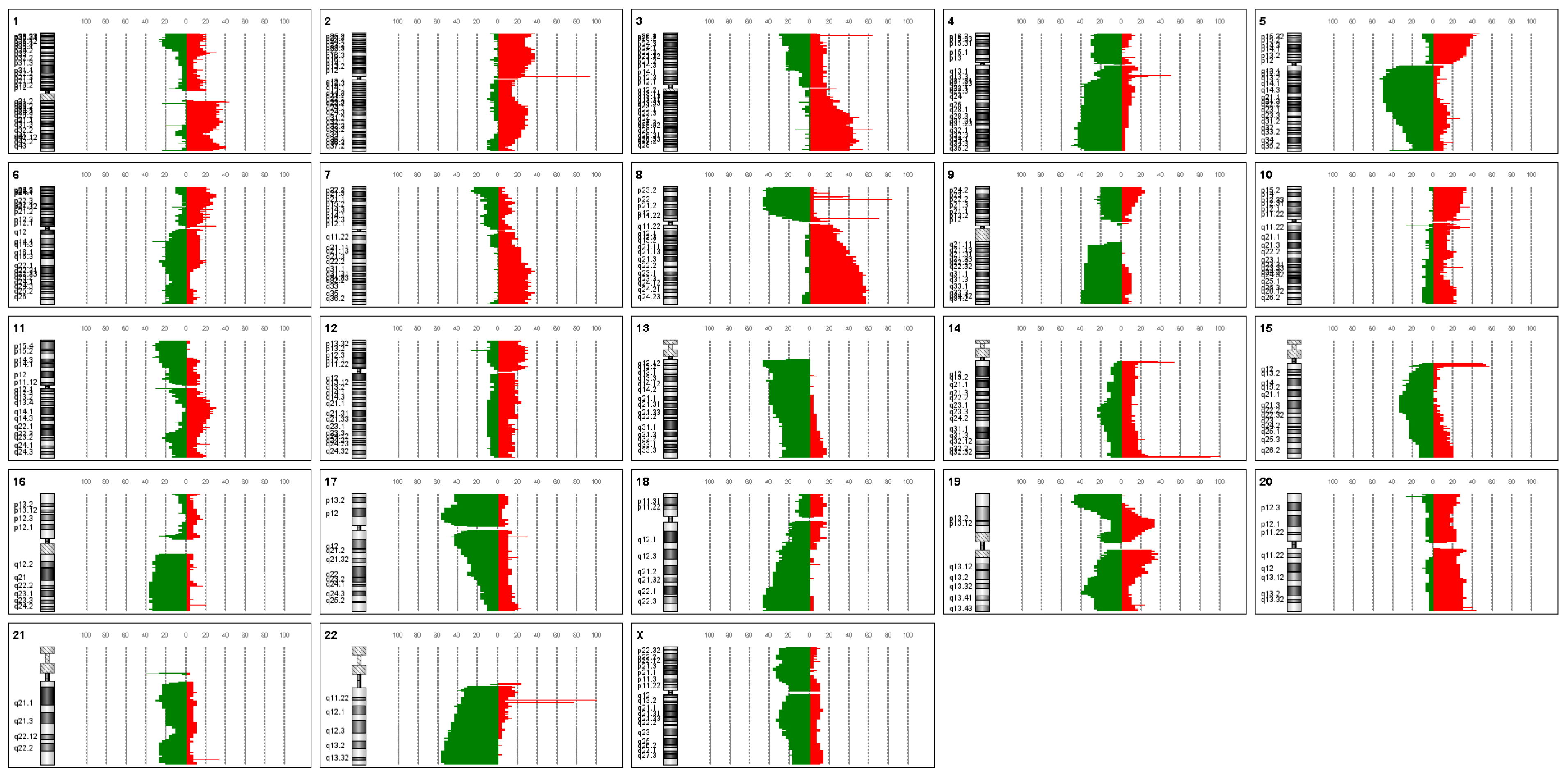
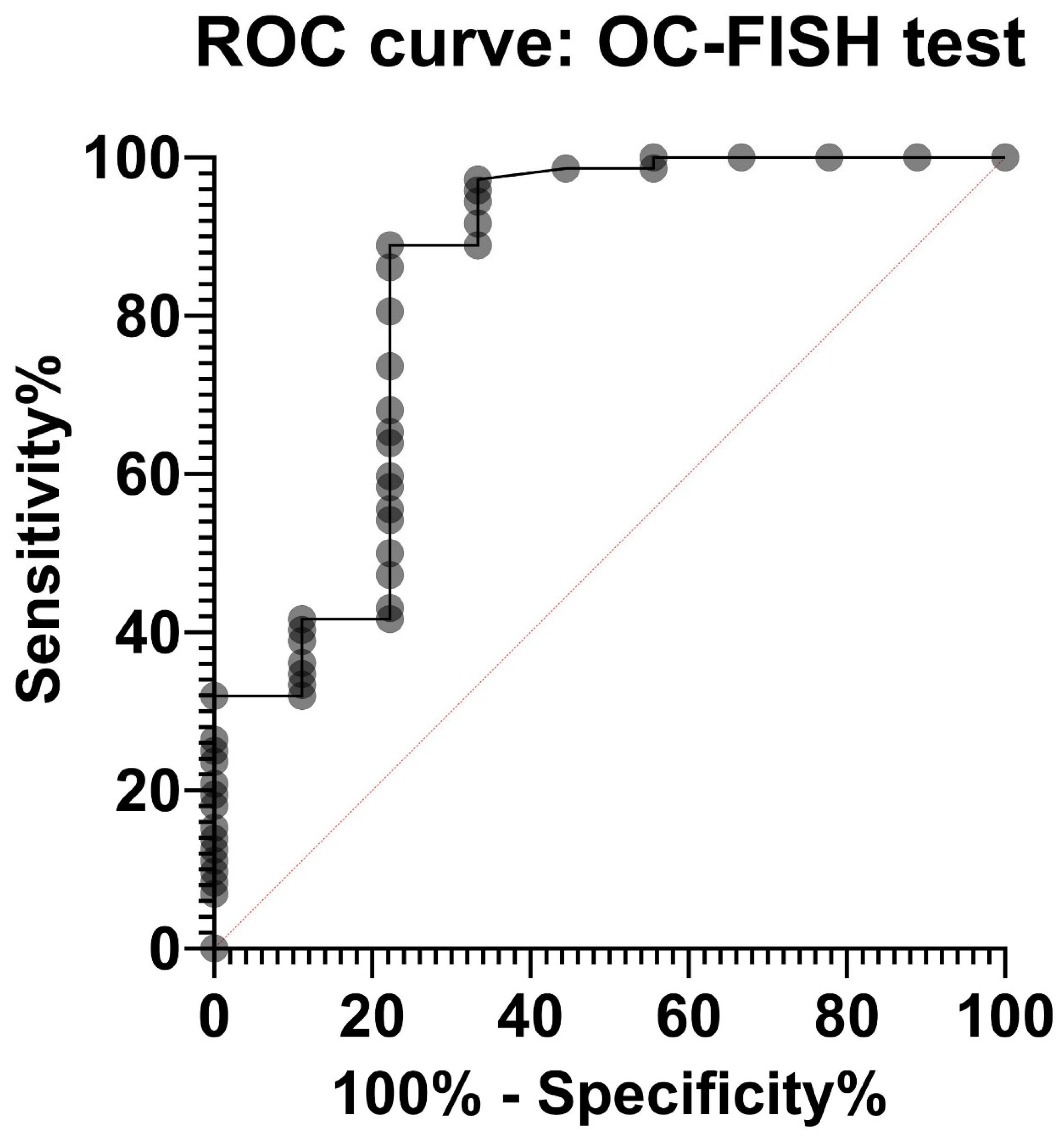
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moschetta, M.; George, A.; Kaye, S.B.; Banerjee, S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Ghanei, M.; Salmaninejad, A.; Rajaie, S.; Hasanzadeh, M.; Pasdar, A. Current insights into the metastasis of epithelial ovarian cancer—Hopes and hurdles. Cell Oncol. 2020, 43, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Shih Ie, M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih Ie, M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Krugmann, J.; Schwarz, C.L.; Melcher, B.; Sterlacci, W.; Ozalinskaite, A.; Lermann, J.; Agaimy, A.; Vieth, M. Malignant ascites occurs most often in patients with high-grade serous papillary ovarian cancer at initial diagnosis: A retrospective analysis of 191 women treated at Bayreuth Hospital, 2006–2015. Arch. Gynecol. Obstet. 2019, 299, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, X.; Chen, D.; Cai, J.; Fu, Y.; Kang, H.; Gao, J.; Gao, K.; Du, N. Intraperitoneal administration of cisplatin plus bevacizumab for the management of malignant ascites in ovarian epithelial cancer: Results of a phase III clinical trial. Med. Oncol. 2015, 32, 292. [Google Scholar] [CrossRef] [PubMed]
- Launonen, I.M.; Vähärautio, A.; Färkkilä, A. The Emerging Role of the Single-Cell and Spatial Tumor Microenvironment in High-Grade Serous Ovarian Cancer. Cold Spring Harb. Perspect. Med. 2023, 13, a041314. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.Z.; Pellman, D.S.; et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef]
- Shorokhova, M.; Nikolsky, N.; Grinchuk, T. Chromothripsis-Explosion in Genetic Science. Cells 2021, 10, 1102. [Google Scholar] [CrossRef] [PubMed]
- Weimer, J.; Huttmann, M.; Nusilati, A.; Andreas, S.; Roseler, J.; Tribian, N.; Rogmans, C.; Stope, M.B.; Dahl, E.; Mustea, A.; et al. Fluorescence in situ hybridization test for detection of endometrial carcinoma cells by non-invasive vaginal swab. J. Cell. Mol. Med. 2023, 27, 379–391. [Google Scholar] [CrossRef]
- Weimer, J.; Kiechle, M.; Senger, G.; Wiedemann, U.; Ovens-Raeder, A.; Schuierer, S.; Kautza, M.; Siebert, R.; Arnold, N. An easy and reliable procedure of microdissection technique for the analysis of chromosomal breakpoints and marker chromosomes. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 1999, 7, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Acar-Perk, B.; Brautigam, K.; Grunewald, R.; Schmutzler, A.; Schem, C.; Arnold, N.K.; Jonat, W.; Weimer, J. The t(14,15) in mouse strain CBA/CaH-T(14;15)6Ca/J causes a break in the ADAMTS12 gene. Comp. Med. 2010, 60, 118–122. [Google Scholar]
- Weimer, J.; Kiechle, M.; Wiedemann, U.; Tonnies, H.; Neitzel, H.; Ruhenstroth, E.; Ovens-Raeder, A.; Arnold, N. Delineation of a complex karyotypic rearrangement by microdissection and CGH in a family affected with split foot. J. Med. Genet. 2000, 37, 442–445. [Google Scholar] [CrossRef]
- Kurbacher, C.M.; Korn, C.; Dexel, S.; Schween, U.; Kurbacher, J.A.; Reichelt, R.; Arenz, P.N. Isolation and culture of ovarian cancer cells and cell lines. Methods Mol. Biol. 2011, 731, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, N.; Herz, A.; Schiepanski, J.H.; Dittrich, J.; Sebens, S.; Dempfle, A.; Feuerborn, J.; Rogmans, C.; Tribian, N.; Flörkemeier, I.; et al. ADAM17 Inhibition Increases the Impact of Cisplatin Treatment in Ovarian Cancer Spheroids. Cancers 2021, 13, 2039. [Google Scholar] [CrossRef]
- Hu, H.; Shikama, Y.; Shichishima, T.; Ikeda, K.; Akutsu, K.; Ono, T.; Kimura, H.; Ogawa, K.; Noji, H.; Takeishi, Y.; et al. A new method for maturity-dependent fractionation of neutrophil progenitors applicable for the study of myelodysplastic syndromes. Biomark. Res. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Onozato, K.; Takano, M.; Tamai, S.; Imoto, I.; Inazawa, J.; Yamada, T.; Matsubara, O. Actinin-4 gene amplification in ovarian cancer: A candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2009, 22, 499–507. [Google Scholar] [CrossRef]
- Román-Lladó, R.; Aguado, C.; Jordana-Ariza, N.; Roca-Arias, J.; Rodríguez, S.; Aldeguer, E.; Garzón-Ibañez, M.; García-Peláez, B.; Vives-Usano, M.; Giménez-Capitán, A.; et al. Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors. J. Mol. Pathol. 2023, 4, 57–68. [Google Scholar] [CrossRef]
- Chrzanowska, N.M.; Kowalewski, J.; Lewandowska, M.A. Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors. Molecules 2020, 25, 1864. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, J.W.; Kim, Y.T.; Kim, J.H.; Kim, S.; Yoon, B.S.; Nam, E.J.; Kim, H.Y. Analysis of chromosomal changes in serous ovarian carcinoma using high-resolution array comparative genomic hybridization: Potential predictive markers of chemoresistant disease. Genes Chromosomes Cancer 2007, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.B.; Fiegler, H.; Jones, A.; Gorman, P.; Roylance, R.R.; Carter, N.P.; Tomlinson, I.P. Analysis of ovarian cancer cell lines using array-based comparative genomic hybridization. J. Pathol. 2005, 205, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.; Iravani, M.; McCluggage, W.G.; Lambros, M.B.; Milanezi, F.; Mackay, A.; Gourley, C.; Geyer, F.C.; Vatcheva, R.; Millar, J.; et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Patil Okaly, G.V.; Panwar, D.; Lingappa, K.B.; Kumari, P.; Anand, A.; Kumar, P.; Chikkalingaiah, M.H.; Kumar, R.V. FISH and HER2/neu equivocal immunohistochemistry in breast carcinoma. Indian J. Cancer 2019, 56, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Akerlund, E.; Gudoityte, G.; Moussaud-Lamodiere, E.; Lind, O.; Bwanika, H.C.; Lehti, K.; Salehi, S.; Carlson, J.; Wallin, E.; Fernebro, J.; et al. The drug efficacy testing in 3D cultures platform identifies effective drugs for ovarian cancer patients. NPJ Precis. Oncol. 2023, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Weber-Matthiesen, K.; Deerberg, J.; Poetsch, M.; Grote, W.; Schlegelberger, B. Clarification of dubious karyotypes in Hodgkin’s disease by simultaneous fluorescence immunophenotyping and interphase cytogenetics (FICTION). Cytogenet. Cell Genet. 1995, 70, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Martin-Subero, J.I.; Chudoba, I.; Harder, L.; Gesk, S.; Grote, W.; Novo, F.J.; Calasanz, M.J.; Siebert, R. Multicolor-FICTION: Expanding the possibilities of combined morphologic, immunophenotypic, and genetic single cell analyses. Am. J. Pathol. 2002, 161, 413–420. [Google Scholar] [CrossRef]
- Summersgill, B.; Clark, J.; Shipley, J. Fluorescence and chromogenic in situ hybridization to detect genetic aberrations in formalin-fixed paraffin embedded material, including tissue microarrays. Nat. Protoc. 2008, 3, 220–234. [Google Scholar] [CrossRef]


| OC-FISH Test on: | Conspicuous Signal Pattern (%) | Histology | FIGO | Grading | Test Result |
|---|---|---|---|---|---|
| male lymphocytes | 10 | true negative | |||
| male lymphocytes | 10 | true negative | |||
| male lymphocytes | 25 | true negative | |||
| LP-3 mesenchyme | 16 | true negative | |||
| LP-3 mesenchyme | 24 | true negative | |||
| LP-3 mesenchyme | 22 | true negative | |||
| OvCa-cells | 38 | n.a. | n.a. | n.a. | true positive |
| OvCa-cells | 67 | n.a. | n.a. | n.a. | true positive |
| OvCa-cells | 55 | n.a. | n.a. | n.a. | true positive |
| OVCAR 3 | 100 | serous | n.a. | 3 | true positive |
| OVCAR 8 | 100 | serous | n.a. | 3 | true positive |
| SCOV 3 | 100 | serous | n.a. | n.a. | true positive |
| RU-0001-OC | 43 | n.a. | n.a. | n.a. | true positive |
| SU-0002-OC | 25 | serous | IIIc | 3 | false negative |
| KA-0003-OC | 65 | serous | IIIb | borderline | true positive |
| WR-0004-OC | 22 | serous papillary | IIIc | 3 | false negative |
| BR-0005-OC | 43 | papillary | IIb | 3 | true positive |
| BI-0006-OC | 39 | serous | IIc | 3 | true positive |
| KS-0007-OC | 32 | serous | IIc | borderline | false negative |
| JH-0008-OC | 50 | serous papillary | IV | n.a. | true positive |
| BI-0009-OC | 46 | serous | IIc | 3 | true positive |
| HK-0011-OC | 51 | endometrioid | IIc | 2 | true positive |
| JE-0012-OC | 49 | endometrioid | IIIc | 3 | true positive |
| RH-0013-OC | 58 | papillary | IIIc | 2 | true positive |
| MH-0014-OC | 43 | serous papillary | n.a. | n.a. | true positive |
| SI-0015-OC | 97 | papillary clear cell | IIIc | 3 | true positive |
| DG-0016-OC | 51 | serous | IV | 3 | true positive |
| MA-0018-OC | 37 | serous papillary | n.a. | 3 | true positive |
| HJ-0019-OC | 47 | papillary | IIIc | 3 | true positive |
| KA-0020-OC | 80 | n.a. | IIIc | 3 | true positive |
| TW-0021-OC | 53 | serous papillary | IIIc | 3 | true positive |
| JD-0022-OC | 85 | serous papillary | IIIc | 3 | true positive |
| JS-0023-OC | 38 | serous papillary | IV | n.a. | true positive |
| KD-0024-OC | 31 | papillary | IIIc | 2 | false negative |
| SM-0028-OC | 49 | serous papillary | IIIc | 3 | true positive |
| JK-0030-OC | 41 | serous papillary | IIIc | 2 | true positive |
| ED-0031-OC | 41 | serous papillary | IIIb | 3 | true positive |
| SB-0032-OC | 39 | serous papillary | IIIb | 3 | true positive |
| TM-0033-OC | 37 | solid | IIIc | 3 | true positive |
| KA-0034-OC | 53 | serous papillary | IIIc | 3 | true positive |
| SD-0035-OC | 75 | mucinous | n.a. | 2 | true positive |
| KH-0036-OC | 84 | serous | n.a. | 3 | true positive |
| FF-0037-OC | 67 | papillary | IV | 2 | true positive |
| SD-0038-OC | 55 | mucinous | n.a. | 2 | true positive |
| PR-0039-OC | 47 | n.a. | Ic | 2 | true positive |
| RR-0040-OC | 63 | serous papillary | Iic | 3 | true positive |
| KR-0041-OC | 61 | papillary | IV | 3 | true positive |
| PK-0042-OC | 47 | serous | IV | 3 | true positive |
| DU-0043-OC | 76 | serous | n.a. | 3 | true positive |
| EA-0044-OC | 96 | serous papillary | IV | 3 | true positive |
| MI-0045-OC | 99 | n.a. | IV | n.a. | true positive |
| BE-0046-OC | 52 | n.a. | n.a. | n.a. | true positive |
| TB-0051-OC | 70 | serous papillary | IV | n.a. | true positive |
| SM-0053-OC | 44 | serous papillary | IV | n.a. | true positive |
| KK-0054-OC | 99 | endometrioid | Ia | 1 | true positive |
| BM-0055-OC | 38 | n.a. | n.a. | n.a. | true positive |
| AK-0056-OC | 61 | serous papillary | IV | 1 | true positive |
| MK-0057-OC | 71 | papillary | IIIc | 3 | true positive |
| TG-0058-OC | 99 | serous papillary | IIIc | 3 | true positive |
| JL-0059-OC | 41 | solid | IIIc | 3 | true positive |
| ZH-0061-OC | 41 | serous papillary | IV | n.a. | true positive |
| BF-0062-OC | 48 | papillary | IIIc | 3 | true positive |
| HH-0063-OC | 51 | serous papillary | IIc | 3 | true positive |
| SK-0064-OC | 44 | serous papillary | IIb | 3 | true positive |
| NG-0066-OC | 43 | serous papillary | n.a. | 2 | true positive |
| WP-0067-OC | 62 | serous | n.a. | borderline | true positive |
| PH-0068-OC | 54 | serous papillary | IV | 3 | true positive |
| BR-0069-OC | 52 | serous | IV | 3 | true positive |
| HM-0070-OC | 95 | endometrioid | Ic | 2 | true positive |
| SA-0071-OC | 44 | serous | IIIc | 1 | true positive |
| DR-0072-OC | 45 | mucinous | IV | 1 | true positive |
| KT-0073-OC | 53 | serous | IIc | borderline | true positive |
| AI-0075-OC | 67 | papillary | IV | 1 | true positive |
| KA-0076-OC | 44 | serous papillary | Ic | borderline | true positive |
| BKM-0077-OC | 67 | serous papillary | IV | 3 | true positive |
| WM-0078-OC | 45 | clear cell | n.a. | 2 | true positive |
| FG-0079-OC | 71 | serous papillary | IV | 2 | true positive |
| EL-0080-OC | 99 | n.a. | n.a. | n.a. | true positive |
| SB-0083-OiCa | 72 | serous papillary | IIIb | 3 | true positive |
| HK-0084-OC | 67 | serous endometrioid clear cell | IIIa | n.a. | true positive |
| BT-0085-OC | 76 | n.a. | n.a. | n.a. | true positive |
| HM-0086-OC | 69 | serous papillary | IIIc | 2 | true positive |
| ZI-0087-OC | 99 | serous papillary | IIIc | 2 | true positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limburg, A.; Qian, X.; Brechtefeld, B.; Hedemann, N.; Flörkemeier, I.; Rogmans, C.; Oliveira-Ferrer, L.; Maass, N.; Arnold, N.; Bauerschlag, D.O.; et al. Generation of a Specific Fluorescence In Situ Hybridization Test for the Detection of Ovarian Carcinoma Cells. Biomedicines 2024, 12, 1171. https://doi.org/10.3390/biomedicines12061171
Limburg A, Qian X, Brechtefeld B, Hedemann N, Flörkemeier I, Rogmans C, Oliveira-Ferrer L, Maass N, Arnold N, Bauerschlag DO, et al. Generation of a Specific Fluorescence In Situ Hybridization Test for the Detection of Ovarian Carcinoma Cells. Biomedicines. 2024; 12(6):1171. https://doi.org/10.3390/biomedicines12061171
Chicago/Turabian StyleLimburg, Amelie, Xueqian Qian, Bernice Brechtefeld, Nina Hedemann, Inken Flörkemeier, Christoph Rogmans, Leticia Oliveira-Ferrer, Nicolai Maass, Norbert Arnold, Dirk O. Bauerschlag, and et al. 2024. "Generation of a Specific Fluorescence In Situ Hybridization Test for the Detection of Ovarian Carcinoma Cells" Biomedicines 12, no. 6: 1171. https://doi.org/10.3390/biomedicines12061171






