Adenosine 5′-Monophosphate-to-Threonine Ratio Promotes Abdominal Aortic Aneurysms via Up-Regulation of HLA-DR on Natural Killer Cells: A Bidirectional Mendelian Randomized Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overall Study Design
2.2. Exposure and Outcome Data Sources
2.3. Instrument Variables
2.4. Mendelian Randomization Analysis
2.5. Metabolite Enrichment
2.6. Statistical Analysis
3. Results
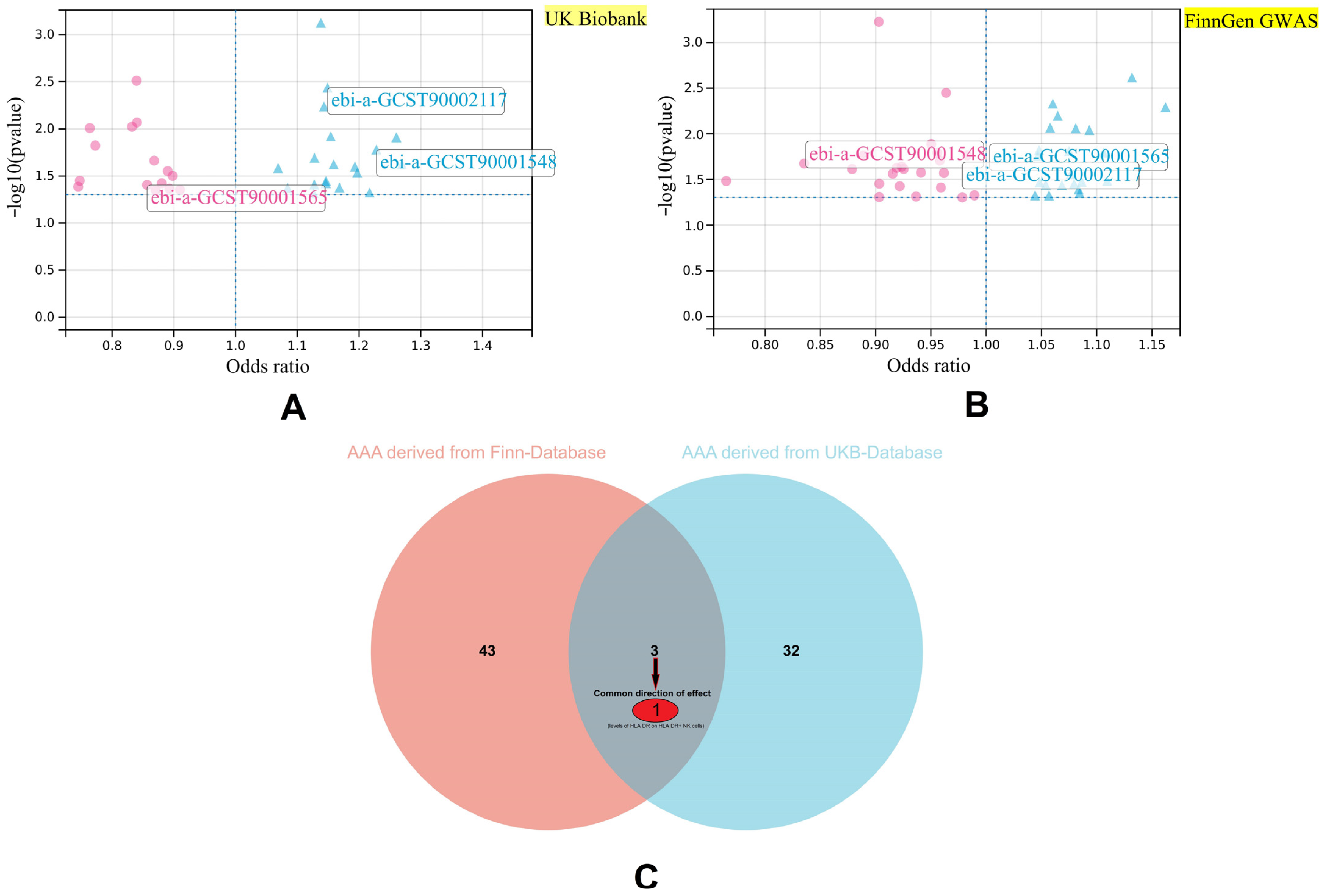
3.1. Key Immune Cell Phenotypes with Causal Relationships with AAA
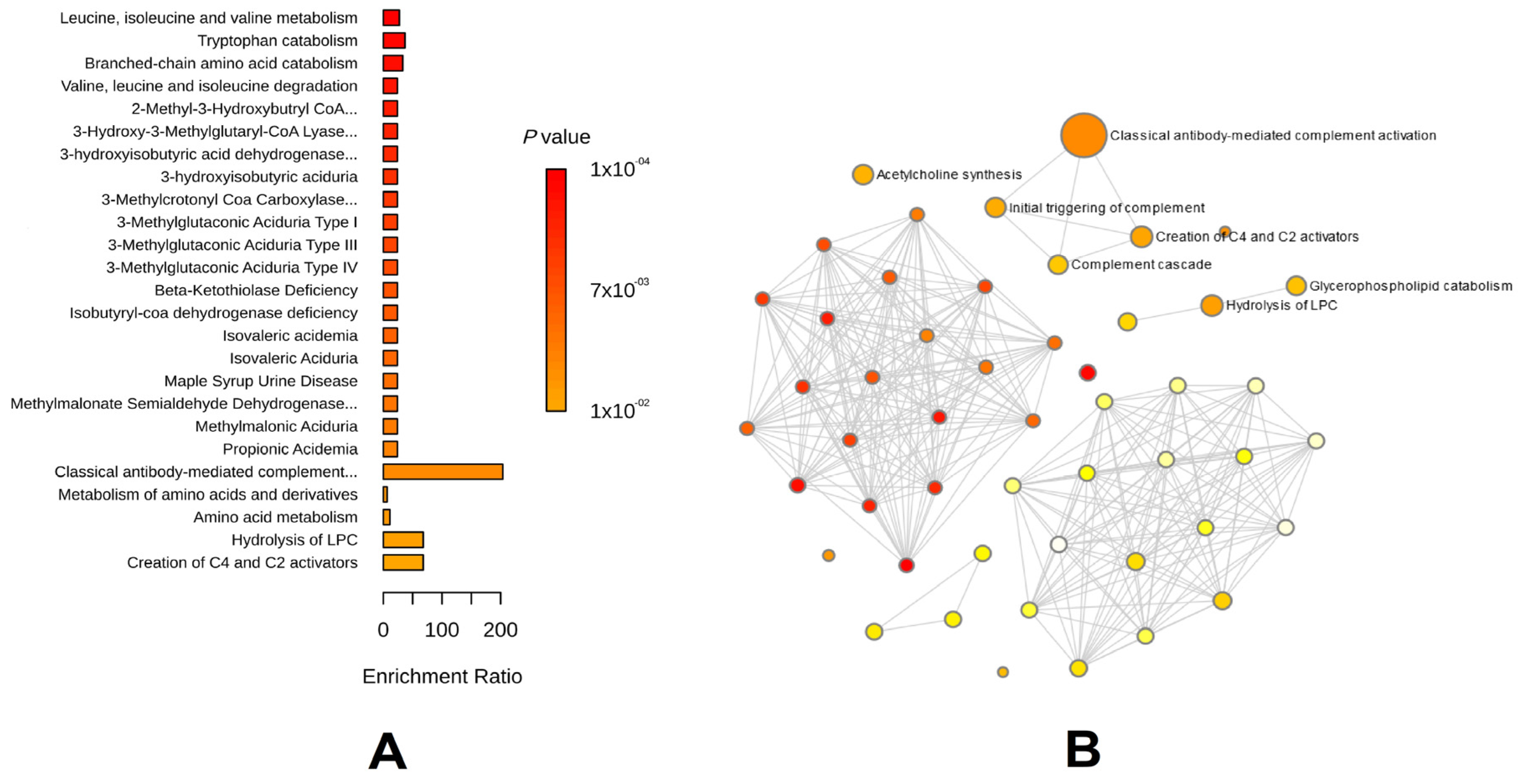
3.2. Key Metabolic Phenotypes of AAA Risk
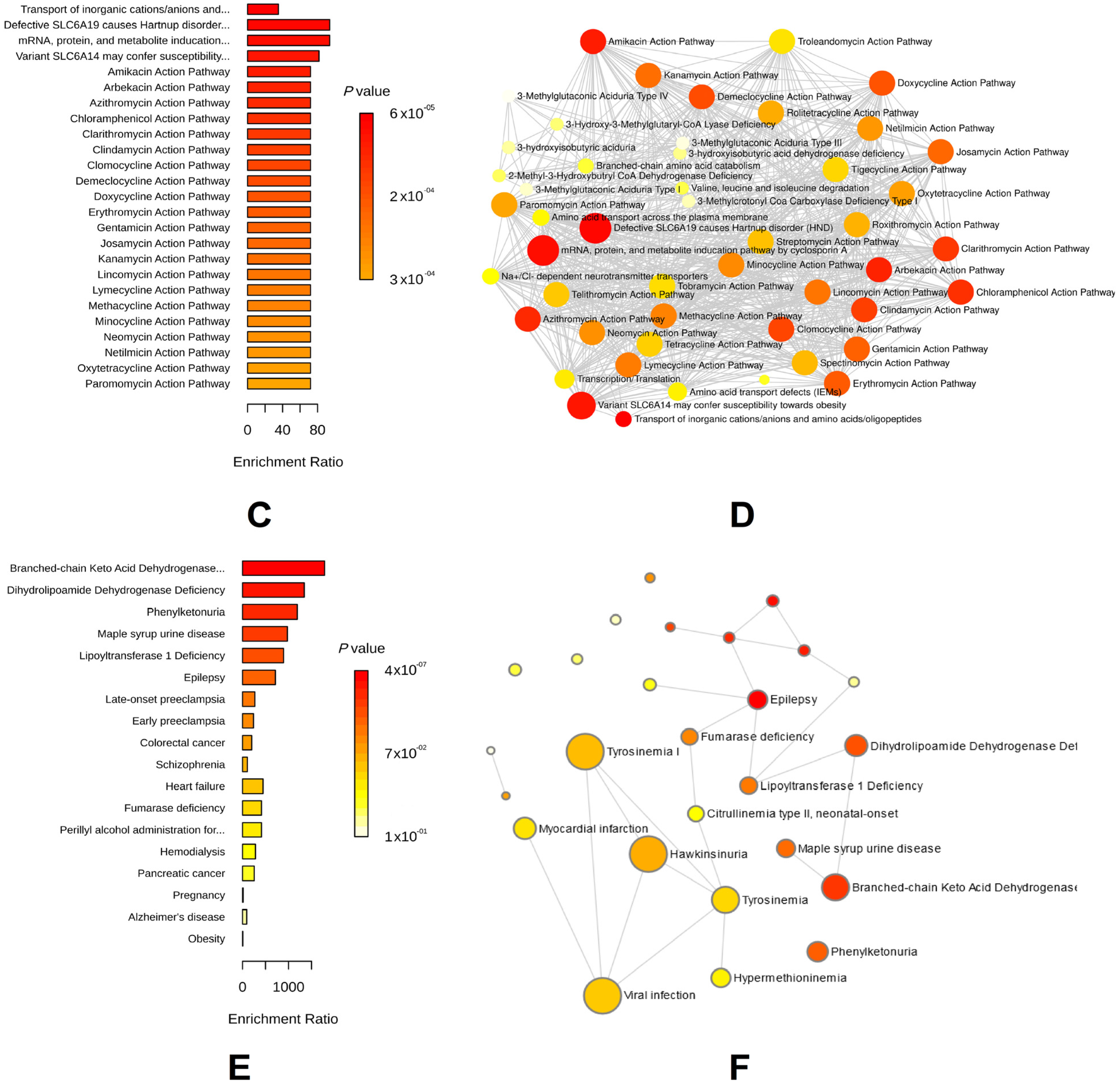
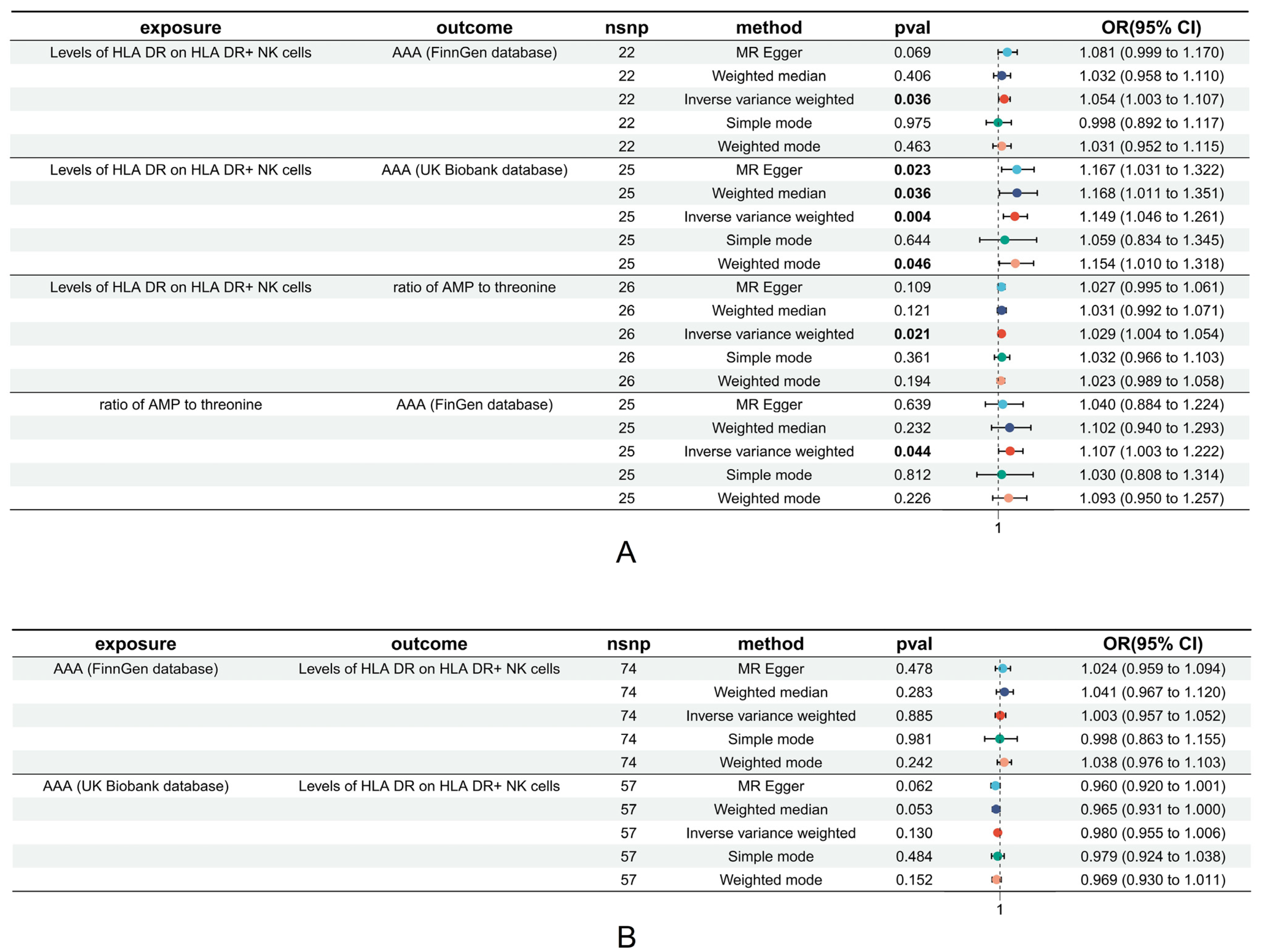
3.3. Causal Relationships between HLA on NK Cell Levels and Risky Metabolic Phenotypes of AAA
3.4. Mediation Effect Estimation
3.5. Heterogeneity and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haque, K.; Bhargava, P. Abdominal Aortic Aneurysm. Am. Fam. Physician 2022, 106, 165–172. [Google Scholar] [PubMed]
- Golledge, J.; Thanigaimani, S.; Powell, J.T.; Tsao, P.S. Pathogenesis and management of abdominal aortic aneurysm. Eur. Heart J. 2023, 44, 2682–2697. [Google Scholar] [CrossRef] [PubMed]
- Huanggu, H.; Yang, D.; Zheng, Y. Blood immunological profile of abdominal aortic aneurysm based on autoimmune injury. Autoimmun. Rev. 2023, 22, 103258. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). 2019. Available online: https://who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 5 July 2020).
- Baman, J.R.; Eskandari, M.K. What Is an Abdominal Aortic Aneurysm? JAMA 2022, 328, 2280. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Tsoi, L.C.; Melvin, W.J.; denDekker, A.; Wasikowski, R.; Joshi, A.D.; Wolf, S.; Obi, A.T.; Billi, A.C.; Xing, X.; et al. Inhibition of macrophage histone demethylase JMJD3 protects against abdominal aortic aneurysms. J. Exp. Med. 2021, 218, e20201839. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, L.; Gu, Y.; Wei, S.; Wang, Z.; Li, M.; Wang, W.; Wang, Z.; Bai, H. Immune checkpoint programmed death-1 mediates abdominal aortic aneurysm and pseudoaneurysm progression. Biomed. Pharmacother. 2021, 142, 111955. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.W.; Conrad, K.A.; Li, X.S. Gut Microbiota-Derived Trimethylamine N-Oxide Contributes to Abdominal Aortic Aneurysm Through Inflammatory and Apoptotic Mechanisms. Circulation 2023, 147, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Tian, Y.; Li, L. T cells in abdominal aortic aneurysm: Immunomodulation and clinical application. Front. Immunol. 2023, 14, 1240132. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhan, Y.; Cai, C.; Yu, D.; Wei, Q.; Quan, L.; Huang, D.; Liu, Y.; Li, Z.; Liu, L.; et al. Common molecular mechanism and immune infiltration patterns of thoracic and abdominal aortic aneurysms. Front. Immunol. 2022, 13, 1030976. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.C.; Davey Smith, G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb. Perspect. Med. 2022, 12, a040501. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Guo, Z. Genetically predicted 486 blood metabolites in relation to risk of colorectal cancer: A Mendelian randomization study. Cancer Med. 2023, 12, 13784–13799. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Butterworth, A.S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Badger, S.A.; Soong, C.V.; O’Donnell, M.E.; Middleton, D. The role of human leukocyte antigen genes in the formation of abdominal aortic aneurysms. J. Vasc. Surg. 2007, 45, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Rong, L.; Jiang, N.; Wayne, A.S.; Xie, J. Targeting HLA-DR loss in hematologic malignancies with an inhibitory chimeric antigen receptor. Mol. Ther. 2022, 30, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Haveman, J.W.; van den Berg, A.P.; Verhoeven, E.L.; Nijsten, M.W.; van den Dungen, J.J.; The, H.T.; Zwaveling, J.H. HLA-DR expression on monocytes and systemic inflammation in patients with ruptured abdominal aortic aneurysms. Crit. Care 2006, 10, R119. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Ayala, J.E.; Escamilla-Tilch, M.; Granados, J.; Hernandez-Dono, S.; Hernandez-Sotelo, K.; Lozano-Corona, R.; Ruiz-Gomez, D.; Garcia-Toca, M.; Hinojosa, C.A. Investigation of an Immunogenetic Profile in Patients with Abdominal Aortic Aneurysms and Possible Applications in Screening and Surveillance. Ann. Vasc. Surg. 2020, 62, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Vucevic, D.; Maravic-Stojkovic, V.; Vasilijic, S.; Borovic-Labudovic, M.; Majstorovic, I.; Radak, D.; Jevtic, M.; Milosavljevic, P.; Colic, M. Inverse production of IL-6 and IL-10 by abdominal aortic aneurysm explant tissues in culture. Cardiovasc. Pathol. 2012, 21, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Whitman, S.C.; Ramsamy, T.A. Participatory role of natural killer and natural killer T cells in atherosclerosis: Lessons learned from in vivo mouse studies. Can. J. Physiol. Pharmacol. 2006, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Schworer, C.M.; Lillvis, J.H.; Stahl, E.; Erdman, R.; Gatalica, Z.; Tromp, G.; Kuivaniemi, H. Immunohistochemical analysis of the natural killer cell cytotoxicity pathway in human abdominal aortic aneurysms. Int. J. Mol. Sci. 2015, 16, 11196–11212. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Sasaki, T.; Matsumoto, K.; Suzuki, K.; Takeshita, M.; Tanemura, S.; Seki, N.; Tsujimoto, H.; Takeuchi, T. Distinct features between HLA-DR+ and HLA-DR- PD-1hi CXCR5-T peripheral helper cells in seropositive rheumatoid arthritis. Rheumatology 2021, 60, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Wada, S.; Ota, H.; Fukushi, Y.; Tanimura, K.; Yoshino, O.; Arase, H.; Yamada, H. Anti-β2-glycoprotein I/HLA-DR antibody in infertility. J. Reprod. Immunol. 2023, 158, 103955. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Lyu, Y.Y.; Zhang, H.Y.; Shen, Z.; Lin, G.Q.; Geng, N.; Wang, Y.L.; Huang, L.; Feng, Z.H.; Guo, X.; et al. Nuclear Receptor NR1D1 Regulates Abdominal Aortic Aneurysm Development by Targeting the Mitochondrial Tricarboxylic Acid Cycle Enzyme Aconitase-2. Circulation 2022, 146, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Zhang, M.; Liang, B.; Zhu, H.; Lee, J.; Viollet, B.; Xia, L.; Zhang, Y.; Zou, M.H. Activation of AMP-activated protein kinase α2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat. Med. 2012, 18, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Lieberg, J.; Wanhainen, A.; Ottas, A.; Vähi, M.; Zilmer, M.; Soomets, U.; Björck, M. Metabolomic Profile of Abdominal Aortic Aneurysm. Metabolites 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, F.; Tang, Y.; Lu, Z.; Chen, Z.; Guo, Q. Adenosine 5′-Monophosphate-to-Threonine Ratio Promotes Abdominal Aortic Aneurysms via Up-Regulation of HLA-DR on Natural Killer Cells: A Bidirectional Mendelian Randomized Analysis. Biomedicines 2024, 12, 1179. https://doi.org/10.3390/biomedicines12061179
Teng F, Tang Y, Lu Z, Chen Z, Guo Q. Adenosine 5′-Monophosphate-to-Threonine Ratio Promotes Abdominal Aortic Aneurysms via Up-Regulation of HLA-DR on Natural Killer Cells: A Bidirectional Mendelian Randomized Analysis. Biomedicines. 2024; 12(6):1179. https://doi.org/10.3390/biomedicines12061179
Chicago/Turabian StyleTeng, Fei, Youyin Tang, Zhangyu Lu, Zheyu Chen, and Qiang Guo. 2024. "Adenosine 5′-Monophosphate-to-Threonine Ratio Promotes Abdominal Aortic Aneurysms via Up-Regulation of HLA-DR on Natural Killer Cells: A Bidirectional Mendelian Randomized Analysis" Biomedicines 12, no. 6: 1179. https://doi.org/10.3390/biomedicines12061179





