Evaluation of Different Decellularization Protocols for Obtaining and Characterizing Canine Cardiac Extracellular Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
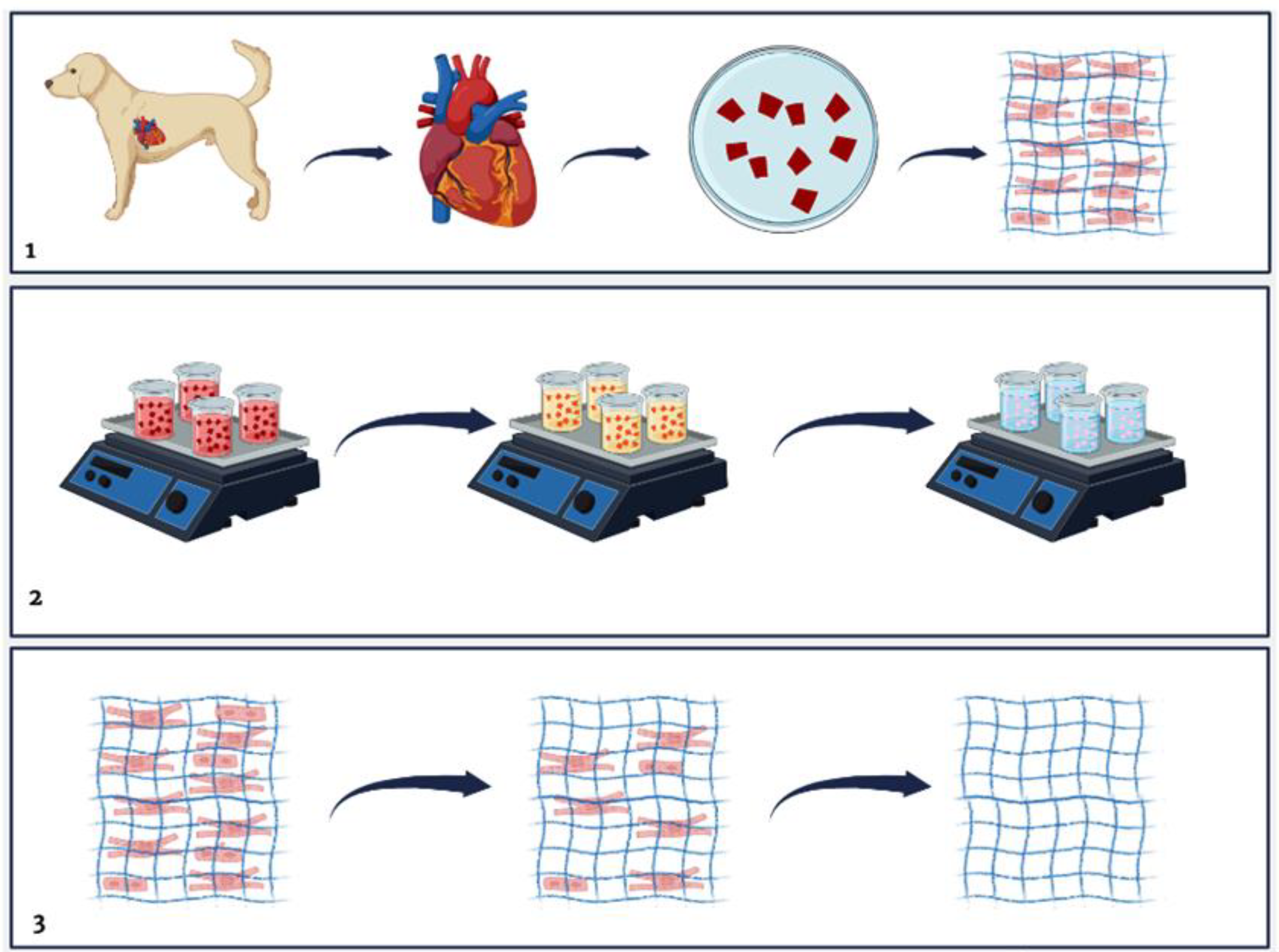
2.2. Decellularization Process
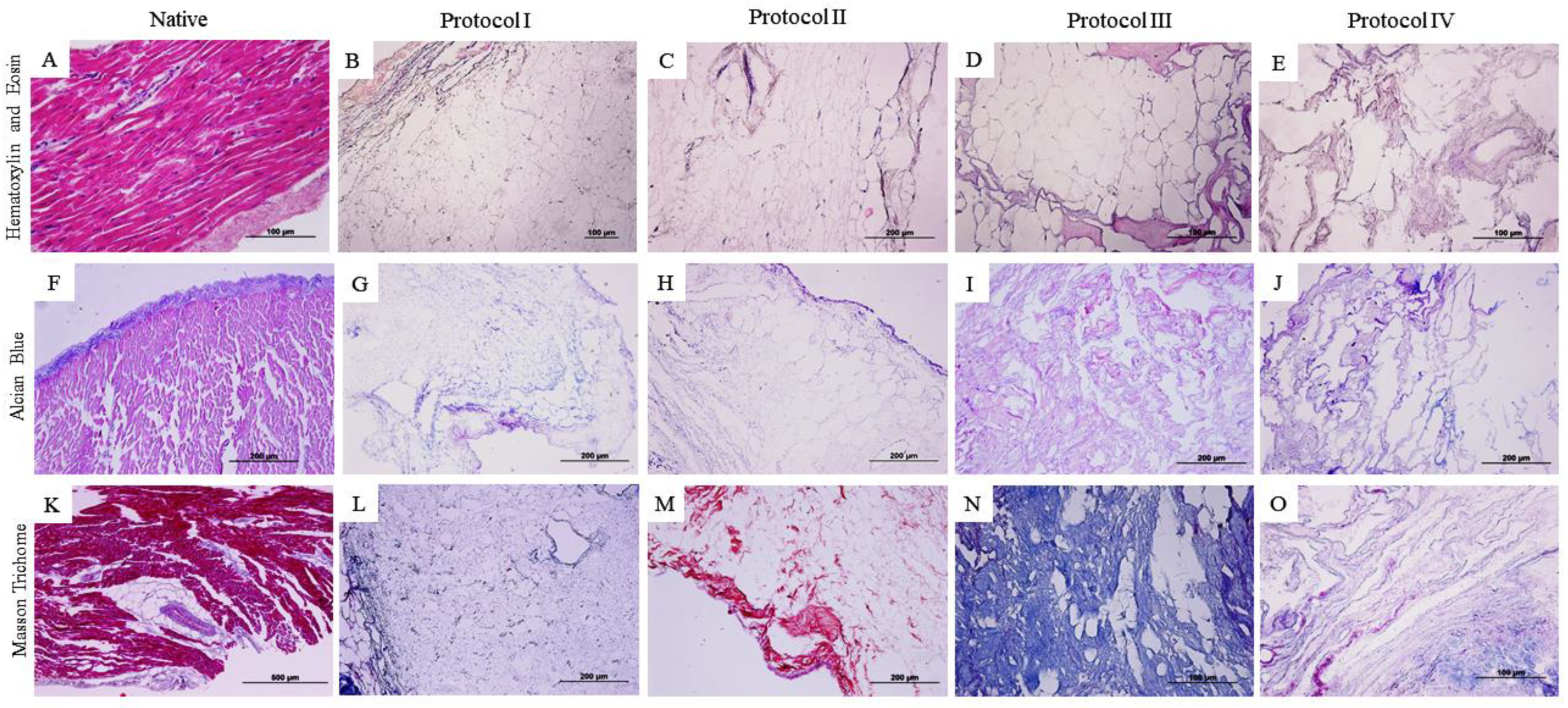
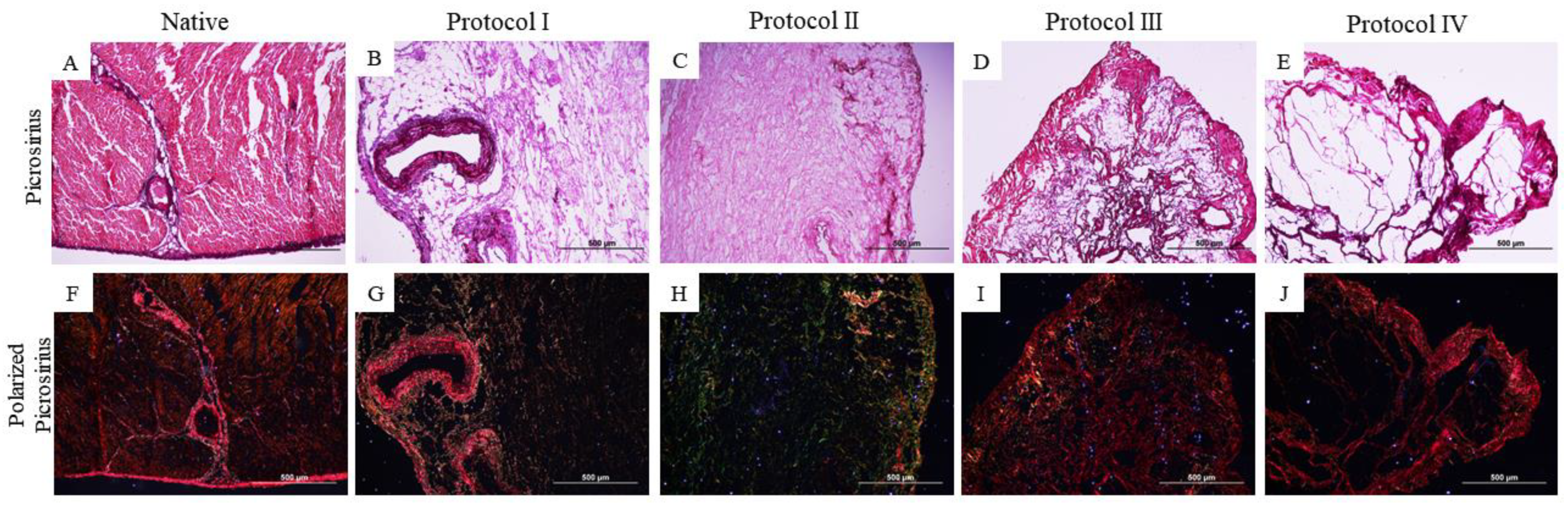
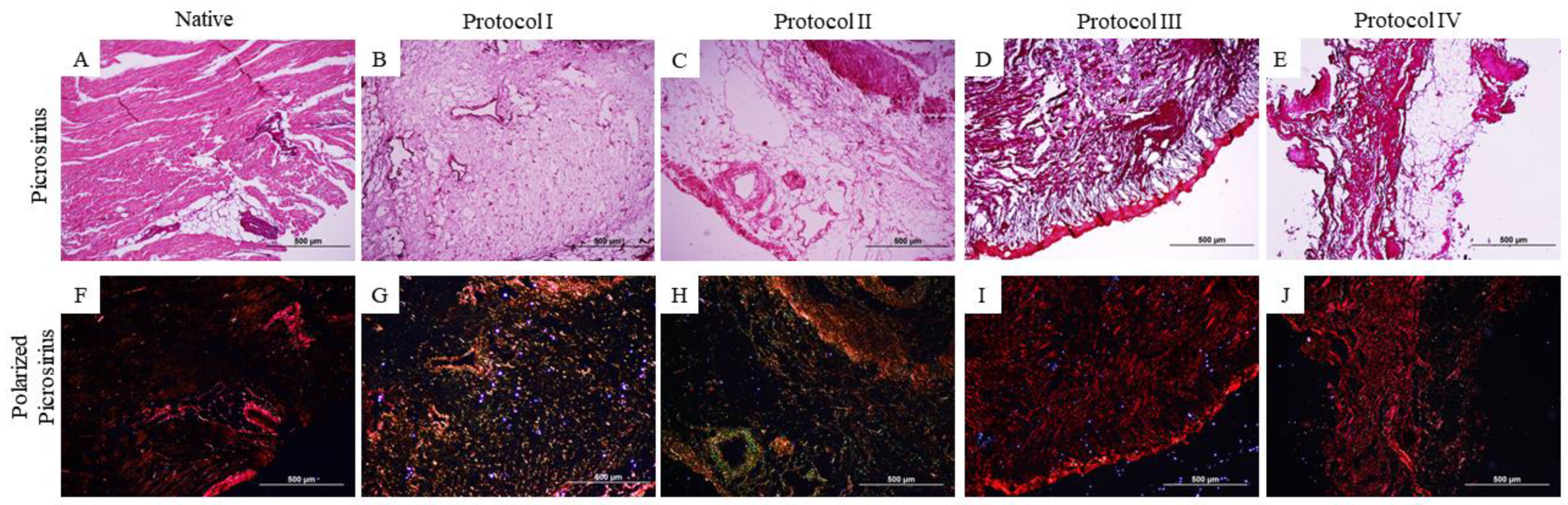
2.3. Qualitative Histological Analysis
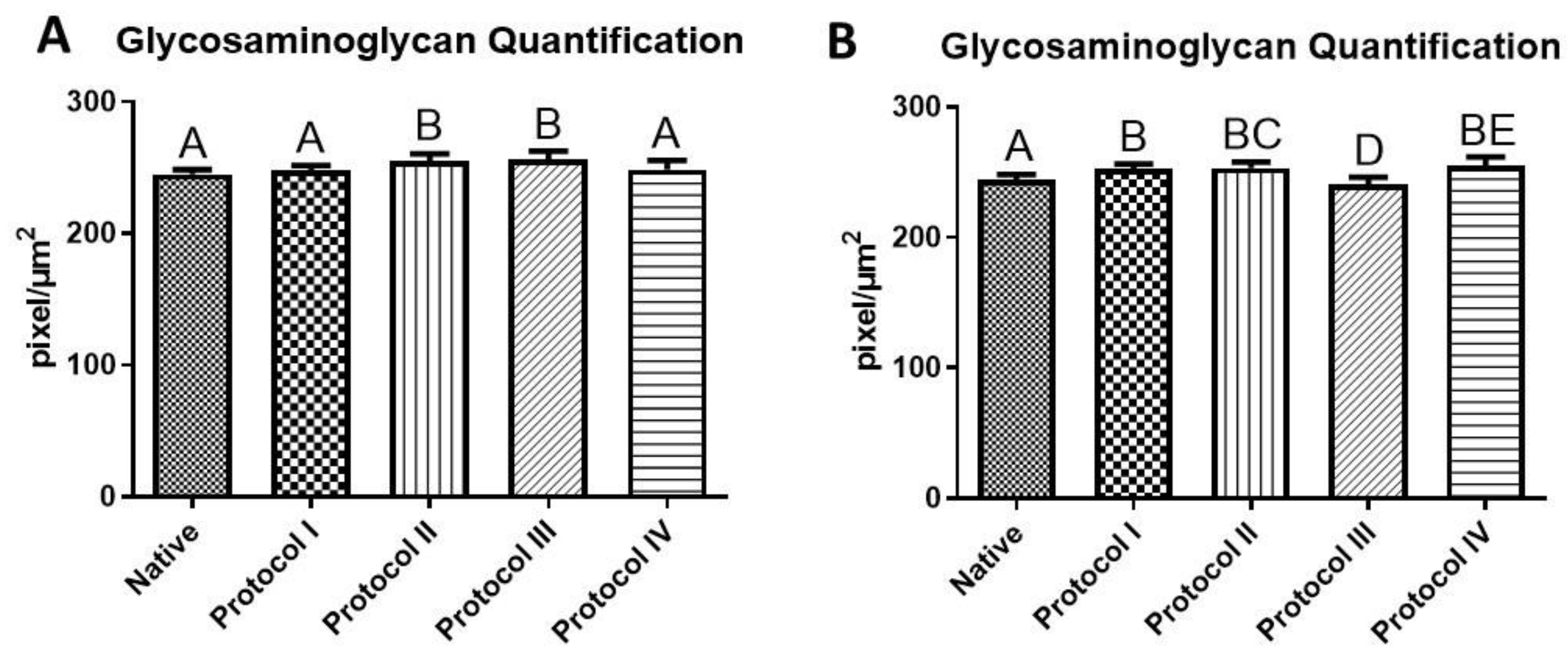
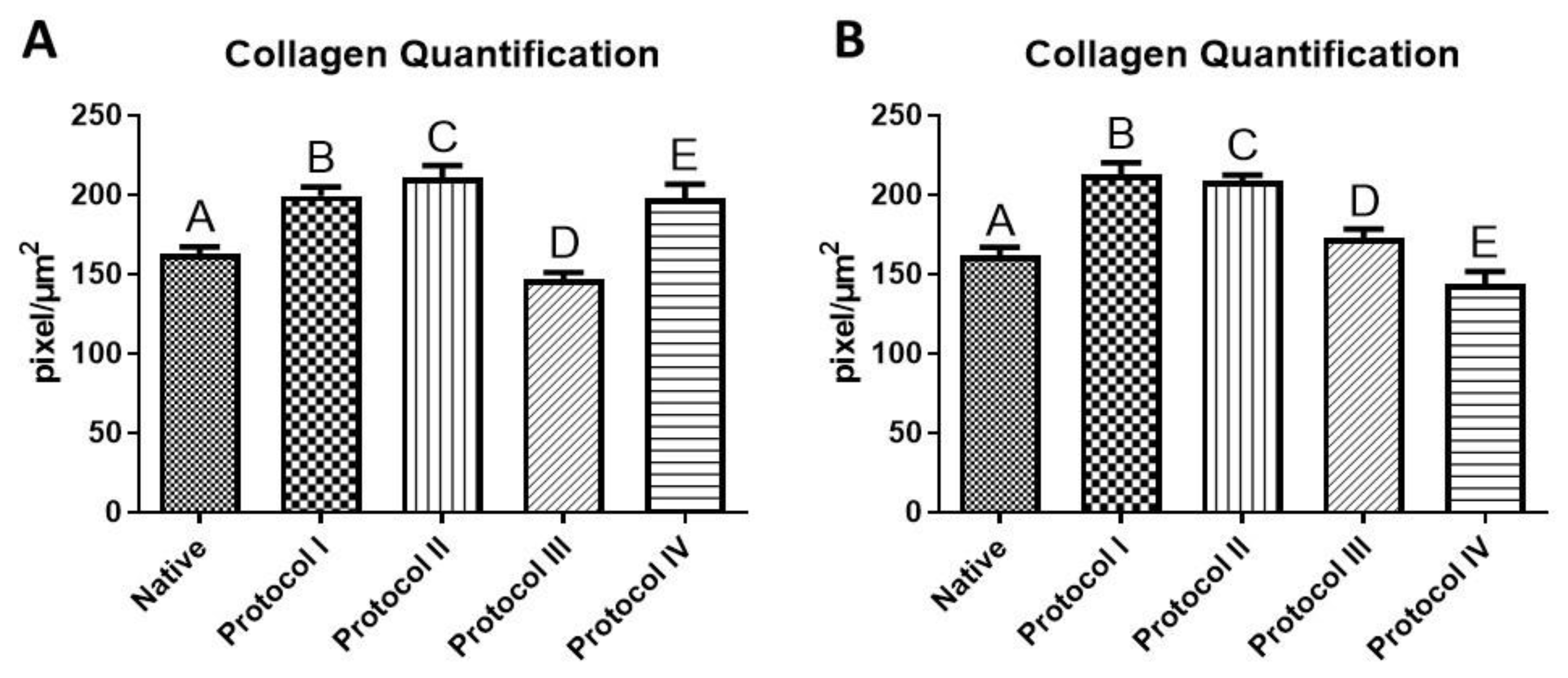
2.4. Quantification of Glycosaminoglycans (GAG) and Collagen
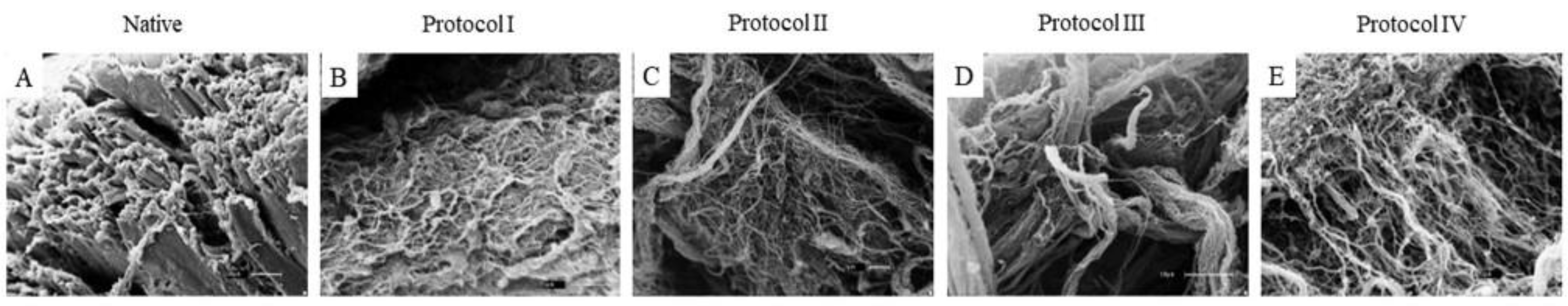
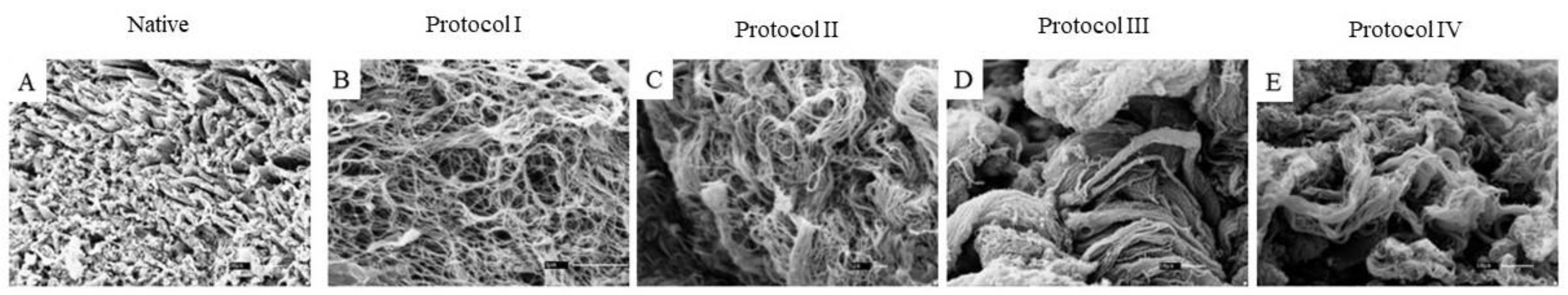
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis of the Quantification of Glycosaminoglycans and Collagen
3. Results
3.1. Qualitative Histological Analysis
3.2. Scanning Electron Microscopy (SEM)
3.3. Quantification of Glycosaminoglycans (GAG)
3.4. Collagen Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baghalishahi, M.; Efthekhar-vaghefi, S.H.; Piryaei, A.; Nematolahi-mahani, S.N.; Mollaei, H.R.; Sadeghi, Y. Cardiac Extracellular Matrix Hydrogel Together with or without Inducer Cocktail Improves Human Adipose Tissue-Derived Stem Cells Differentiation into Cardiomyocyte–like Cells. Biochem. Biophys. Res. Commun. 2018, 502, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef]
- WHO. WHO World Health Statistics 2019: The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 20 September 2021).
- Aubert, O.; Yoo, D.; Zielinski, D.; Cozzi, E.; Cardillo, M.; Dürr, M.; Domínguez-Gil, B.; Coll, E.; Da Silva, M.I.; Sallinen, V.; et al. COVID-19 Pandemic and Worldwide Organ Transplantation: A Population-Based Study. Lancet Public Health 2021, 6, e709–e719. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; de Bakker, D.E.M.; Bakkers, J. Cardiac Regenerative Capacity: An Evolutionary Afterthought? Cell. Mol. Life Sci. 2021, 78, 5107. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, Y.; Zhang, K.; Ke, S.; Wang, Y.; Wang, J.; Wang, H. Recent Advances in Decellularized Matrix-Derived Materials for Bioink and 3D Bioprinting. Gels 2023, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, M.; Dorkoosh, F. Cardiac Tissue Engineering, Biomaterial Scaffolds, and Their Fabrication Techniques. Polym. Adv. Technol. 2021, 32, 2290–2305. [Google Scholar] [CrossRef]
- Hyun, C.; Filippich, L.J. Molecular Genetics of Sudden Cardiac Death in Small Animals—A Review. Vet. J. 2006, 171, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.L. Animal Models of Ventricular Arrhythmias. Pharmacol. Ther. 2007, 113, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Hulsmann, J.; Aubin, H.; Oberle, F.; Schutterle, N.; Bandesha, S.; Ijima, M.; Lichtenberg, A.; Akhyari, P. Mechanistics of Biomass Discharge during Whole-Heart Decellularization. Biomed. Mater. 2018, 13, 035014. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/Chitosan/Alginate Ternary Scaffolds for Cardiac Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of Neonatal and Adult Mammalian Heart Regeneration by the MiR-15 Family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef]
- Silva, I.G.R.d.; Pantoja, B.T.d.S.; Almeida, G.H.D.R.; Carreira, A.C.O.; Miglino, M.A. Bacterial Cellulose and ECM Hydrogels: An Innovative Approach for Cardiovascular Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3955. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-Decellularized Matrix: Using Nature’s Platform to Engineer a Bioartificial Heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Liguori, G.R.; Liguori, T.T.A.; de Moraes, S.R.; Sinkunas, V.; Terlizzi, V.; van Dongen, J.A.; Sharma, P.K.; Moreira, L.F.P.; Harmsen, M.C. Molecular and Biomechanical Clues From Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020, 8, 520. [Google Scholar] [CrossRef]
- Kieda, J.; Shakeri, A.; Landau, S.; Wang, E.Y.; Zhao, Y.; Lai, B.F.; Okhovatian, S.; Wang, Y.; Jiang, R.; Radisic, M. Advances in Cardiac Tissue Engineering and Heart-on-a-Chip. J. Biomed. Mater. Res. Part A 2024, 112, 492–511. [Google Scholar] [CrossRef]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Tenreiro, M.F.; Louro, A.F.; Alves, P.M.; Serra, M. Next Generation of Heart Regenerative Therapies: Progress and Promise of Cardiac Tissue Engineering. NPJ Regen. Med. 2021, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of Fibroblast Cell Sheets for Natural Extracellular Matrix Scaffold Preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Shih, T.R.; Lin, H.L.; Chen, Y.M.; Sung, J.H.; Lu, C.H.; Wei, J.R.; Wang, Z.W.; et al. Decellularization and Recellularization Technologies in Tissue Engineering. Cell Transplant. 2014, 23, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. Int. J. Mol. Sci. 2022, 23, 13040. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.H.; Stamm, C. Human Cardiac Extracellular Matrix Supports Myocardial Lineage Commitment of Pluripotent Stem Cells. Eur. J. Cardio-Thoracic Surg. 2015, 47, 416–425. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Tan, Y.H.; Helms, H.R.; Nakayama, K.H. Decellularization Strategies for Regenerating Cardiac and Skeletal Muscle Tissues. Front. Bioeng. Biotechnol. 2022, 10, 831300. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Wang, B.; Shah, M.; Williams, L.N.; de Jongh Curry, A.L.; Hong, Y.; Zhang, G.; Liao, J. Acellular Myocardial Scaffolds and Slices Fabrication, and Method for Applying Mechanical and Electrical Simulation to Tissue Construct. Methods Mol. Biol. 2022, 2485, 55. [Google Scholar] [CrossRef] [PubMed]
- Perea-Gil, I.; Uriarte, J.J.; Prat-Vidal, C.; Gálvez-Montón, C.; Roura, S.; Llucià-Valldeperas, A.; Soler-Botija, C.; Farré, R.; Navajas, D.; Bayes-Genis, A. In Vitro Comparative Study of Two Decellularization Protocols in Search of an Optimal Myocardial Scaffold for Recellularization. Am. J. Transl. Res. 2015, 7, 558. [Google Scholar] [PubMed]
- Ye, X.; Wang, H.; Gong, W.; Li, S.; Li, H.; Wang, Z.; Zhao, Q. Impact of Decellularization on Porcine Myocardium as Scaffold for Tissue Engineered Heart Tissue. J. Mater. Sci. Mater. Med. 2016, 27, 70. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Choi, Y.-H.; Saric, T.; Anić, P.; Morawietz, L.; Stamm, C. Preparation of Cardiac Extracellular Matrix Scaffolds by Decellularization of Human Myocardium. J. Biomed. Mater. Res. Part A 2014, 102, 3263–3272. [Google Scholar] [CrossRef]
- Gómez-Cid, L.; López-Donaire, M.L.; Velasco, D.; Marín, V.; González, M.I.; Salinas, B.; Cussó, L.; García, Á.; Bravo, S.B.; Fernández-Santos, M.E.; et al. Cardiac Extracellular Matrix Hydrogel Enriched with Polyethylene Glycol Presents Improved Gelation Time and Increased On-Target Site Retention of Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9226. [Google Scholar] [CrossRef] [PubMed]
- Jeffords, M.E.; Wu, J.; Shah, M.; Hong, Y.; Zhang, G. Tailoring Material Properties of Cardiac Matrix Hydrogels to Induce Endothelial Differentiation of Human Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 11053–11061. [Google Scholar] [CrossRef]
- Roosens, A.; Somers, P.; De Somer, F.; Carriel, V.; Van Nooten, G.; Cornelissen, R. Impact of Detergent-Based Decellularization Methods on Porcine Tissues for Heart Valve Engineering. Ann. Biomed. Eng. 2016, 44, 2827–2839. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of Physical Methods in Decellularization of Animal Tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Liguori, T.T.A.; Liguori, G.R.; van Dongen, J.A.; Moreira, L.F.P.; Harmsen, M.C. Bioactive Decellularized Cardiac Extracellular Matrix-Based Hydrogel as a Sustained-Release Platform for Human Adipose Tissue-Derived Stromal Cell-Secreted Factors. Biomed. Mater. 2021, 16, 025022. [Google Scholar] [CrossRef]
- Rajabi-Zeleti, S.; Jalili-Firoozinezhad, S.; Azarnia, M.; Khayyatan, F.; Vahdat, S.; Nikeghbalian, S.; Khademhosseini, A.; Baharvand, H.; Aghdami, N. The Behavior of Cardiac Progenitor Cells on Macroporous Pericardium-Derived Scaffolds. Biomaterials 2014, 35, 970–982. [Google Scholar] [CrossRef]
- Mesquita, F.C.P.; Morrissey, J.; Lee, P.F.; Monnerat, G.; Xi, Y.; Andersson, H.; Nogueira, F.C.S.; Domont, G.B.; Sampaio, L.C.; Hochman-Mendez, C.; et al. Cues from Human Atrial Extracellular Matrix Enrich the Atrial Differentiation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Biomater. Sci. 2021, 9, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
- Sakina, R.; Llucià-Valldeperas, A.; Henriques Lourenço, A.; Harichandan, A.; Gelsomino, S.; Wieringa, P.; Mota, C.; Moroni, L. Decellularization of Porcine Heart Tissue to Obtain Extracellular Matrix Based Hydrogels. Methods Cell Biol. 2020, 157, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Mashayekhan, S.; Baheiraei, N.; Pourjavadi, A. Biohybrid Oxidized Alginate/Myocardial Extracellular Matrix Injectable Hydrogels with Improved Electromechanical Properties for Cardiac Tissue Engineering. Int. J. Biol. Macromol. 2021, 180, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D.; Hancko, A.; Shakya, P.; Hill, R.; Saviola, A.J.; Hansen, K.C.; Davis, M.E.; Christman, K.L. Characterization of Decellularized Left and Right Ventricular Myocardial Matrix Hydrogels and Their Effects on Cardiac Progenitor Cells. J. Mol. Cell. Cardiol. 2022, 171, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical Imaging Predicts Mechanical Properties During Decellularization of Cardiac Tissue. Tissue Eng. Part C Methods 2013, 19, 802. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Al-Hejailan, R.; Weigel, T.; Schürlein, S.; Berger, C.; Al-Mohanna, F.; Hansmann, J. Decellularization of Full Heart—Optimizing the Classical Sodium-Dodecyl-Sulfate-Based Decellularization Protocol. Bioengineering 2022, 9, 147. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Bujak, M.; Zymek, P.; Ren, G.; Entman, M.L.; Frangogiannis, N.G. Extracellular Matrix Remodeling in Canine and Mouse Myocardial Infarcts. Cell Tissue Res. 2006, 324, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Meșină, M. Optimization Techniques of Single-Detergent Based Protocols for Heart Tissue Decellularization. Curr. Health Sci. J. 2023, 49, 156–162. [Google Scholar] [CrossRef]
- Theodoro, T.R.; Matos, L.L.; Cavalheiro, R.P.; Justo, G.Z.; Nader, H.B.; Pinhal, M.A.S. Crosstalk between tumor cells and lymphocytes modulates heparanase expression. J. Transl. Med. 2019, 17, 103. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; de Rodrigues, A.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- de Moraes, R.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Garcia, C.F.; Galdeano, E.A.; Maia, F.L.M.; Machado, E.G.; Munhoz, M.d.A.e.S.; Buchaim, D.V.; Fernandes, V.A.R.; et al. Viability of Collagen Matrix Grafts Associated with Nanohydroxyapatite and Elastin in Bone Repair in the Experimental Condition of Ovariectomy. Int. J. Mol. Sci. 2023, 24, 15727. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.R.; Maia, F.L.M.; Iatecola, A.; Massimino, L.C.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Da Rocha, D.N.; Mariano, E.D.; Hirata, M.C.; Ferreira, J.R.M.; et al. In Vivo Evaluation of Collagen and Chitosan Scaffold, Associated or Not with Stem Cells, in Bone Repair. J. Funct. Biomater. 2023, 14, 357. [Google Scholar] [CrossRef]

| Animal | Race | Age |
|---|---|---|
| 1 | Dachshund | 1 year |
| 2 | NDB | ≈14 years old |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, I.G.R.; Miglino, M.A.; de Souza, S.S.; Buchaim, D.V.; Buchaim, R.L. Evaluation of Different Decellularization Protocols for Obtaining and Characterizing Canine Cardiac Extracellular Matrix. Biomedicines 2024, 12, 1190. https://doi.org/10.3390/biomedicines12061190
da Silva IGR, Miglino MA, de Souza SS, Buchaim DV, Buchaim RL. Evaluation of Different Decellularization Protocols for Obtaining and Characterizing Canine Cardiac Extracellular Matrix. Biomedicines. 2024; 12(6):1190. https://doi.org/10.3390/biomedicines12061190
Chicago/Turabian Styleda Silva, Izabela Gabriela Rodrigues, Maria Angelica Miglino, Samara Silva de Souza, Daniela Vieira Buchaim, and Rogerio Leone Buchaim. 2024. "Evaluation of Different Decellularization Protocols for Obtaining and Characterizing Canine Cardiac Extracellular Matrix" Biomedicines 12, no. 6: 1190. https://doi.org/10.3390/biomedicines12061190








