Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes
Abstract
1. Introduction
1.1. Major Depressive Disorder (MDD) and Type 2 Diabetes (T2D) Prevalence
1.2. MDD and T2D Comorbidity
1.3. Hypothalamic–Pituitary–Adrenal (HPA) Axis and Stress
1.4. Resilience, Stress, CRH System, and Depression
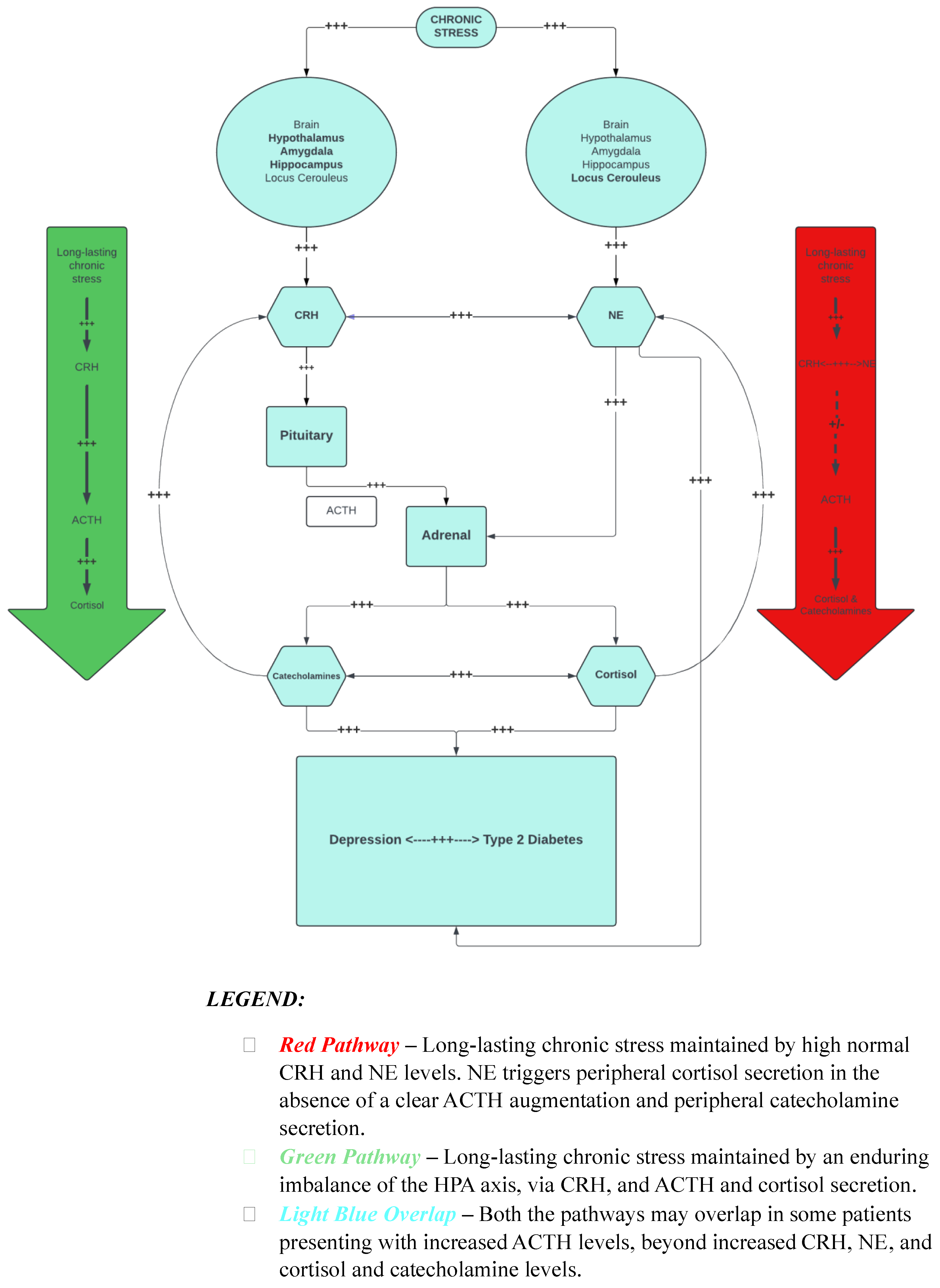
1.5. CRH System, Stress, and CRH–Norepinephrine–CRH Circuit
2. Stress, Depressive Symptoms, T2D, and Our Hypothesis
3. CRHR1 Genetic Studies
4. CRHR2 Genetic Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 3 March 2024).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas. Diabetes around the World in 2021. Available online: https://diabetesatlas.org/#:~:text=Diabetes%20around%20the%20world%20in%202021%3A,%2D%20and%20middle%2Dincome%20countries (accessed on 22 May 2024).
- Yip, W.C.Y.; Sequeira, I.R.; Plank, L.D.; Poppitt, S.D. Prevalence of Pre-Diabetes across Ethnicities: A Review of Impaired Fasting Glucose (IFG) and Impaired Glucose Tolerance (IGT) for Classification of Dysglycaemia. Nutrients 2017, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Demakakos, P.; Pierce, M.B.; Hardy, R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults: The English longitudinal study of aging. Diabetes Care 2010, 33, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Rustad, J.K.; Musselman, D.L.; Nemeroff, C.B. The relationship of depression and diabetes: Pathophysiological and treatment implications. Psychoneuroendocrinology 2011, 36, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, A.; Mensi, R.; Douki, W.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Valtorta, F.; Gaha, L.; Comai, S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J. Biol. Psychiatry 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Fakhoury, M. Revisiting the Serotonin Hypothesis: Implications for Major Depressive Disorders. Mol. Neurobiol. 2016, 53, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Mangold, D.; Marino, E.; Javors, M. The cortisol awakening response predicts subclinical depressive symptomatology in Mexican American adults. J. Psychiatr. Res. 2011, 45, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Suthers, K.; Ferrucci, L.; Simonsick, E.M.; Ble, A.; Schrager, M.; Bandinelli, S.; Lauretani, F.; Giannelli, S.V.; Penninx, B.W. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology 2007, 32, 151–159. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Moller-Leimkuhler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef]
- Yokoyama, K.; Yamada, T.; Mitani, H.; Yamada, S.; Pu, S.; Yamanashi, T.; Matsumura, H.; Nakagome, K.; Kaneko, K. Relationship between hypothalamic-pituitary-adrenal axis dysregulation and insulin resistance in elderly patients with depression. Psychiatry Res. 2015, 226, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Kang, C.; Yuan, J.; Zhang, Y.; Wei, Y.; Xu, L.; Zhou, F.; Fan, X.; Yang, J. Neuroendocrine abnormalities associated with untreated first episode patients with major depressive disorder and bipolar disorder. Psychoneuroendocrinology 2019, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Cataldo Bascunan, L.R.; Lyons, C.; Bennet, H.; Artner, I.; Fex, M. Serotonergic regulation of insulin secretion. Acta Physiol. 2019, 225, e13101. [Google Scholar] [CrossRef] [PubMed]
- Bennet, H.; Balhuizen, A.; Medina, A.; Dekker Nitert, M.; Ottosson Laakso, E.; Essen, S.; Spegel, P.; Storm, P.; Krus, U.; Wierup, N.; et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides 2015, 71, 113–120. [Google Scholar] [CrossRef]
- Bjorntorp, P. Alterations in the ageing corticotropic stress-response axis. Novartis Found. Symp. 2002, 242, 46–58; discussion 58–65. [Google Scholar]
- Lehrer, H.M.; Dubois, S.K.; Maslowsky, J.; Laudenslager, M.L.; Steinhardt, M.A. Hair cortisol concentration and glycated hemoglobin in African American adults. Psychoneuroendocrinology 2016, 72, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sunena; Mishra, D.N. Stress Etiology of Type 2 Diabetes. Curr. Diabetes Rev. 2022, 18, e240222201413. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Akre, S.; Chakole, S.; Wanjari, M.B. Stress-Induced Diabetes: A Review. Cureus 2022, 14, e29142. [Google Scholar] [CrossRef]
- Koumantarou Malisiova, E.; Mourikis, I.; Darviri, C.; Nicolaides, N.C.; Zervas, I.M.; Papageorgiou, C.; Chrousos, G.P. Hair cortisol concentrations in mental disorders: A systematic review. Physiol. Behav. 2021, 229, 113244. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Vitale, E.; Triggiani, V. Chronic Stress as a Risk Factor for Type 2 Diabetes: Endocrine, Metabolic, and Immune Implications. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mosili, P.; Mkhize, B.C.; Sibiya, N.H.; Ngubane, P.S.; Khathi, A. Review of the direct and indirect effects of hyperglycemia on the HPA axis in T2DM and the co-occurrence of depression. BMJ Open Diabetes Res. Care 2024, 12, e003218. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Bedont, J.L.; Blackshaw, S. Constructing the suprachiasmatic nucleus: A watchmaker’s perspective on the central clockworks. Front. Syst. Neurosci. 2015, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Vaughan, J.; Perrin, M. Corticotropin-Releasing Factor (CRF) Family of Ligands and Their Receptors. Endocrinol. 1997, 7, 3S–9S. [Google Scholar] [CrossRef]
- Smith, G.W.; Aubry, J.M.; Dellu, F.; Contarino, A.; Bilezikjian, L.M.; Gold, L.H.; Chen, R.; Marchuk, Y.; Hauser, C.; Bentley, C.A.; et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998, 20, 1093–1102. [Google Scholar] [CrossRef]
- Oler, J.A.; Fox, A.S.; Shelton, S.E.; Rogers, J.; Dyer, T.D.; Davidson, R.J.; Shelledy, W.; Oakes, T.R.; Blangero, J.; Kalin, N.H. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 2010, 466, 864–868. [Google Scholar] [CrossRef]
- Keck, M.E. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids 2006, 31, 241–250. [Google Scholar] [CrossRef]
- Naert, G.; Ixart, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell Neurosci. 2011, 46, 55–66. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. Corticotropin-releasing hormone and arginine vasopressin in depression focus on the human postmortem hypothalamus. Vitam. Horm. 2010, 82, 339–365. [Google Scholar] [CrossRef]
- Gold, P.W. Endocrine Factors in Key Structural and Intracellular Changes in Depression. Trends Endocrinol. Metab. 2021, 32, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Wilhelm, F.H.; Kossowsky, J.; Holsboer-Trachsler, E.; Schneider, S. Children suffering from separation anxiety disorder (SAD) show increased HPA axis activity compared to healthy controls. J. Psychiatr. Res. 2010, 45, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kolber, B.J.; Boyle, M.P.; Wieczorek, L.; Kelley, C.L.; Onwuzurike, C.C.; Nettles, S.A.; Vogt, S.K.; Muglia, L.J. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J. Neurosci. 2010, 30, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Kopin, I.J.; Lake, R.C.; Ziegler, M. Plasma levels of norepinephrine. Ann. Intern. Med. 1978, 88, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Lemche, E.; Giampietro, V.P.; Surguladze, S.A.; Amaro, E.J.; Andrew, C.M.; Williams, S.C.; Brammer, M.J.; Lawrence, N.; Maier, M.A.; Russell, T.A.; et al. Human attachment security is mediated by the amygdala: Evidence from combined fMRI and psychophysiological measures. Hum. Brain Mapp. 2006, 27, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Asakura, M.; Nagashima, H.; Fujii, S.; Sasuga, Y.; Misonoh, A.; Hasegawa, H.; Osada, K. Influences of chronic stress on central nervous systems. Nihon Shinkei Seishin Yakurigaku Zasshi 2000, 20, 97–105. [Google Scholar] [PubMed]
- Sutoh, M.; Kasuya, E.; Yayou, K.; Ohtani, F.; Kobayashi, Y. Intravenous tryptophan administration attenuates cortisol secretion induced by intracerebroventricular injection of noradrenaline. Anim. Sci. J. 2016, 87, 266–270. [Google Scholar] [CrossRef]
- Gold, P.W.; Kling, M.A.; Khan, I.; Calabrese, J.R.; Kalogeras, K.; Post, R.M.; Avgerinos, P.C.; Loriaux, D.L.; Chrousos, G.P. Corticotropin releasing hormone: Relevance to normal physiology and to the pathophysiology and differential diagnosis of hypercortisolism and adrenal insufficiency. Adv. Biochem. Psychopharmacol. 1987, 43, 183–200. [Google Scholar]
- Clark, A.J.; Metherell, L.A. Mechanisms of disease: The adrenocorticotropin receptor and disease. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Fisher, L.A.; Spiess, J.; Rivier, C.; Rivier, J.; Vale, W. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology 1982, 111, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.W.; Lightly, E.R.; Milner, S.W.; Williams, B.C. Catecholamine stimulation of cortisol secretion by 3-day primary cultures of purified zona fasciculata/reticularis cells isolated from bovine adrenal cortex. Mol. Cell Endocrinol. 1988, 57, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Gallucci, W.T.; Chrousos, G.P.; Gold, P.W. Catecholamine effects upon rat hypothalamic corticotropin-releasing hormone secretion in vitro. J. Clin. Investig. 1988, 82, 839–846. [Google Scholar] [CrossRef]
- Al-Damluji, S.; Perry, L.; Tomlin, S.; Bouloux, P.; Grossman, A.; Rees, L.H.; Besser, G.M. Alpha-adrenergic stimulation of corticotropin secretion by a specific central mechanism in man. Neuroendocrinology 1987, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Kling, M.A.; Munson, P.J.; Listwak, S.; Licinio, J.; Prolo, P.; Karp, B.; McCutcheon, I.E.; Geracioti, T.D., Jr.; DeBellis, M.D.; et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. USA 2000, 97, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 35, 722–729. [Google Scholar] [CrossRef]
- Bruehl, H.; Rueger, M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Javier, E.; Arentoft, A.; Wolf, O.T.; Convit, A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 2439–2445. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Srinivasan, K. Maternal Nutrition and Offspring Stress Response-Implications for Future Development of Non-Communicable Disease: A Perspective from India. Front. Psychiatry 2019, 10, 795. [Google Scholar] [CrossRef]
- Pivonello, R.; De Leo, M.; Vitale, P.; Cozzolino, A.; Simeoli, C.; De Martino, M.C.; Lombardi, G.; Colao, A. Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology 2010, 92 (Suppl. S1), S77–S81. [Google Scholar] [CrossRef]
- Qi, L.; Kraft, P.; Hunter, D.J.; Hu, F.B. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum. Mol. Genet. 2008, 17, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, E.M.; Koltsida, G.; Farakla, I.; Papanikolaou, A.; Critselis, E.; Mantzou, E.; Zoumakis, E.; Kolaitis, G.; Chrousos, G.P.; Charmandari, E. Psychological vulnerability to stress in carriers of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hormones 2017, 16, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Torlontano, M.; Scillitani, A.; Arosio, M.; Bacci, S.; Di Lembo, S.; Epaminonda, P.; Augello, G.; Enrini, R.; Ambrosi, B.; et al. Association of subclinical hypercortisolism with type 2 diabetes mellitus: A case-control study in hospitalized patients. Eur. J. Endocrinol. 2005, 153, 837–844. [Google Scholar] [CrossRef]
- Heuser, I.; Yassouridis, A.; Holsboer, F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 1994, 28, 341–356. [Google Scholar] [CrossRef]
- Heuser, I.J.; Gotthardt, U.; Schweiger, U.; Schmider, J.; Lammers, C.H.; Dettling, M.; Holsboer, F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol. Aging 1994, 15, 227–231. [Google Scholar] [CrossRef]
- Heuser, I.; Lammers, C.H. Stress and the brain. Neurobiol. Aging 2003, 24 (Suppl. S1), S69–S76; discussion S81–S62. [Google Scholar] [CrossRef]
- Amin, M.; Syed, S.; Wu, R.; Postolache, T.T.; Gragnoli, C. Familial Linkage and Association of the NR3C1 Gene with Type 2 Diabetes and Depression Comorbidity. Int. J. Mol. Sci. 2022, 23, 11951. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Ott, J.; Wu, R.; Postolache, T.T.; Gragnoli, C. Implication of Melanocortin Receptor Genes in the Familial Comorbidity of Type 2 Diabetes and Depression. Int. J. Mol. Sci. 2022, 23, 8350. [Google Scholar] [CrossRef]
- Adam, E.K.; Kumari, M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef]
- Phillips, D.I.; Barker, D.J.; Fall, C.H.; Seckl, J.R.; Whorwood, C.B.; Wood, P.J.; Walker, B.R. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome? J. Clin. Endocrinol. Metab. 1998, 83, 757–760. [Google Scholar] [CrossRef]
- Misra, M.; Bredella, M.A.; Tsai, P.; Mendes, N.; Miller, K.K.; Klibanski, A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E385–E392. [Google Scholar] [CrossRef]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr. Pract. 2009, 15, 469–474. [Google Scholar] [CrossRef]
- Kumari, M.; Chandola, T.; Brunner, E.; Kivimaki, M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2010, 95, 4415–4423. [Google Scholar] [CrossRef]
- Kumari, M.; Shipley, M.; Stafford, M.; Kivimaki, M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 2011, 96, 1478–1485. [Google Scholar] [CrossRef]
- Hackett, R.A.; Steptoe, A.; Kumari, M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. Metab. 2014, 99, 4625–4631. [Google Scholar] [CrossRef]
- Carroll, T.; Raff, H.; Findling, J.W. Late-night salivary cortisol for the diagnosis of Cushing syndrome: A meta-analysis. Endocr. Pract. 2009, 15, 335–342. [Google Scholar] [CrossRef]
- Liu, H.; Bravata, D.M.; Cabaccan, J.; Raff, H.; Ryzen, E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin. Endocrinol. 2005, 63, 642–649. [Google Scholar] [CrossRef]
- Adam, E.K.; Hawkley, L.C.; Kudielka, B.M.; Cacioppo, J.T. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. USA 2006, 103, 17058–17063. [Google Scholar] [CrossRef]
- Bruehl, H.; Wolf, O.T.; Convit, A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 2009, 34, 815–821. [Google Scholar] [CrossRef]
- Ursache, A.; Wedin, W.; Tirsi, A.; Convit, A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology 2012, 37, 1270–1276. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Vieira, A.R.; Moreira, R.O.; Coutinho, W.F.; Carraro, L.M.; Moreira, D.M.; Pasquali, R.; Meirelles, R.M. The potential role of increased adrenal volume in the pathophysiology of obesity-related type 2 diabetes. J. Endocrinol. Investig. 2006, 29, 159–163. [Google Scholar] [CrossRef]
- Timpl, P.; Spanagel, R.; Sillaber, I.; Kresse, A.; Reul, J.M.; Stalla, G.K.; Blanquet, V.; Steckler, T.; Holsboer, F.; Wurst, W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Genet. 1998, 19, 162–166. [Google Scholar] [CrossRef]
- Refojo, D.; Schweizer, M.; Kuehne, C.; Ehrenberg, S.; Thoeringer, C.; Vogl, A.M.; Dedic, N.; Schumacher, M.; von Wolff, G.; Avrabos, C.; et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 2011, 333, 1903–1907. [Google Scholar] [CrossRef]
- Rogers, J.; Raveendran, M.; Fawcett, G.L.; Fox, A.S.; Shelton, S.E.; Oler, J.A.; Cheverud, J.; Muzny, D.M.; Gibbs, R.A.; Davidson, R.J.; et al. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol. Psychiatry 2013, 18, 700–707. [Google Scholar] [CrossRef]
- Heim, C.; Bradley, B.; Mletzko, T.C.; Deveau, T.C.; Musselman, D.L.; Nemeroff, C.B.; Ressler, K.J.; Binder, E.B. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front. Behav. Neurosci. 2009, 3, 41. [Google Scholar] [CrossRef]
- Wasserman, D.; Wasserman, J.; Rozanov, V.; Sokolowski, M. Depression in suicidal males: Genetic risk variants in the CRHR1 gene. Genes. Brain Behav. 2009, 8, 72–79. [Google Scholar] [CrossRef]
- Ressler, K.J.; Bradley, B.; Mercer, K.B.; Deveau, T.C.; Smith, A.K.; Gillespie, C.F.; Nemeroff, C.B.; Cubells, J.F.; Binder, E.B. Polymorphisms in CRHR1 and the serotonin transporter loci: Gene x gene x environment interactions on depressive symptoms. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153B, 812–824. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, F.; Wang, G.; Xiao, Z.; Wang, H.; Tang, J.; Wang, X.; Qiu, D.; Liu, W.; Cao, Z.; et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci. Lett. 2006, 404, 358–362. [Google Scholar] [CrossRef]
- Licinio, J.; O’Kirwan, F.; Irizarry, K.; Merriman, B.; Thakur, S.; Jepson, R.; Lake, S.; Tantisira, K.G.; Weiss, S.T.; Wong, M.L. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol. Psychiatry 2004, 9, 1075–1082. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, F.; Wang, G.; Xiao, Z.; Tang, J.; Liu, W.; Wang, H.; Liu, H.; Wang, X.; Wu, Y.; et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci. Lett. 2007, 414, 155–158. [Google Scholar] [CrossRef]
- Del Bosque-Plata, L.; Amin, M.; Gonzalez-Ramirez, R.; Wu, R.; Postolache, T.T.; Vergare, M.; Gordon, D.; Gragnoli, C. LD block disorder-specific pleiotropic roles of novel CRHR1 in type 2 diabetes and depression disorder comorbidity. Eur. Arch. Psychiatry Clin. Neurosci. 2023. [Google Scholar] [CrossRef]
- Spurdle, A.B.; Thompson, D.J.; Ahmed, S.; Ferguson, K.; Healey, C.S.; O’Mara, T.; Walker, L.C.; Montgomery, S.B.; Dermitzakis, E.T.; Fahey, P.; et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat. Genet. 2011, 43, 451–454. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lee, K.E.; Duggal, P.; Moore, E.L.; Wilson, A.F.; Klein, R.; Bailey-Wilson, J.E.; Klein, B.E. Genome-wide linkage analysis of multiple metabolic factors: Evidence of genetic heterogeneity. Obesity 2009, 18, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Huising, M.O.; van der Meulen, T.; Vaughan, J.M.; Matsumoto, M.; Donaldson, C.J.; Park, H.; Billestrup, N.; Vale, W.W. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc. Natl. Acad. Sci. USA 2010, 107, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.C.; Kesterson, R.A.; Heldwein, K.A.; Stevens, S.L.; Heard, A.D.; Hollis, J.H.; Murray, S.E.; Hill, J.K.; Pantely, G.A.; Hohimer, A.R.; et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000, 24, 403–409. [Google Scholar] [CrossRef]
- Bale, T.L.; Contarino, A.; Smith, G.W.; Chan, R.; Gold, L.H.; Sawchenko, P.E.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000, 24, 410–414. [Google Scholar] [CrossRef]
- Kishimoto, T.; Radulovic, J.; Radulovic, M.; Lin, C.R.; Schrick, C.; Hooshmand, F.; Hermanson, O.; Rosenfeld, M.G.; Spiess, J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000, 24, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, Y.; Chen, A. Urocortins: Emerging metabolic and energy homeostasis perspectives. Trends Endocrinol. Metab. 2008, 19, 122–129. [Google Scholar] [CrossRef]
- Grunddal, K.V.; Trammell, S.A.J.; Baech-Laursen, C.; Andersen, D.B.; Xu, S.F.S.; Andersen, H.; Gillum, M.P.; Ghiasi, S.M.; Novak, I.; Tyrberg, B.; et al. Opposing roles of the entero-pancreatic hormone urocortin-3 in glucose metabolism in rats. Diabetologia 2022, 65, 1018–1031. [Google Scholar] [CrossRef]
- Li, H.; Page, A.J. Activation of CRF2 receptor increases gastric vagal afferent mechanosensitivity. J. Neurophysiol. 2019, 122, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.L.; Massart, J.; Schonke, M.; De Castro Barbosa, T.; Guo, L.; Wade, M.; Alsina-Fernandez, J.; Miles, R.; Ryan, A.; Bauer, S.; et al. Modified UCN2 Peptide Acts as an Insulin Sensitizer in Skeletal Muscle of Obese Mice. Diabetes 2019, 68, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, P.M.; Cleasby, M.E.; Kuperman, Y.; Morton, N.M.; Kelly, P.A.; Brownstein, D.G.; Mustard, K.J.; Vaughan, J.M.; Carter, R.N.; Hahn, C.N.; et al. Urocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high-fat diet. Diabetologia 2011, 54, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Paruthiyil, S.; Hagiwara, S.I.; Kundassery, K.; Bhargava, A. Sexually dimorphic metabolic responses mediated by CRF(2) receptor during nutritional stress in mice. Biol. Sex. Differ. 2018, 9, 49. [Google Scholar] [CrossRef]
- Ishitobi, Y.; Nakayama, S.; Yamaguchi, K.; Kanehisa, M.; Higuma, H.; Maruyama, Y.; Ninomiya, T.; Okamoto, S.; Tanaka, Y.; Tsuru, J.; et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Papiol, S.; Arias, B.; Gasto, C.; Gutierrez, B.; Catalan, R.; Fananas, L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J. Affect. Disord. 2007, 104, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, S.; Hattersley, A.T.; Hitman, G.A.; Walker, M.; Levy, J.C.; Sampson, M.; O’rahilly, S.; Frayling, T.M.; Bell, J.I.; Lathrop, G.M.; et al. A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (The Diabetes UK Warren 2 Repository): Analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am. J. Hum. Genet. 2001, 69, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Leak, T.S.; Langefeld, C.D.; Keene, K.L.; Gallagher, C.J.; Lu, L.; Mychaleckyj, J.C.; Rich, S.S.; Freedman, B.I.; Bowden, D.W.; Sale, M.M. Chromosome 7p linkage and association study for diabetes related traits and type 2 diabetes in an African-American population enriched for nephropathy. BMC Med. Genet. 2010, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.H.; Lam, V.K.; So, W.Y.; Ma, R.C.; Chan, J.C.; Ng, M.C. Genome-wide linkage scan for factors of metabolic syndrome in a Chinese population. BMC Genet. 2010, 11, 14. [Google Scholar] [CrossRef]
- Camp, N.J.; Lowry, M.R.; Richards, R.L.; Plenk, A.M.; Carter, C.; Hensel, C.H.; Abkevich, V.; Skolnick, M.H.; Shattuck, D.; Rowe, K.G.; et al. Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 135B, 85–93. [Google Scholar] [CrossRef]
- Kremeyer, B.; Garcia, J.; Muller, H.; Burley, M.W.; Herzberg, I.; Parra, M.V.; Duque, C.; Vega, J.; Montoya, P.; Lopez, M.C.; et al. Genome-wide linkage scan of bipolar disorder in a colombian population isolate replicates Loci on chromosomes 7p21-22, 1p31, 16p12 and 21q21-22 and identifies a novel locus on chromosome 12q. Hum. Hered. 2010, 70, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Hamshere, M.L.; Schulze, T.G.; Schumacher, J.; Corvin, A.; Owen, M.J.; Jamra, R.A.; Propping, P.; Maier, W.; Orozco y Diaz, G.; Mayoral, F.; et al. Mood-incongruent psychosis in bipolar disorder: Conditional linkage analysis shows genome-wide suggestive linkage at 1q32.3, 7p13 and 20q13.31. Bipolar Disord. 2009, 11, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Pregelj, P. Psychosis and depression—A neurobiological view. Psychiatr. Danub. 2009, 21 (Suppl. S1), S102–S105. [Google Scholar]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Amin, M.; Ott, J.; Gordon, D.; Wu, R.; Postolache, T.T.; Vergare, M.; Gragnoli, C. Comorbidity of Novel CRHR2 Gene Variants in Type 2 Diabetes and Depression. Int. J. Mol. Sci. 2022, 23, 9819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrelli, M.; Goparaju, P.; Postolache, T.T.; del Bosque-Plata, L.; Gragnoli, C. Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes. Biomedicines 2024, 12, 1187. https://doi.org/10.3390/biomedicines12061187
Perrelli M, Goparaju P, Postolache TT, del Bosque-Plata L, Gragnoli C. Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes. Biomedicines. 2024; 12(6):1187. https://doi.org/10.3390/biomedicines12061187
Chicago/Turabian StylePerrelli, Michele, Pruthvi Goparaju, Teodor T. Postolache, Laura del Bosque-Plata, and Claudia Gragnoli. 2024. "Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes" Biomedicines 12, no. 6: 1187. https://doi.org/10.3390/biomedicines12061187
APA StylePerrelli, M., Goparaju, P., Postolache, T. T., del Bosque-Plata, L., & Gragnoli, C. (2024). Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes. Biomedicines, 12(6), 1187. https://doi.org/10.3390/biomedicines12061187






