Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure–Activity Relationships
Abstract
:1. Introduction
2. Naturally Occurring Coumarins with Anti-Breast Cancer Efficacy
2.1. Simple Coumarins with Anti-Breast Cancer Potential
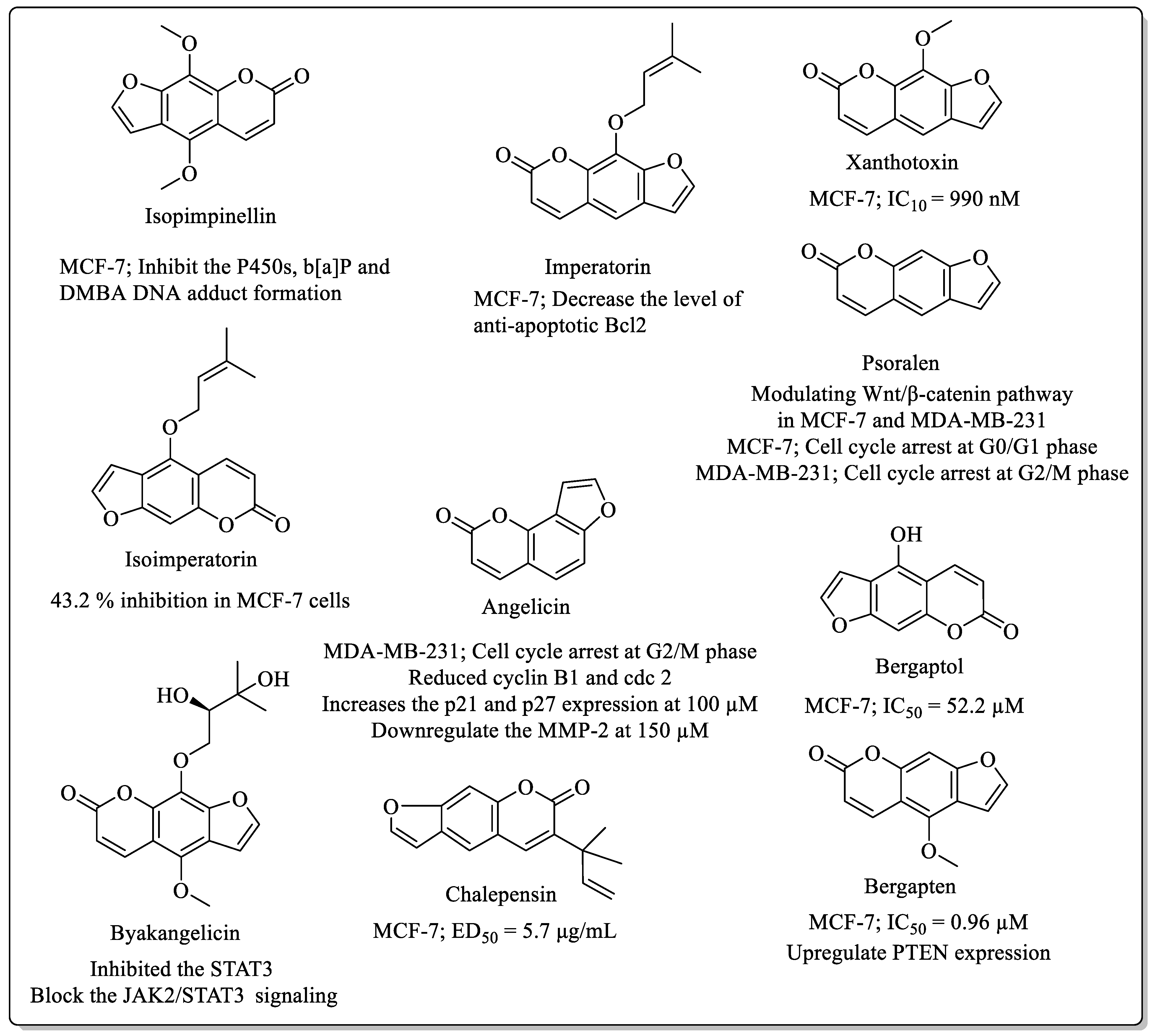
2.2. Furanocoumarins Coumarins with Anti-Breast Cancer Potential
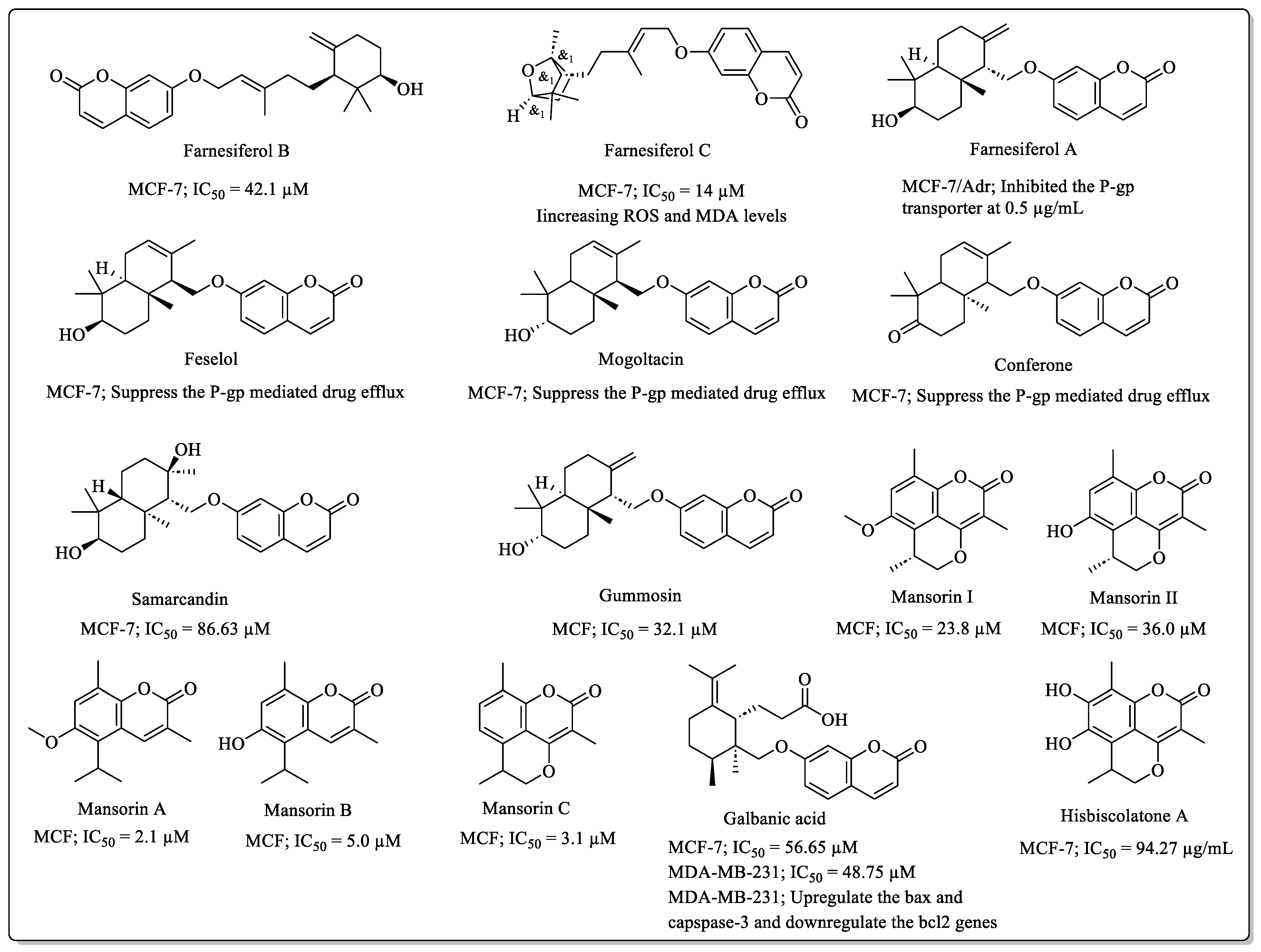
2.3. Sesquiterpene Coumarins with Anti-Breast Cancer Potential
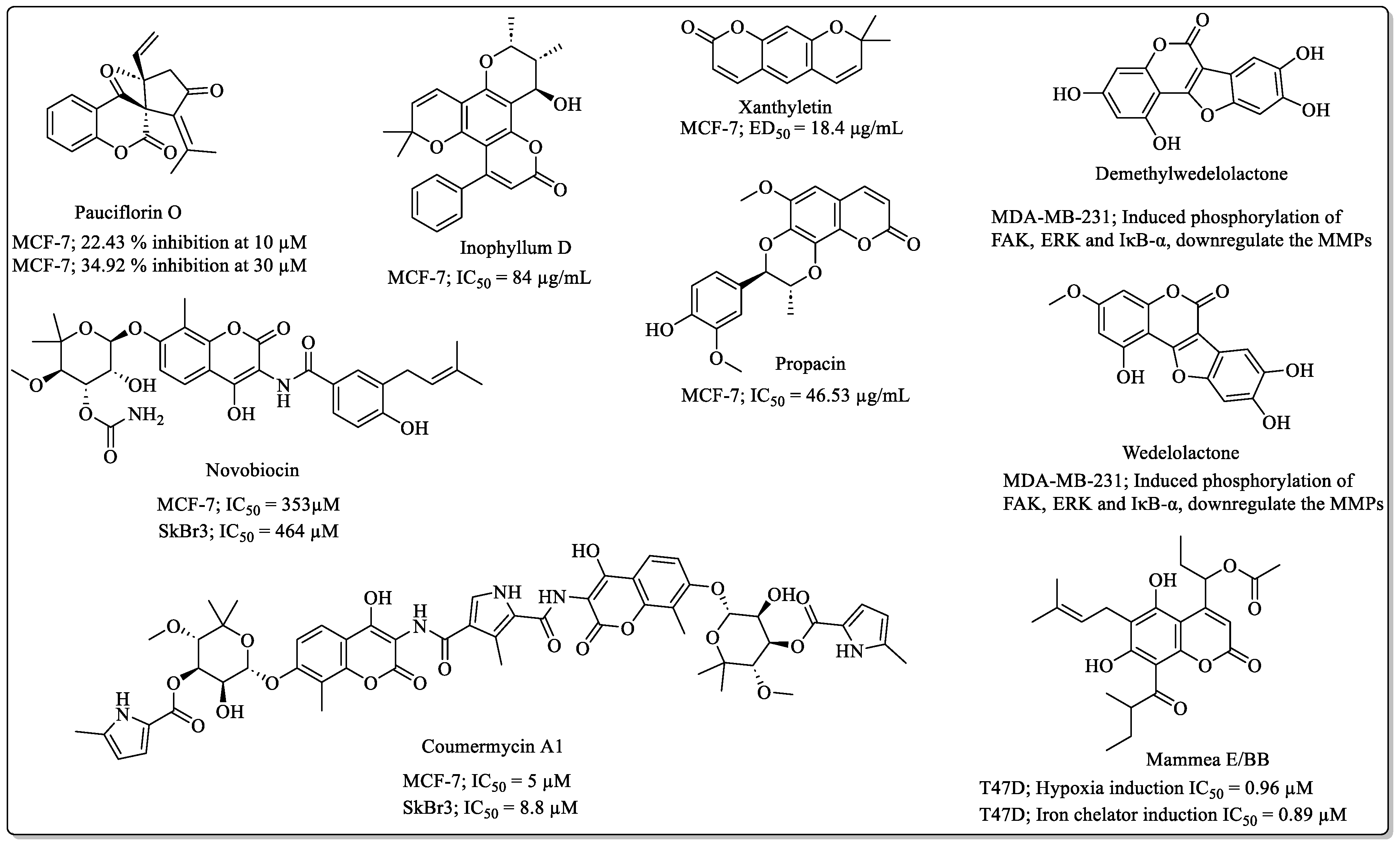
2.4. Miscellaneous Naturally Occurring Coumarins with Anti-Breast Cancer Potential
3. Synthetic Coumarin-Inspired Derivatives with Anti-Breast Cancer Efficacy
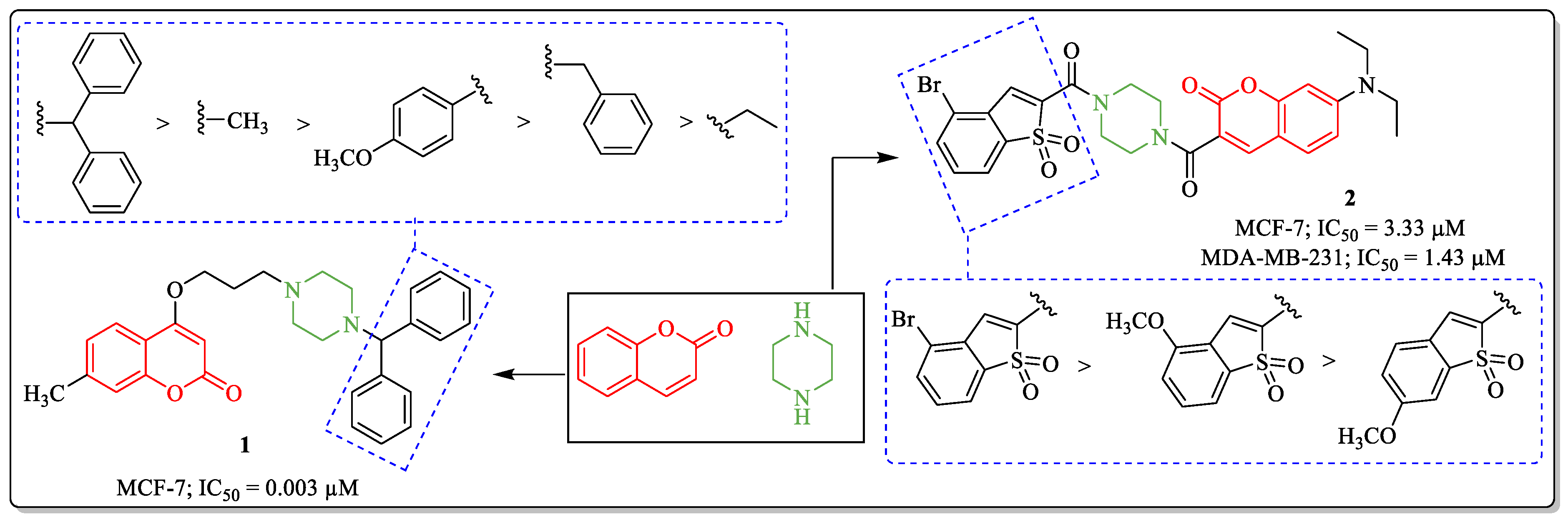
3.1. Coumarin and Piperazine Conjugates as Anti-Breast Cancer Agents
3.2. Coumarin and Piperidine Conjugates as Anti-Breast Cancer Agents
3.3. Bis-Coumarin Derivatives as Anti-Breast Cancer Agents
3.4. Indole–Coumarin Derivatives as Anti-Breast Cancer Agents
3.5. Coumarin and Isatin Conjugates as Anti-Breast Cancer Agents
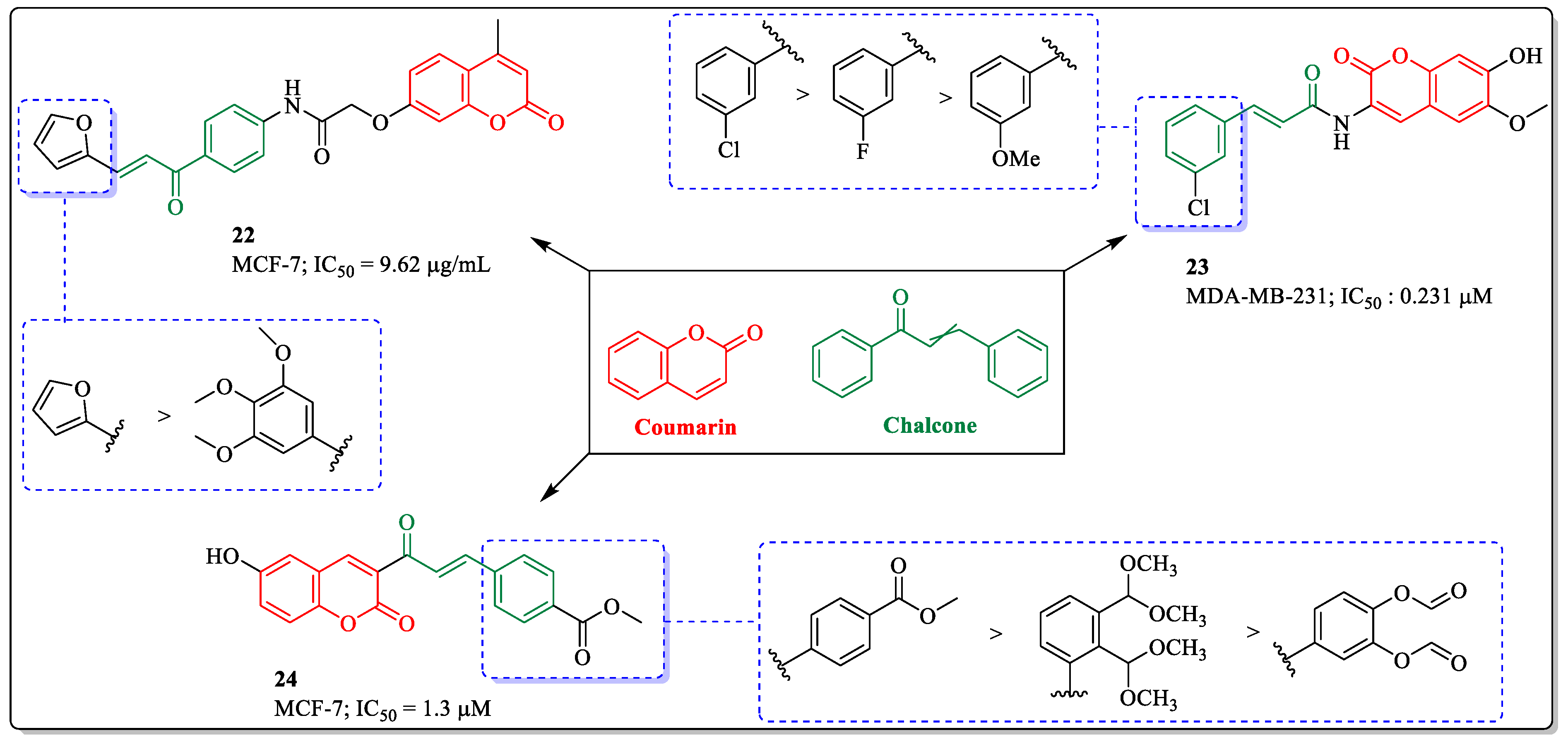
3.6. Coumarin and Chalcone Conjugates as Anti-Breast Cancer Agents
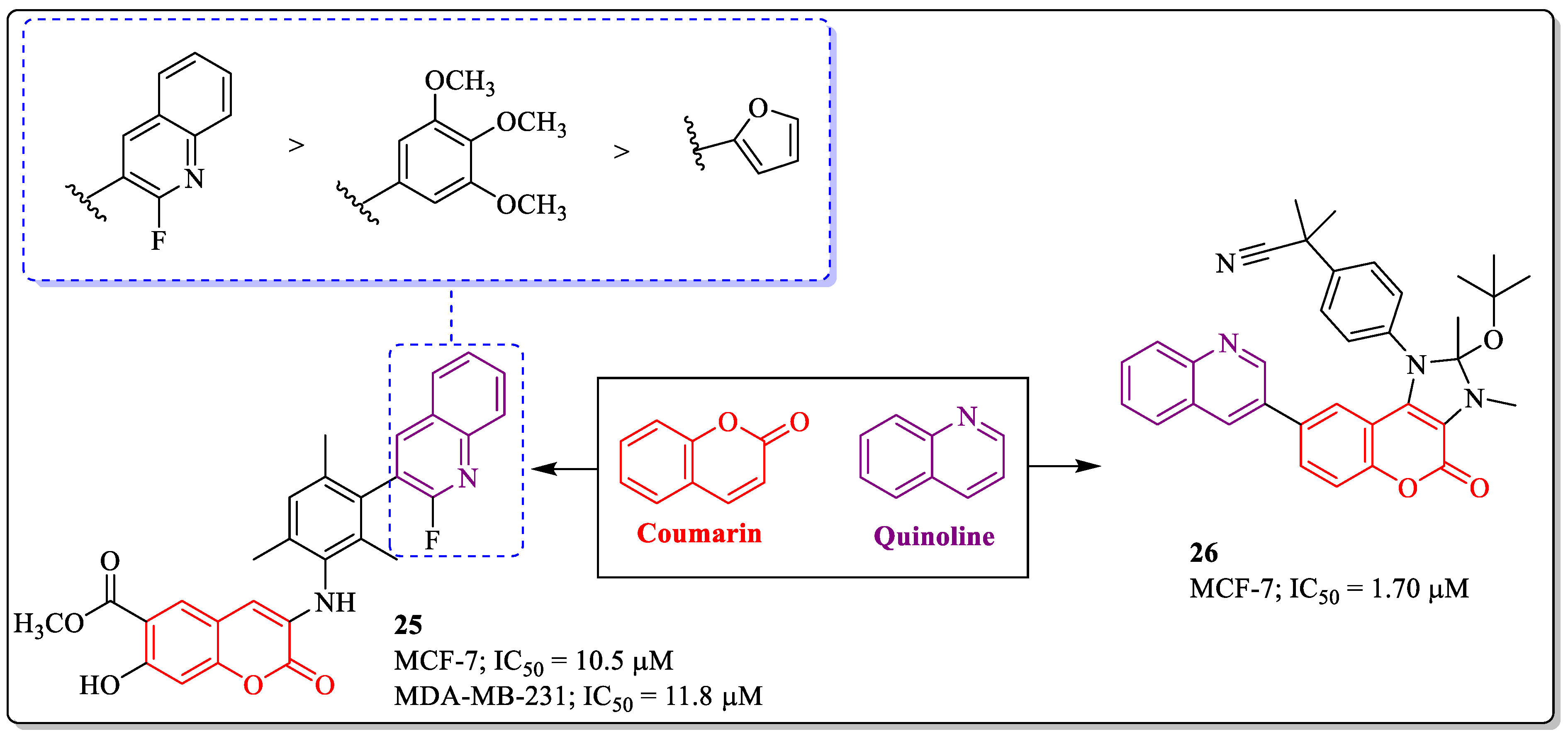
3.7. Coumarin and Quinoline Hybrids as Anti-Breast Cancer Agents
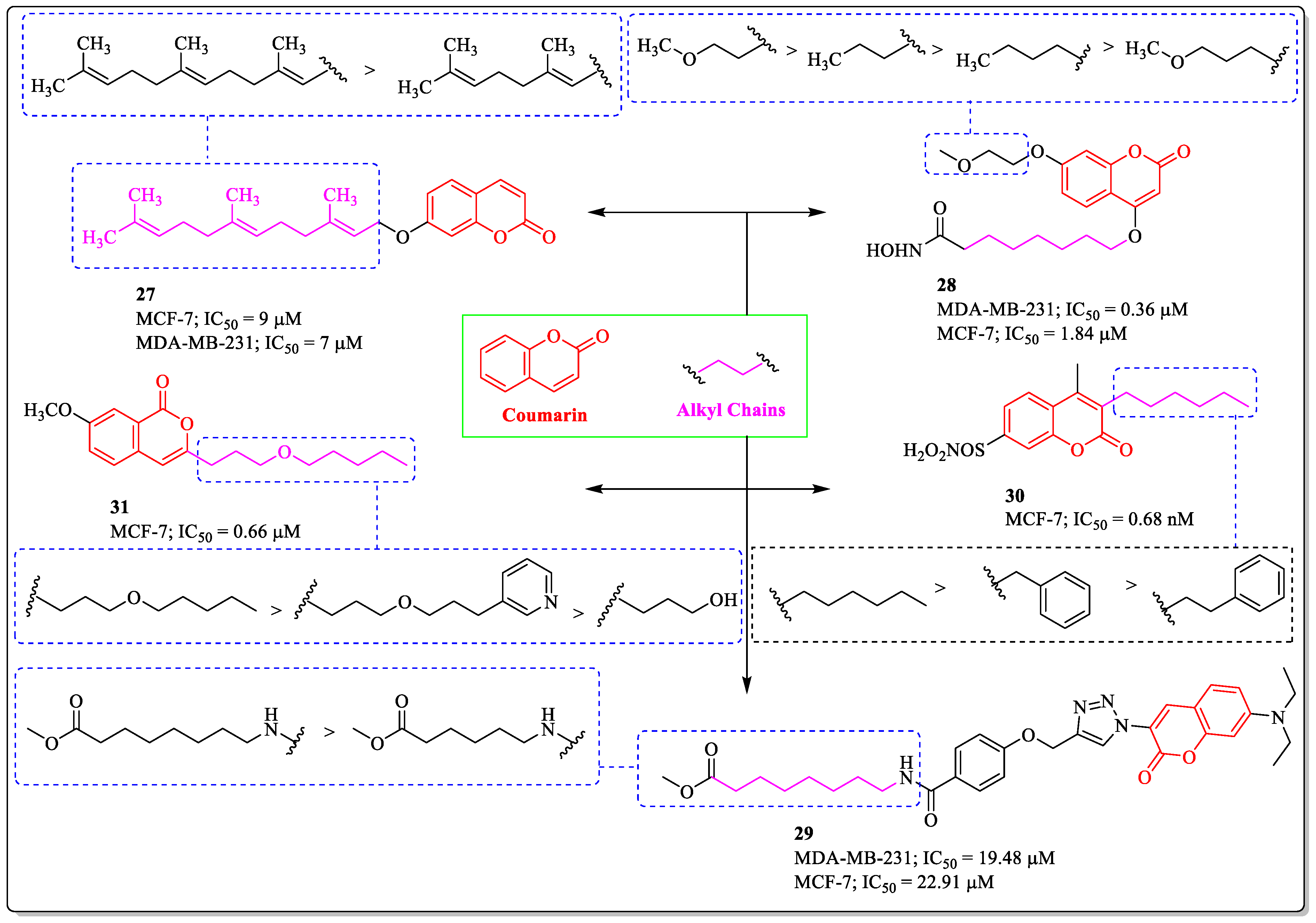
3.8. Coumarin-Appended Alkyl Chains as Anti-Breast Cancer Agents
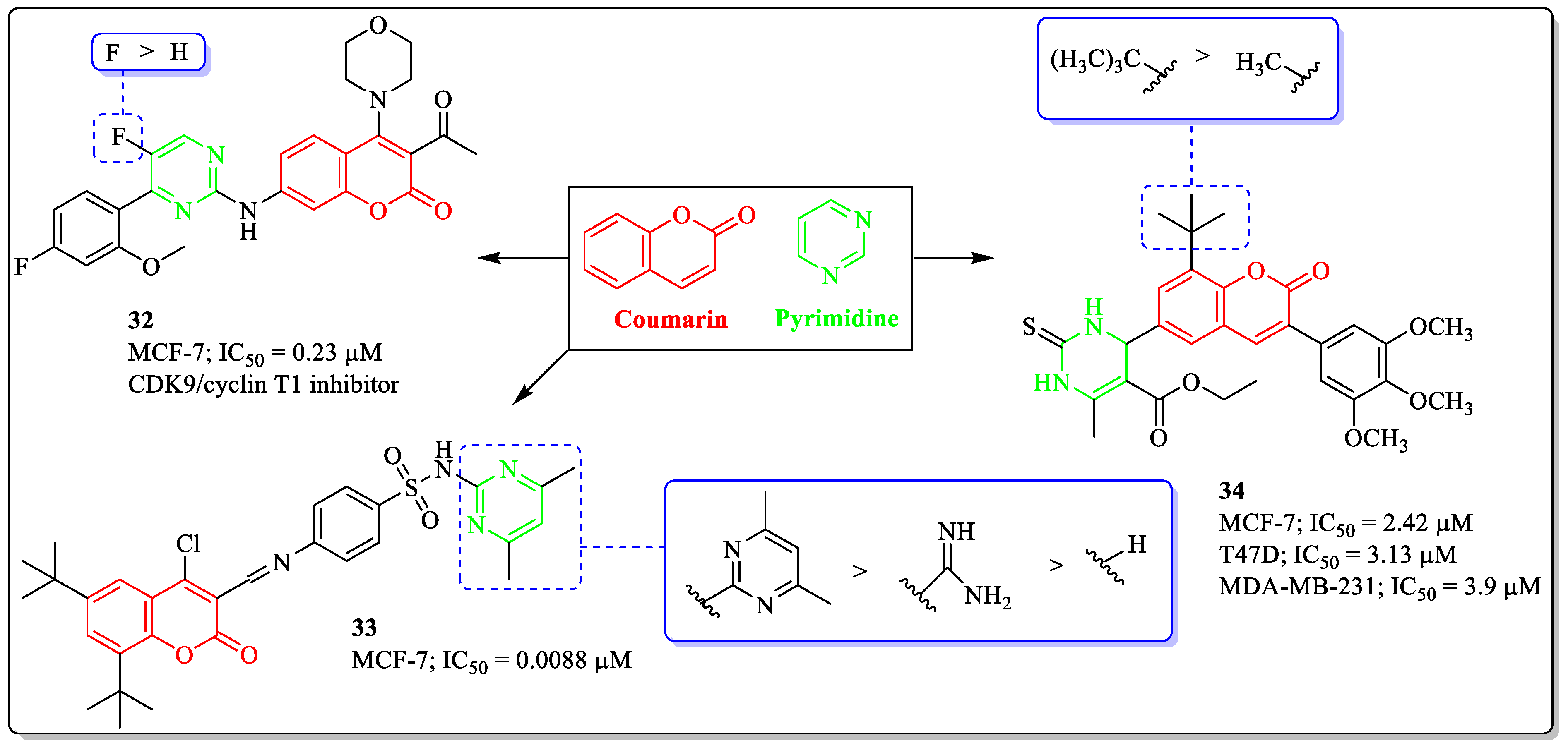
3.9. Coumarin and Pyrimidine Hybrids as Anti-Breast Cancer Agents
3.10. Coumarin and Pyridine Hybrids as Anti-Breast Cancer Agents
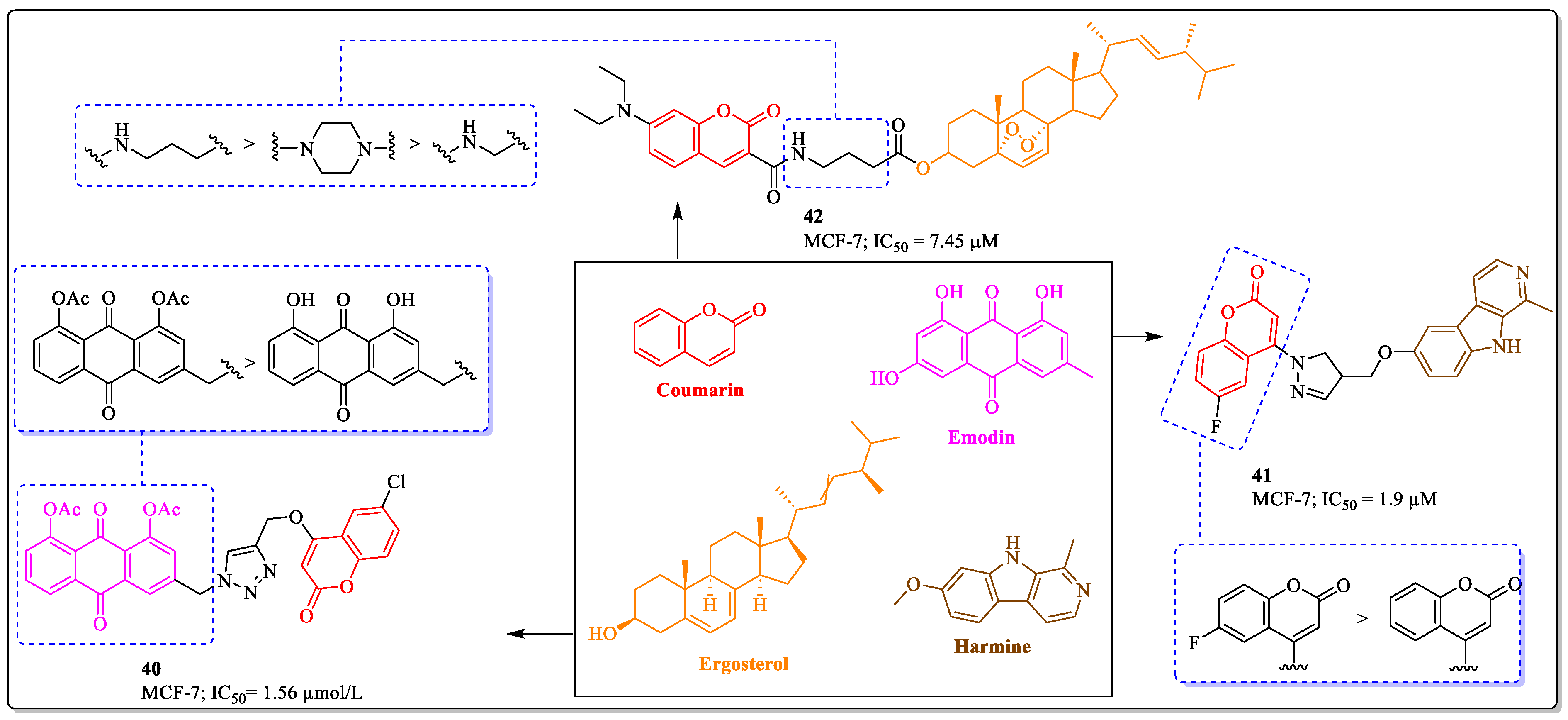
3.11. Coumarins Clubbed with Aloe Emodin, Harmine, and Ergosterol as Anti-Breast Cancer Agents
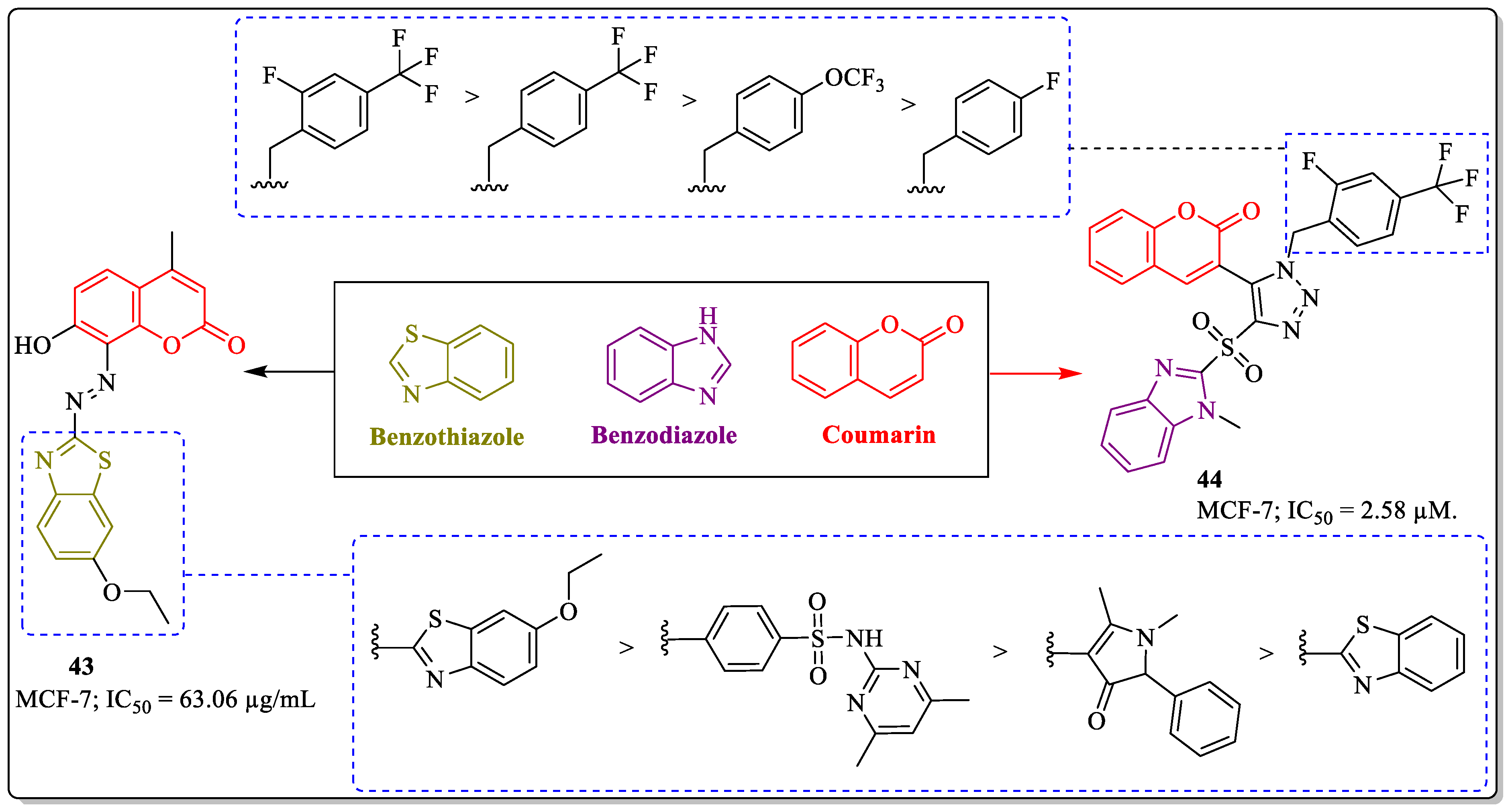
3.12. Coumarin-Clubbed Benzothiazole/Benzodiazole Derivatives as Anti-Breast Cancer Agents
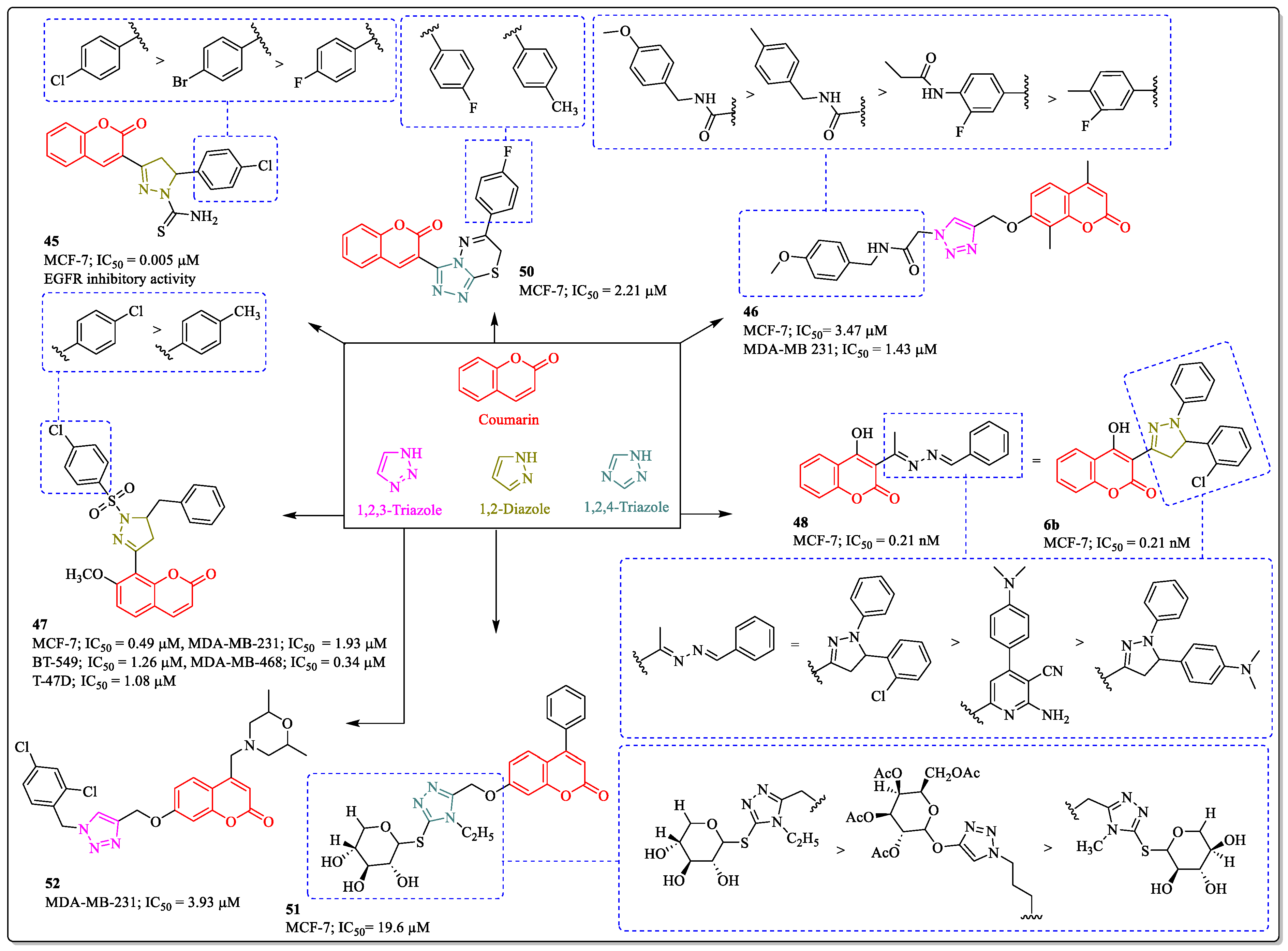
3.13. Coumarin-Clubbed Diazole and Triazole Hybrids as Anti-Breast Cancer Agents
3.14. Coumarin-Clubbed Thiazole Derivatives as Anti-Breast Cancer Agents
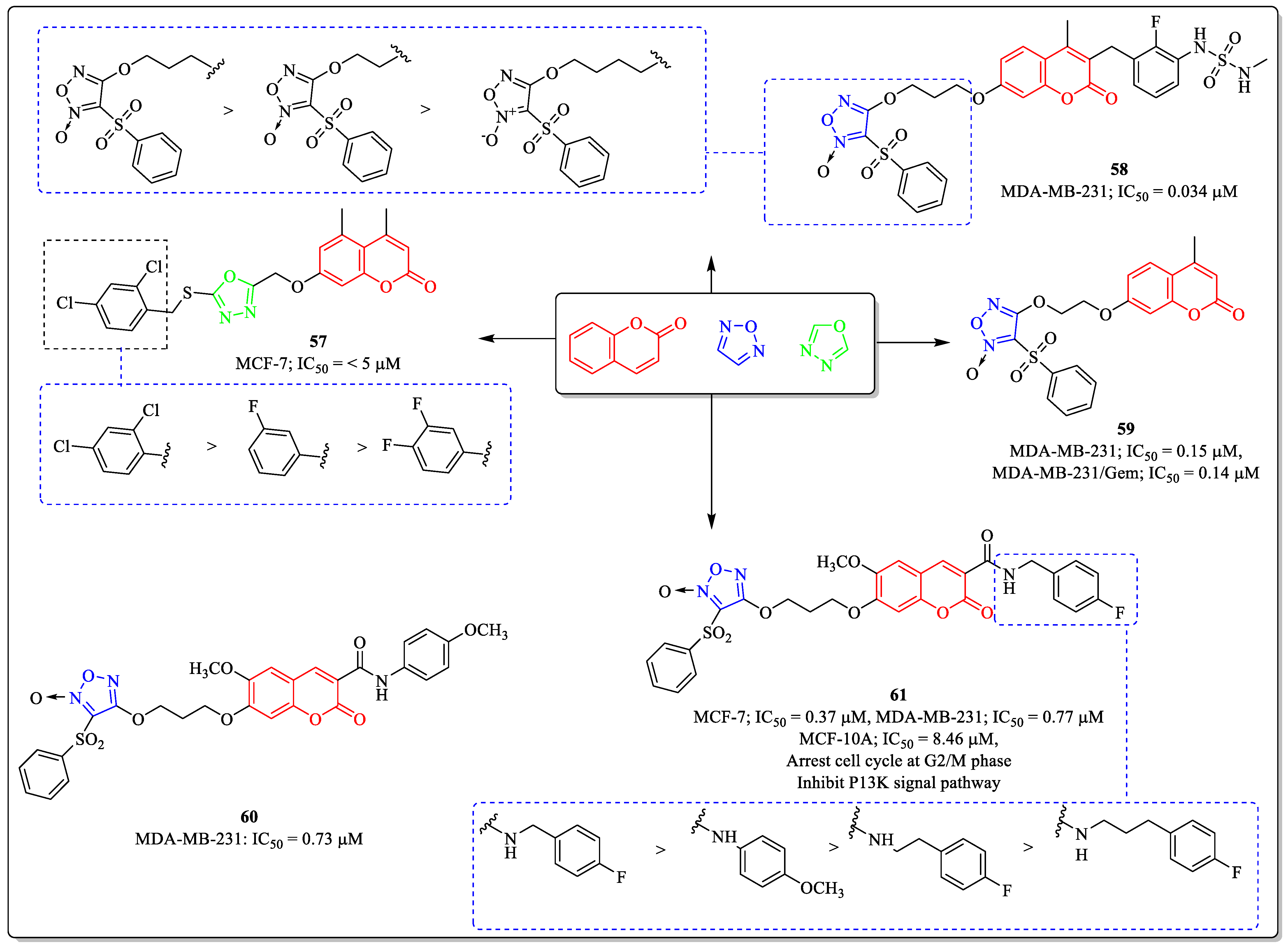
3.15. Coumarin-Clubbed Oxadiazole Derivatives as Anti-Breast Cancer Agents
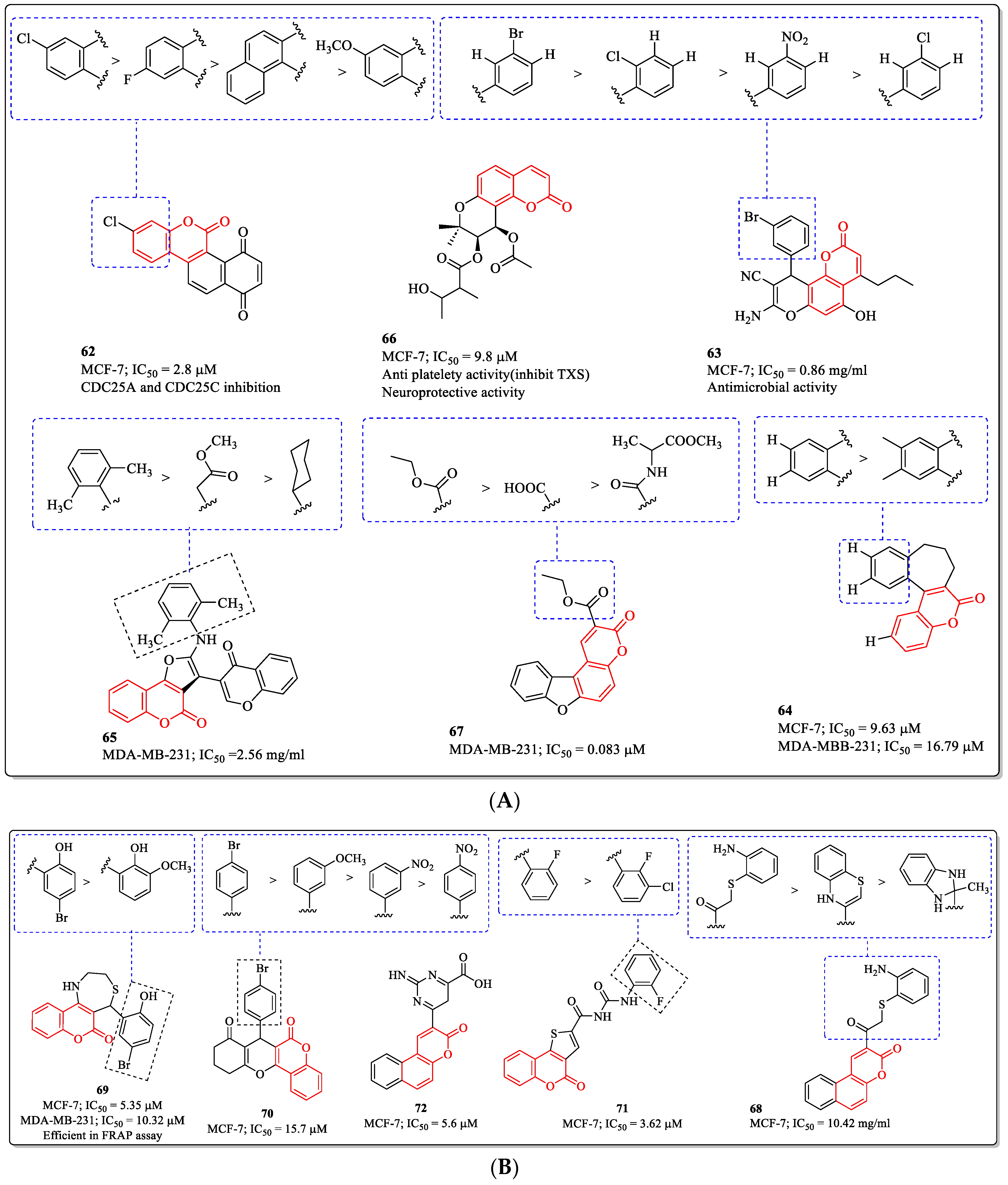
3.16. Fused Coumarin Derivatives as Anti-Breast Cancer Agents
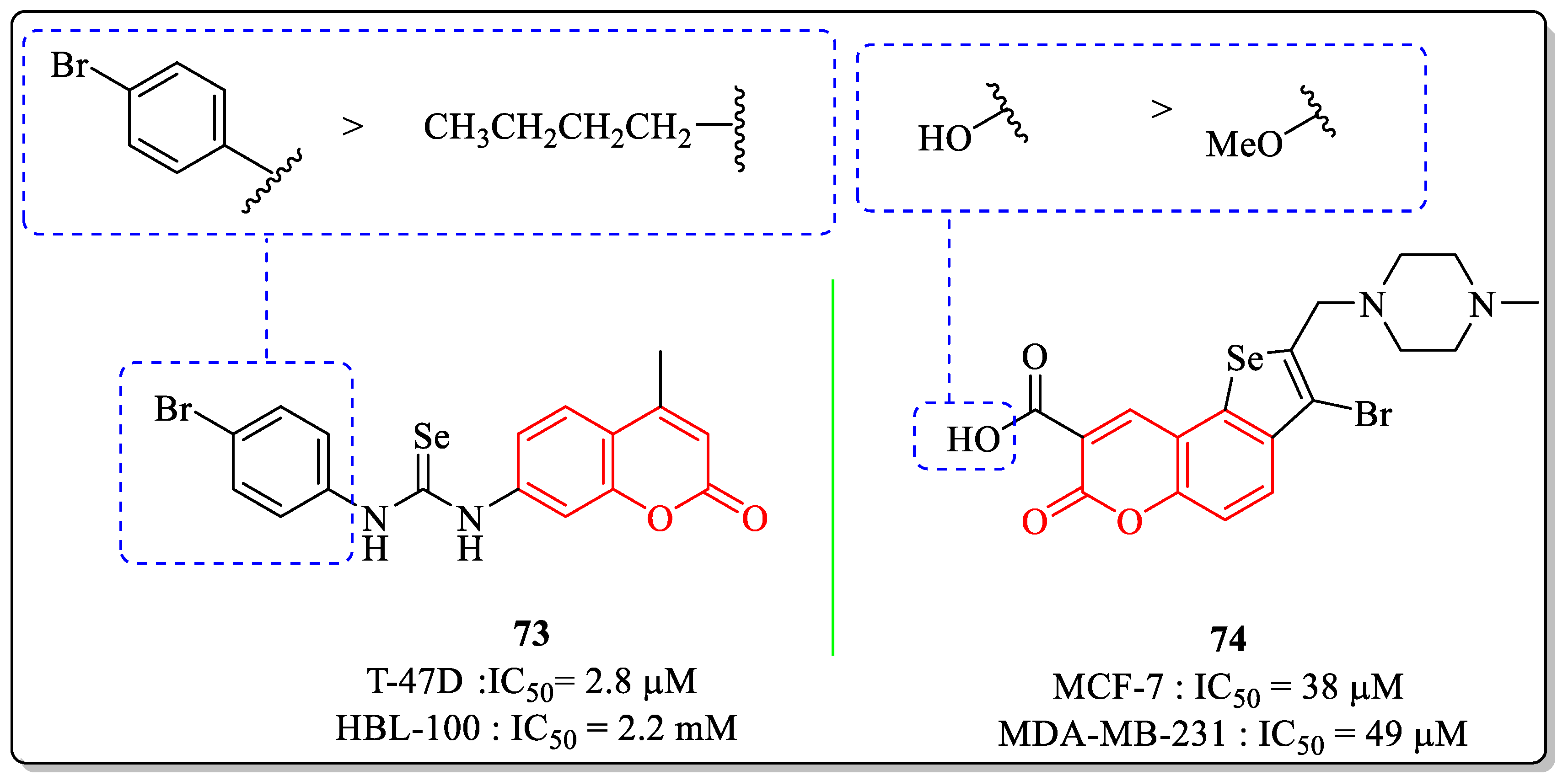
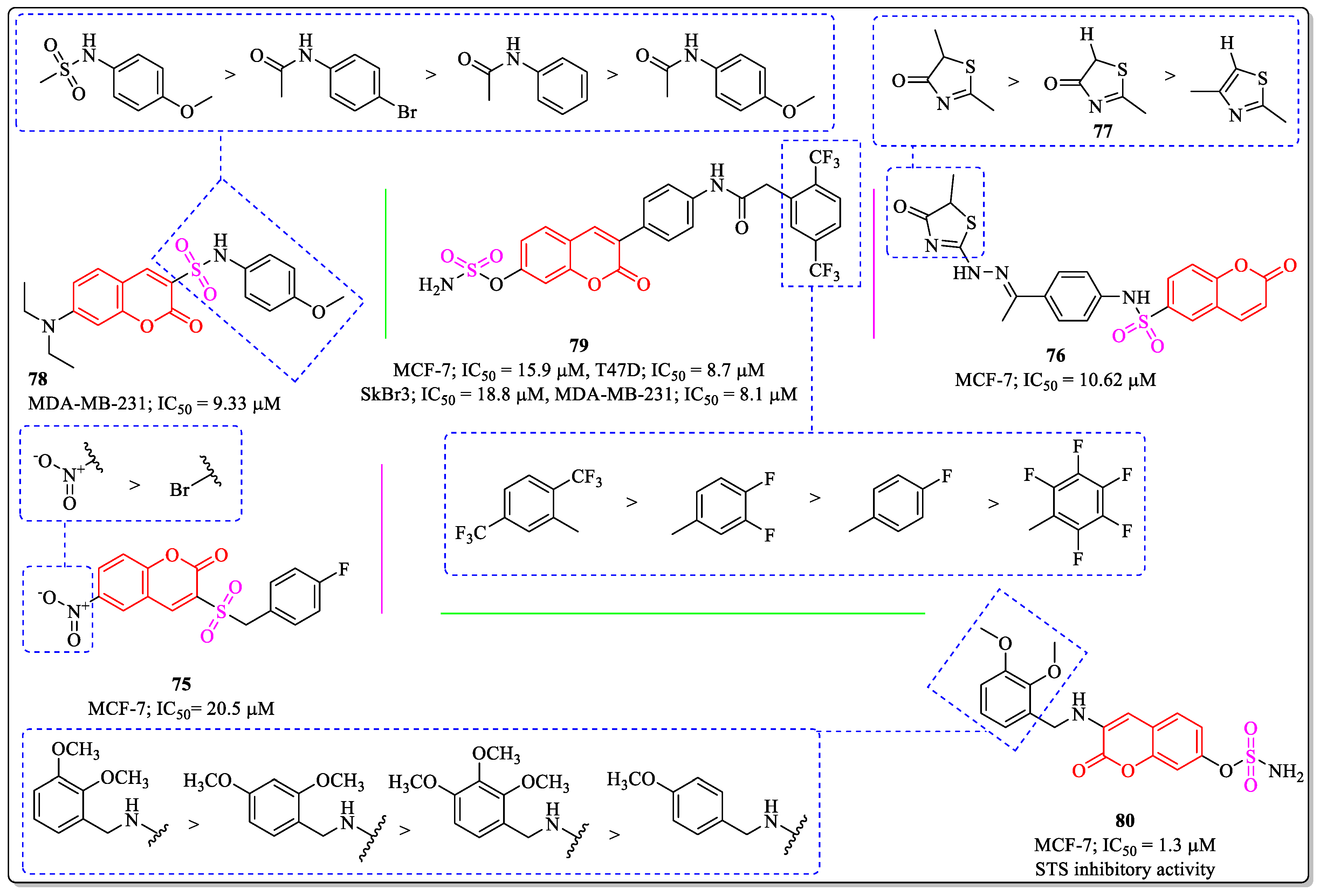
3.17. Coumarin-Appended Selenium Derivatives as Anti-Breast Cancer Agents
3.18. Coumarin-Appended Sulphoxide Derivatives as Anti-Breast Cancer Agents
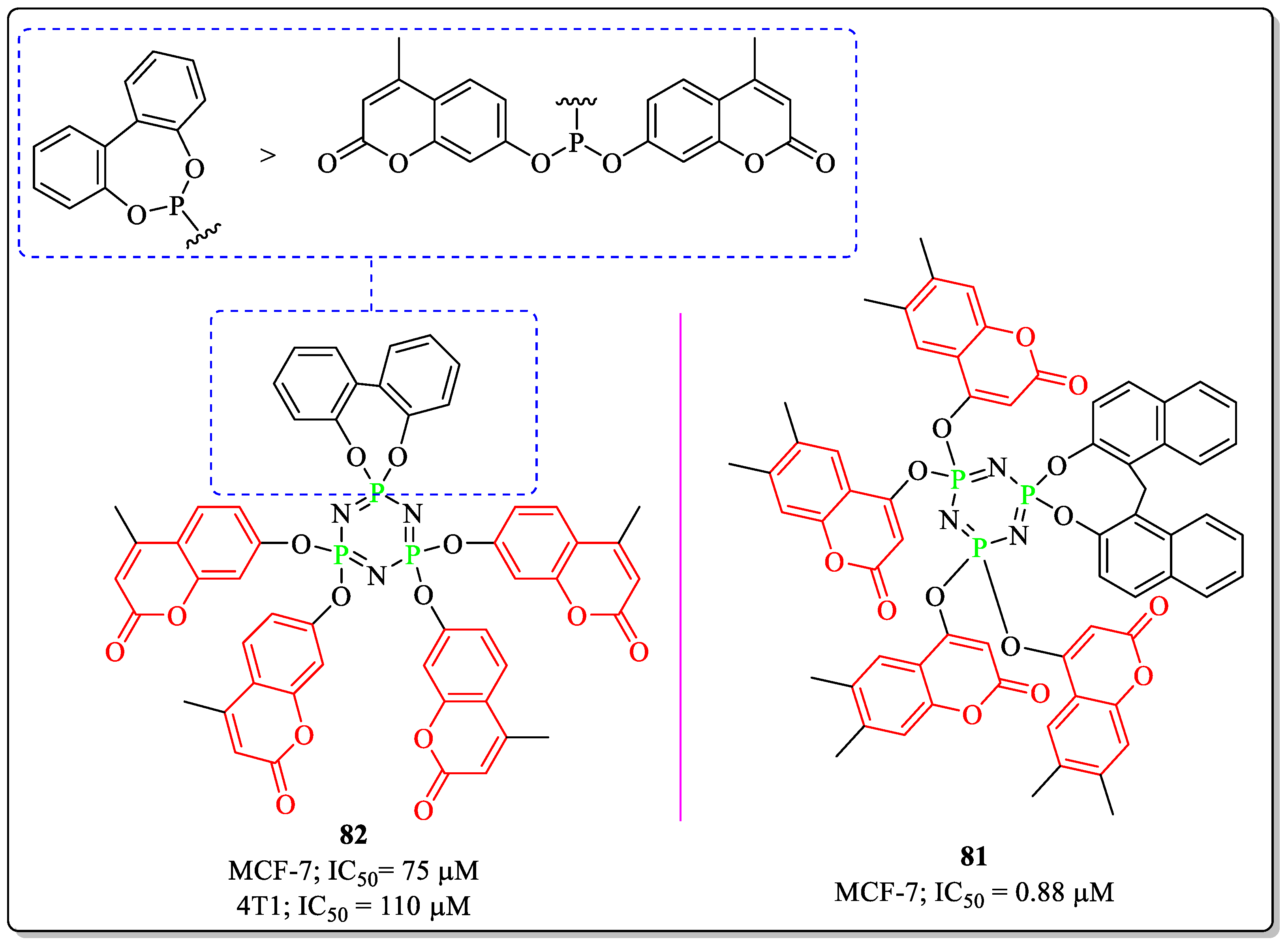
3.19. Coumarin-Appended Phosphorus Derivatives as Anti-Breast Cancer Agents
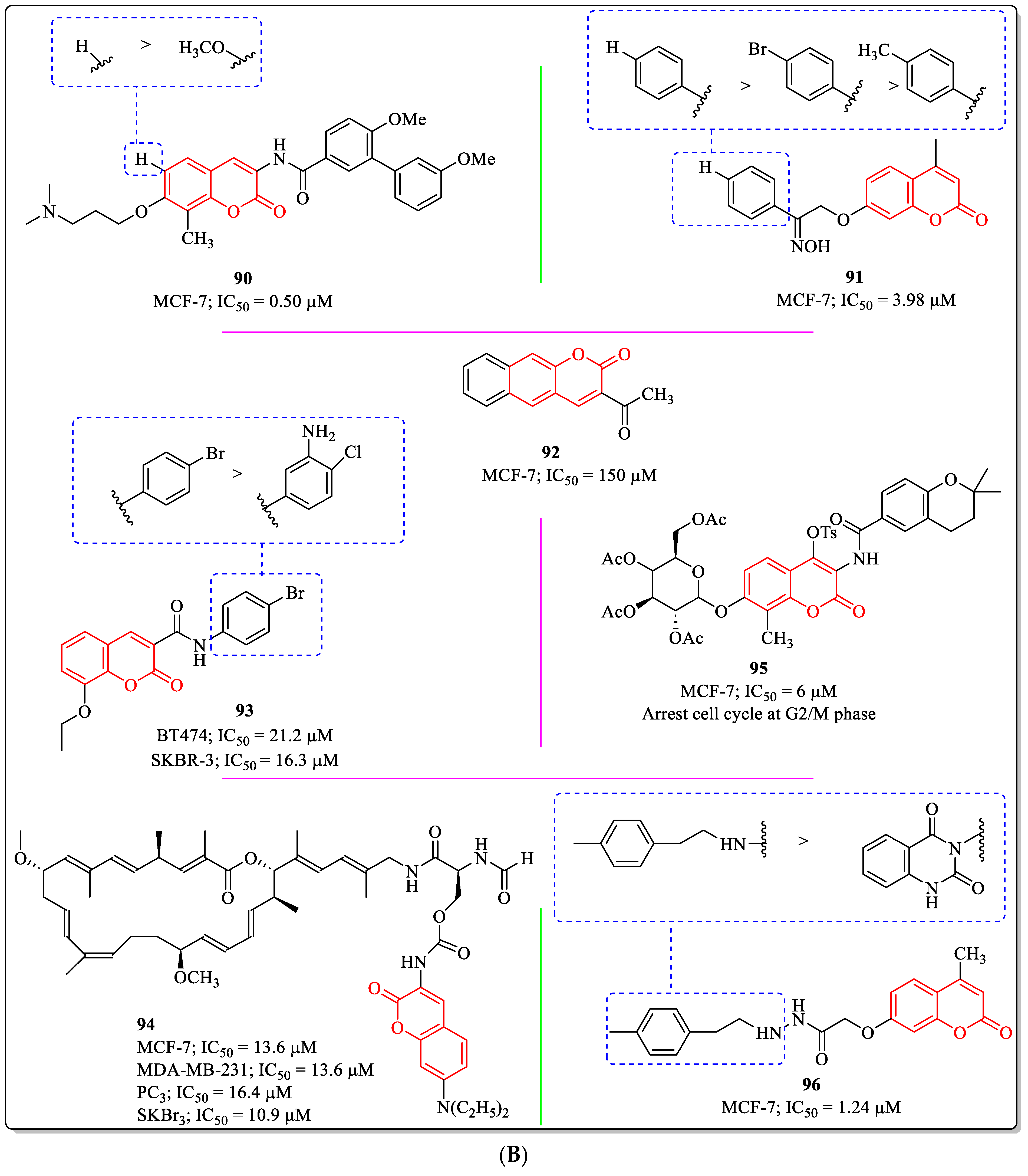
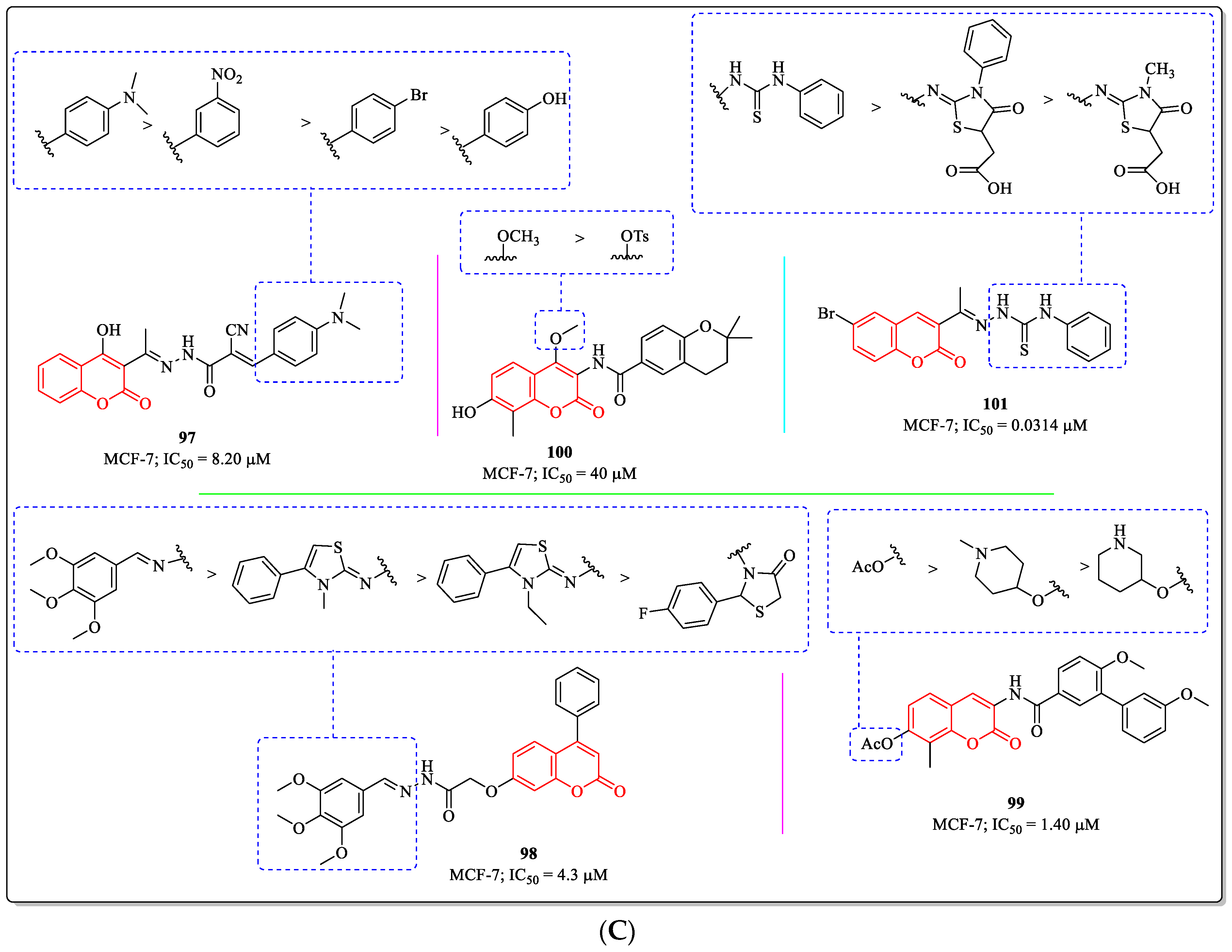
3.20. Miscellaneous Coumarin Derivatives as Anti-Breast Cancer Agents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rippe, J.M.; Angelopoulos, T.J. Lifestyle strategies for risk factor reduction, prevention and treatment of cardiovascular disease. In Lifestyle Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 19–36. [Google Scholar]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global increase in breast cancer incidence: Risk factors and preventive measures. BioMed Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef] [PubMed]
- Chodosh, L.A. Breast cancer: Current state and future promise. Breast Cancer Res. 2011, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, K.; Sharma, A.; Sharma, S.; Batra, K.; Joshi, K.; Singh, B.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Mechanistic insight and structure activity relationship of isatin-based derivatives in development of anti-breast cancer agents. Mol. Cell. Biochem. 2023, 479, 1165–1198. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, H.; Arora, S.; Bedi, P.M.S. Design, synthesis, and biological evaluation of novel morpholinated isatin–quinoline hybrids as potent anti-breast cancer agents. Arch. Pharm. 2022, 355, 2100368. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1-and BRCA2-Associated Hereditary Breast and Ovarian Cancer; University of Washington: Seattle, MA, USA, 2022. [Google Scholar]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp. Hematol. Oncol. 2023, 12, 3. [Google Scholar] [CrossRef]
- Harris, E.E. Precision medicine for breast cancer: The paths to truly individualized diagnosis and treatment. Int. J. Breast Cancer 2018, 2018, 4809183. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Sahoo, C.R.; Padhy, R.N. Role of hormone receptors and HER2 as prospective molecular markers for breast cancer: An update. Genes Dis. 2022, 9, 648–658. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Wang, X.; Hao, J. Small-molecule inhibitors of breast cancer-related targets: Potential therapeutic agents for breast cancer. Eur. J. Med. Chem. 2021, 210, 112954. [Google Scholar] [CrossRef] [PubMed]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther. 2020, 12, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Shrestha, R.M.; Yadav, P.N. Anticancer mechanism of coumarin-based derivatives. Eur. J. Med. Chem. 2024, 267, 116179. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, J.V.; Rana, A.; Bhagat, K.; Gulati, H.K.; Kumar, R.; Salwan, R.; Bhagat, K.; Kaur, G.; Singh, N. Monocarbonyl curcumin-based molecular hybrids as potent antibacterial agents. ACS Omega 2019, 4, 11673–11684. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, S.; Arora, S.; Attri, S.; Kaur, P.; Gulati, H.K.; Bhagat, K.; Kumar, N.; Singh, H.; Singh, J.V. New coumarin-benzotriazole based hybrid molecules as inhibitors of acetylcholinesterase and amyloid aggregation. Bioorg. Med. Chem. Lett. 2020, 30, 127477. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, K.; Singh, J.V.; Sharma, A.; Kaur, A.; Kumar, N.; Gulati, H.K.; Singh, A.; Singh, H.; Bedi, P.M.S. Novel series of triazole containing coumarin and isatin based hybrid molecules as acetylcholinesterase inhibitors. J. Mol. Struct. 2021, 1245, 131085. [Google Scholar] [CrossRef]
- Bhagat, K.; Bhagat, J.; Gupta, M.K.; Singh, J.V.; Gulati, H.K.; Singh, A.; Kaur, K.; Kaur, G.; Sharma, S.; Rana, A. Design, synthesis, antimicrobial evaluation, and molecular modeling studies of novel indolinedione–coumarin molecular hybrids. ACS Omega 2019, 4, 8720–8730. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, K.; Kaur, K.; Sharma, A.; Mohana, P.; Prajapati, J.; Kaur, U.; Goswami, D.; Arora, S.; Chadha, R. Discovery of triazole tethered thymol/carvacrol-coumarin hybrids as new class of α-glucosidase inhibitors with potent in vivo antihyperglycemic activities. Eur. J. Med. Chem. 2024, 263, 115948. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Thornes, R.; Daly, L.; Lynch, G.; Breslin, B.; Browne, H.; Browne, H.; Corrigan, T.; Daly, P.; Edwards, G.; Gaffney, E. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J. Cancer Res. Clin. Oncol. 1994, 120, S32–S34. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Shaheen, H.M.; Nyemb, J.N.; Segueni, N.; George, J.; Patil, V.R.; Batiha, G.E.-S. Anticancer Properties and Clinical Trials of Coumarins: A Review. Free Radic. Antioxid. 2022, 12, 41–48. [Google Scholar] [CrossRef]
- Song, F.; Huo, X.; Guo, Z. Anti-breast cancer potential of natural and synthetic coumarin derivatives. Curr. Top. Med. Chem. 2021, 21, 1692–1709. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Shi, X.; Sun, L. Isolation and purification of esculetin from the seeds of Euphorbia lathyris L. using high-speed counter-current chromatography. Se Pu Chin. J. Chromatogr. 2010, 28, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Su, Z.; Yili, A.; Xiao, Z.; Hang, B.; Aisa, H. Isolation of esculetin from Cichorium glandulosum by high-speed countercurrent chromatography. Chem. Nat. Compd. 2007, 43, 109. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yin, C.; Zhang, Y.; Guo, G.; Zhao, C.; Wang, O.; Xiang, Y.; Zhang, X.; Liang, G. Osthole inhibits triple negative breast cancer cells by suppressing STAT3. J. Exp. Clin. Cancer Res. 2018, 37, 322. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Kim, C.W.; Kim, H.S. Scoparone attenuates PD-L1 expression in human breast cancer cells by MKP-3 upregulation. Anim. Cells Syst. 2024, 28, 55–65. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Li, J.; Zhao, X.; Cui, Y.; Eerdun, C. Scoparone inhibits breast cancer cell viability through the NF-κB signaling pathway. Exp. Ther. Med. 2023, 26, 328. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Davari, A.-S.; Iranshahi, M.; Sabouri-Rad, S.; Najaran, Z.T. Comparative analysis of the cytotoxic effect of 7-prenyloxycoumarin compounds and herniarin on MCF-7 cell line. Avicenna J. Phytomed. 2015, 5, 520. [Google Scholar]
- Rashmi, R.; Prakash, N.; Swamy, H.N.; Swamy, M. Cytotoxicity Screening of Esculin with or without Piperine. J. Entomol. Zool. Stud. 2020, 8, 230–233. [Google Scholar]
- Rashmi, R.; Prakash, N.; Narayana Swamy, H.; Narayana Swamy, M.; Rathnamma, D.; Suguna Rao, A.; Sahadev, A.; Santhosh, C.; Sunilchandra, U.; Naveen Kumar, S. Evaluation of anticancer efficacy of umbelliferone with or without piperine. J. Entomol. Zool. Stud. 2020, 8, 225–229. [Google Scholar]
- Wang, R.; Zhou, W.; Wang, J.; Liu, Y.; Chen, Y.; Jiang, S.; Luo, X.; Stupack, D.G.; Luo, N. Role of hyaluronan and glucose on 4-methylumbelliferone-inhibited cell proliferation in breast carcinoma cells. Anticancer Res. 2015, 35, 4799–4805. [Google Scholar] [PubMed]
- Charmforoshan, E.; Karimi, E.; Oskoueian, E.; Es-Haghi, A.; Iranshahi, M. Inhibition of human breast cancer cells (MCF-7 cell line) growth via cell proliferation, migration, and angiogenesis by auraptene of Ferula szowitsiana root extract. J. Food Meas. Charact. 2019, 13, 2644–2653. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Yan, Y.; Wang, H. Effect of fraxetin on proliferation and apoptosis in breast cancer cells. Oncol. Lett. 2017, 14, 7374–7378. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, M.H.; Guetat, A.; Bouajila, J.; Alzahrani, A.K.; Basha, J. Deverra tortuosa (Desf.) DC from Saudi Arabia as a new source of marmin and furanocoumarins derivatives with α-glucosidase, antibacterial and cytotoxic activities. Heliyon 2021, 7, e06656. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, H.E.; Reed, M.J.; DiGiovanni, J. Naturally occurring coumarins inhibit human cytochromes P450 and block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct formation in MCF-7 cells. Chem. Res. Toxicol. 2003, 16, 415–422. [Google Scholar] [CrossRef]
- Mirzaei, S.A.; Dehkordi, N.G.; Ghamghami, M.; Amiri, A.H.; Abdolahinia, E.D.; Elahian, F. ABC-transporter blockage mediated by xanthotoxin and bergapten is the major pathway for chemosensitization of multidrug-resistant cancer cells. Toxicol. Appl. Pharmacol. 2017, 337, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, C.; Hua, Y.; Cheng, K.; Zhang, Y.; Liu, J.; Han, Y.; Liu, S.; Zhang, G.; Xu, S. Psoralen induced cell cycle arrest by modulating Wnt/β-catenin pathway in breast cancer cells. Sci. Rep. 2018, 8, 14001. [Google Scholar] [CrossRef]
- Hsiao, C.; Chien-Yu, C.; Yu-Hsuan, L.; I-Fen, C. Angelicin inhibits the growth and migration of triple-negative breast cancer cells. Pak. J. Pharm. Sci. 2023, 36, 51–57. [Google Scholar]
- Anaya, A.L.; Macías-Rubalcava, M.; Cruz-Ortega, R.; García-Santana, C.; Sánchez-Monterrubio, P.N.; Hernández-Bautista, B.E.; Mata, R. Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico. Phytochemistry 2005, 66, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.-C.; Qu, X.; Yu, H.-F.; Zhang, H.-M.; Wang, Z.-H.; Zhang, Z.-T. Antitumor and apoptotic effects of bergaptol are mediated via mitochondrial death pathway and cell cycle arrest in human breast carcinoma cells. Bangladesh J. Pharmacol. 2016, 11, 489–494. [Google Scholar] [CrossRef]
- Iranshahy, M.; Farhadi, F.; Paknejad, B.; Zareian, P.; Iranshahi, M.; Karami, M.; Abtahi, S.R. Gummosin, a sesquiterpene coumarin from Ferula assa-foetida is preferentially cytotoxic to human breast and prostate cancer cell lines. Avicenna J. Phytomed. 2019, 9, 446. [Google Scholar] [PubMed]
- Hasanzadeh, D.; Mahdavi, M.; Dehghan, G.; Charoudeh, H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017, 4, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hanafi-Bojd, M.Y.; Iranshahi, M.; Mosaffa, F.; Tehrani, S.O.; Kalalinia, F.; Behravan, J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Medica 2011, 77, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Barthomeuf, C.; Bayet-Robert, M.; Chollet, P.; Davoodi, D.; Piacente, S.; Rezaee, R.; Sahebkar, A. Drimane-type sesquiterpene coumarins from Ferula gummosa fruits enhance doxorubicin uptake in doxorubicin-resistant human breast cancer cell line. J. Tradit. Complement. Med. 2014, 4, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Karimi, E.; Oskoueian, E.; Iranshahi, M.; Neamati, A. Galbanic acid: Induced antiproliferation in estrogen receptor-negative breast cancer cells and enhanced cellular redox state in the human dermal fibroblasts. J. Biochem. Mol. Toxicol. 2019, 33, e22402. [Google Scholar] [CrossRef] [PubMed]
- Tra, N.T.; Ha, N.T.T.; Cham, B.T.; Anh, L.T.T.; Yen, L.T.H.; Giang, B.L.; Anh, D.T.T.; Tuyen, N.V.; Kiem, P.V. A new benzofuran derivative from the stems of Helicteres hirsuta. Nat. Prod. Commun. 2019, 14, 1934578X19858814. [Google Scholar] [CrossRef]
- Krstić, G.; Saidu, M.B.; Barta, A.; Vágvölgyi, M.t.; Ali, H.; Zupkó, I.; Berkecz, R.; Gallah, U.S.; Rédei, D.; Hohmann, J. Anticancer Meroterpenoids from Centrapalus pauciflorus leaves: Chromone-and 2,4-Chromadione-Monoterpene Derivatives. ACS Omega 2023, 8, 31389–31398. [Google Scholar] [CrossRef]
- Taher, M.; Aminuddin, A.; Susanti, D.; Aminudin, N.I.; On, S.; Ahmad, F.; Hamidon, H. Cytotoxic, anti-inflammatory and adipogenic effects of inophyllum D, calanone, isocordato-oblongic acid, and morelloflavone on cell lines. Nat. Prod. Sci. 2016, 22, 122–128. [Google Scholar] [CrossRef]
- Fakai, M.I.; Abd Malek, S.N.; Karsani, S.A. Induction of apoptosis by chalepin through phosphatidylserine externalisations and DNA fragmentation in breast cancer cells (MCF7). Life Sci. 2019, 220, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Burlison, J.A.; Blagg, B.S. Synthesis and evaluation of coumermycin A1 analogues that inhibit the Hsp90 protein folding machinery. Org. Lett. 2006, 8, 4855–4858. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.G.; Zou, J.N.; Wang, S.Z.; Zhang, T.C.; Xi, T. Novobiocin decreases SMYD3 expression and inhibits the migration of MDA-MB-231 human breast cancer cells. IUBMB Life 2010, 62, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lin, W.-L.; Chen, N.-F.; Chuang, S.-K.; Tseng, T.-H. Demethylwedelolactone derivatives inhibit invasive growth in vitro and lung metastasis of MDA-MB-231 breast cancer cells in nude mice. Eur. J. Med. Chem. 2012, 56, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Mahdi, F.; Jekabsons, M.B.; Nagle, D.G.; Zhou, Y.-D. Mammea E/BB, an isoprenylated dihydroxycoumarin protonophore that potently uncouples mitochondrial electron transport, disrupts hypoxic signaling in tumor cells. J. Nat. Prod. 2010, 73, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Girase, P.S.; Dhawan, S.; Kumar, V.; Shinde, S.R.; Palkar, M.B.; Karpoormath, R. An appraisal of anti-mycobacterial activity with structure-activity relationship of piperazine and its analogues: A review. Eur. J. Med. Chem. 2021, 210, 112967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-H.; Guo, H.-Y.; Deng, H.; Li, J.; Quan, Z.-S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzym. Inhib. Med. Chem. 2021, 36, 1165–1197. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Mukherjee, S.; Bhatt, H.; Rajani, D.; Ahmad, I.; Patel, H.; Kumari, P. Synthesis, docking, and biological investigations of new coumarin-piperazine hybrids as potential antibacterial and anticancer agents. J. Mol. Struct. 2023, 1276, 134755. [Google Scholar] [CrossRef]
- Cai, G.; Yu, W.; Song, D.; Zhang, W.; Guo, J.; Zhu, J.; Ren, Y.; Kong, L. Discovery of fluorescent coumarin-benzo[b]thiophene 1,1-dioxide conjugates as mitochondria-targeting antitumor STAT3 inhibitors. Eur. J. Med. Chem. 2019, 174, 236–251. [Google Scholar] [CrossRef]
- Singh, K.; Pal, R.; Khan, S.A.; Kumar, B.; Akhtar, M.J. Insights into the structure activity relationship of nitrogen-containing heterocyclics for the development of antidepressant compounds: An updated review. J. Mol. Struct. 2021, 1237, 130369. [Google Scholar] [CrossRef]
- Abdelshaheed, M.M.; Fawzy, I.M.; El-Subbagh, H.I.; Youssef, K.M. Piperidine nucleus in the field of drug discovery. Future J. Pharm. Sci. 2021, 7, 188. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Zhang, G.; Wu, C.; Tang, Z.; Liu, L.; You, Q.; Xiang, H. Novel SERMs based on 3-aryl-4-aryloxy-2H-chromen-2-one skeleton-A possible way to dual ERα/VEGFR-2 ligands for treatment of breast cancer. Eur. J. Med. Chem. 2017, 140, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Umar, S.; Shah, N.N.; Balkrishnan, S.; Soman, S.S. Design, Synthesis, and Anticancer Activity of 3H–benzo[f]chromen-3-one Derivatives. J. Heterocycl. Chem. 2017, 54, 2501–2510. [Google Scholar] [CrossRef]
- Dube, P.N.; Waghmare, M.N.; Mokale, S.N. Synthesis, in Vitro, and in Vivo Biological Evaluation and Molecular Docking Analysis of Novel 3-(3-oxo-substitutedphenyl-3-)4-(2-(piperidinyl) ethoxy) phenyl) propyl)-2H-chromen-2-one Derivatives as Anti-breast Cancer Agents. Chem. Biol. Drug Des. 2016, 87, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Moroni, E.; Yan, B.; Colombo, G.; Blagg, B.S. 3D-QSAR-assisted design, synthesis, and evaluation of novobiocin analogues. ACS Med. Chem. Lett. 2013, 4, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, B.; Peterson, L.B.; Blagg, B.S. 3-Arylcoumarin derivatives manifest anti-proliferative activity through Hsp90 inhibition. ACS Med. Chem. Lett. 2012, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Reddy Kusuma, B.; Blagg, B.S. Synthesis and evaluation of noviose replacements on novobiocin that manifest antiproliferative activity. ACS Med. Chem. Lett. 2010, 1, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Pršir, K.; Horak, E.; Kralj, M.; Uzelac, L.; Liekens, S.; Steinberg, I.M.; Krištafor, S. Design, Synthesis, Spectroscopic Characterisation and In Vitro Cytostatic Evaluation of Novel Bis(coumarin-1,2,3-triazolyl) benzenes and Hybrid Coumarin-1,2,3-triazolyl-aryl Derivatives. Molecules 2022, 27, 637. [Google Scholar] [CrossRef]
- Khudhayer Oglah, M.; Fakri Mustafa, Y. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2020, 29, 479–486. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, L.; Yao, Y.; Li, S.; Lv, M.; Wang, K.; Li, X.; Chen, H. Anticancer activity and DNA binding property of the dimers of triphenylethylene–coumarin hybrid with two amino side chains. Med. Chem. Res. 2015, 24, 2314–2324. [Google Scholar] [CrossRef]
- Kusuma, B.R.; Peterson, L.B.; Zhao, H.; Vielhauer, G.; Holzbeierlein, J.; Blagg, B.S. Targeting the heat shock protein 90 dimer with dimeric inhibitors. J. Med. Chem. 2011, 54, 6234–6253. [Google Scholar] [CrossRef] [PubMed]
- Achar, G.; Shahini, C.; Patil, S.A.; Małecki, J.G.; Budagumpi, S. Coumarin-substituted 1,2,4-triazole-derived silver (I) and gold (I) complexes: Synthesis, characterization and anticancer studies. New J. Chem. 2019, 43, 1216–1229. [Google Scholar] [CrossRef]
- Kumar, S.; Ritika. A brief review of the biological potential of indole derivatives. Future J. Pharm. Sci. 2020, 6, 121. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D.; Joseph, M.M.; Salam, A.A.A.; Sreelekha, T. Indole-coumarin-thiadiazole hybrids: An appraisal of their MCF-7 cell growth inhibition, apoptotic, antimetastatic and computational Bcl-2 binding potential. Eur. J. Med. Chem. 2017, 136, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.R.; Sunil, D.; Ajees, A.A.; Pai, K.; Biswas, S. N′-((2-(6-Bromo-2-oxo-2H-chromen-3-yl)-1H-indol-3-yl) methylene) benzohydrazide as a probable Bcl-2/Bcl-xL inhibitor with apoptotic and anti-metastatic potential. Eur. J. Med. Chem. 2016, 120, 134–147. [Google Scholar] [CrossRef]
- Galayev, O.; Garazd, Y.; Garazd, M.; Lesyk, R. Synthesis and anticancer activity of 6-heteroarylcoumarins. Eur. J. Med. Chem. 2015, 105, 171–181. [Google Scholar] [CrossRef]
- Mazzei, M.; Miele, M.; Nieddu, E.; Barbieri, F.; Bruzzo, C.; Alama, A. Unsymmetrical methylene derivatives of indoles as antiproliferative agents. Eur. J. Med. Chem. 2001, 36, 915–923. [Google Scholar] [CrossRef]
- Fan, Y.L.; Huang, Z.P.; Liu, M. Isatin–Coumarin Hybrids Tethered via Diethylene Glycol: Design, Synthesis, and Their In Vitro Antitumor Activities. J. Heterocycl. Chem. 2018, 55, 2722–2726. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Deng, J.L.; Wang, Q.; Lv, Z.S.; Fan, Y.L. Design, synthesis, and evaluation of tetraethylene glycol tethered isatin–coumarin hybrids as novel anticancer agents. J. Heterocycl. Chem. 2019, 56, 400–405. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Gao, F.; Wang, Y.L.; Zhang, F.; Bai, L.Y.; Deng, J.L.; Wang, Q.; Fan, Y.L. Design, Synthesis, and Evaluation of Tetraethylene Glycol-Tethered Isatin–1,2,3-Triazole–Coumarin Hybrids as Novel Anticancer Agents. J. Heterocycl. Chem. 2019, 56, 1127–1132. [Google Scholar] [CrossRef]
- Diao, Q.P.; Guo, H.; Wang, G.Q. Design, Synthesis, and In Vitro Anticancer Activities of Diethylene Glycol Tethered Isatin-1,2,3-triazole-coumarin Hybrids. J. Heterocycl. Chem. 2019, 56, 1667–1671. [Google Scholar] [CrossRef]
- Go, M.; Wu, X.; Liu, X. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S. Antimitotic chalcones and related compounds as inhibitors of tubulin assembly. Anti-Cancer Agents Med. Chem. 2009, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- El-Sherief, H.A.; Abuo-Rahma, G.E.-D.A.; Shoman, M.E.; Beshr, E.A.; Abdel-baky, R.M. Design and synthesis of new coumarin–chalcone/NO hybrids of potential biological activity. Med. Chem. Res. 2017, 26, 3077–3090. [Google Scholar] [CrossRef]
- Li, L.; Zhao, P.; Hu, J.; Liu, J.; Liu, Y.; Wang, Z.; Xia, Y.; Dai, Y.; Chen, L. Synthesis, in vitro and in vivo antitumor activity of scopoletin-cinnamic acid hybrids. Eur. J. Med. Chem. 2015, 93, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Molaverdi, F.; Khoobi, M.; Emami, S.; Alipour, M.; Firuzi, O.; Foroumadi, A.; Dehghan, G.; Miri, R.; Shaki, F.; Jafarpour, F. Polyoxygenated cinnamoylcoumarins as conformationally constrained analogs of cytotoxic diarylpentanoids: Synthesis and biological activity. Eur. J. Med. Chem. 2013, 68, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, X.; Wang, F.; He, H.; Qi, B. Discovery of 4-((4-(4-(3-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl) ureido)-2-fluorophenoxy)-6-methoxyquinolin-7-yl) oxy)-N,N-diethylpiperidine-1-carboxamide as kinase inhibitor for the treatment of colorectal cancer. Bioorg. Chem. 2021, 106, 104511. [Google Scholar] [CrossRef]
- Karnik, K.S.; Sarkate, A.P.; Tiwari, S.V.; Azad, R.; Burra, P.V.; Wakte, P.S. Computational and Synthetic approach with Biological Evaluation of Substituted Quinoline derivatives as small molecule L858R/T790M/C797S triple mutant EGFR inhibitors targeting resistance in Non-Small Cell Lung Cancer (NSCLC). Bioorg. Chem. 2021, 107, 104612. [Google Scholar] [CrossRef]
- Elbadawi, M.M.; Eldehna, W.M.; Wang, W.; Agama, K.K.; Pommier, Y.; Abe, M. Discovery of 4-alkoxy-2-aryl-6,7-dimethoxyquinolines as a new class of topoisomerase I inhibitors endowed with potent in vitro anticancer activity. Eur. J. Med. Chem. 2021, 215, 113261. [Google Scholar] [CrossRef] [PubMed]
- Khelifi, I.; Naret, T.; Renko, D.; Hamze, A.; Bernadat, G.; Bignon, J.; Lenoir, C.; Dubois, J.; Brion, J.-D.; Provot, O. Design, synthesis and anticancer properties of IsoCombretaQuinolines as potent tubulin assembly inhibitors. Eur. J. Med. Chem. 2017, 127, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Ghate, M.; Vyas, V.K. CoMFA and CoMSIA studies on 6, 7-disubstituted-4-phenoxyquinoline derivatives as c-Met kinase inhibitors and anticancer agents. Med. Chem. Res. 2015, 24, 4078–4092. [Google Scholar] [CrossRef]
- Lipeeva, A.V.; Zakharov, D.O.; Gatilov, Y.V.; Pokrovskii, M.A.; Pokrovskii, A.G.; Shults, E.E. Design and Synthesis of 3-(N-Substituted) aminocoumarins as Anticancer Agents from 3-Bromopeuruthenicin. ChemistrySelect 2019, 4, 10197–10201. [Google Scholar] [CrossRef]
- Han, X.; Luo, J.; Wu, F.; Hou, X.; Yan, G.; Zhou, M.; Zhang, M.; Pu, C.; Li, R. Synthesis and biological evaluation of novel 2,3-dihydrochromeno[3,4-d]imidazol-4(1H)-one derivatives as potent anticancer cell proliferation and migration agents. Eur. J. Med. Chem. 2016, 114, 232–243. [Google Scholar] [CrossRef]
- Gkionis, L.; Kavetsou, E.; Kalospyros, A.; Manousakis, D.; Garzon Sanz, M.; Butterworth, S.; Detsi, A.; Tirella, A. Investigation of the cytotoxicity of bioinspired coumarin analogues towards human breast cancer cells. Mol. Divers. 2021, 25, 307–321. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, F.; Han, L.; Qu, Y.; Ge, D.; Zhang, H. Development of coumarin-based hydroxamates as histone deacetylase inhibitors with antitumor activities. Molecules 2020, 25, 717. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.N.; Evangelista, F.C.; Seckler, D.; Marques, D.R.; Freitas, T.R.; Nunes, R.R.; Oliveira, J.T.; Ribeiro, R.I.; Santos, H.B.; Thome, R.G. Synthesis, cytotoxic activity, and mode of action of new Santacruzamate A analogs. Med. Chem. Res. 2018, 27, 2397–2413. [Google Scholar] [CrossRef]
- Ganeshapillai, D.; Woo, L.L.; Thomas, M.P.; Purohit, A.; Potter, B.V. C-3-and C-4-substituted bicyclic coumarin sulfamates as potent steroid sulfatase inhibitors. ACS Omega 2018, 3, 10748–10772. [Google Scholar] [CrossRef]
- Guimarães, K.G.; de Freitas, R.P.; Ruiz, A.L.; Fiorito, G.F.; de Carvalho, J.E.; da Cunha, E.F.; Ramalho, T.C.; Alves, R.B. Synthesis, antiproliferative activities, and computational evaluation of novel isocoumarin and 3,4-dihydroisocoumarin derivatives. Eur. J. Med. Chem. 2016, 111, 103–113. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, T.M.; Mayer, T.U.; Coughlin, M.L.; Mitchison, T.J. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 2000, 150, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Wang, X.; Huang, J.; Li, S.; Liu, C.; Dong, R.; Zhu, G.; Duan, C.; Jiang, F. Discovery of coumarin derivatives as potent and selective cyclin-dependent kinase 9 (CDK9) inhibitors with high antitumour activity. Eur. J. Med. Chem. 2020, 200, 112424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Qin, Y.-J.; Wang, P.-F.; Yang, Y.-A.; Wen, Q.; Zhang, X.; Qiu, H.-Y.; Duan, Y.-T.; Wang, Y.-T.; Sang, Y.-L. Sulfonamides containing coumarin moieties selectively and potently inhibit carbonic anhydrases II and IX: Design, synthesis, inhibitory activity and 3D-QSAR analysis. Eur. J. Med. Chem. 2013, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Avula, S.R.; Sharma, K.; Palnati, G.R.; Bathula, S.R. Discovery of coumarin–monastrol hybrid as potential antibreast tumor-specific agent. Eur. J. Med. Chem. 2013, 60, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.H.; Deeb, E.A. Synthesis, anticancer assessment on human breast, liver and colon carcinoma cell lines and molecular modeling study using novel pyrazolo[4,3-c]pyridine derivatives. Bioorg. Chem. 2018, 77, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.A. The Bipyridinium Herbicides; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Aruchamy, B.; Kuruburu, M.G.; Bovilla, V.R.; Madhunapantula, S.V.; Drago, C.; Benny, S.; Presanna, A.T.; Ramani, P. Design, Synthesis, and Anti-Breast Cancer Potential of Imidazole–Pyridine Hybrid Molecules In Vitro and Ehrlich Ascites Carcinoma Growth Inhibitory Activity Assessment In Vivo. ACS Omega 2023, 8, 40287–40298. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H.A. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab. J. Chem. 2022, 15, 103846. [Google Scholar] [CrossRef]
- Altaher, A.M.; Adris, M.A.; Aliwaini, S.H.; Awadallah, A.M.; Morjan, R.Y. The Anticancer Effects of Novel Imidazo[1,2-a]pyridine Compounds against HCC1937 Breast Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 2943. [Google Scholar] [CrossRef]
- Fayed, E.A.; Sabour, R.; Harras, M.F.; Mehany, A.B. Design, synthesis, biological evaluation and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 2019, 28, 1284–1297. [Google Scholar] [CrossRef]
- Hassan, A.; Sarg, M.; El Deeb, M.; Bayoumi, A.; El Rabeb, S. Facile synthesis and anticancer activity study of novel series of substituted and fused coumarin derivatives. J. Heterocycl. Chem. 2018, 55, 1426–1443. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Hemdan, M.M.; Atta-Allah, S.R. An Efficient One-Pot Synthesis of New Coumarin Derivatives as Potent Anticancer Agents under Microwave Irradiation. J. Heterocycl. Chem. 2017, 54, 3519–3526. [Google Scholar] [CrossRef]
- Mohareb, R.M.; MegallyAbdo, N.Y. Uses of 3-(2-Bromoacetyl)-2H-chromen-2-one in the Synthesis of Heterocyclic Compounds Incorporating Coumarin: Synthesis, Characterization and Cytotoxicity. Molecules 2015, 20, 11535–11553. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Fleita, D.H.; Sakka, O.K. Novel synthesis of hydrazide-hydrazone derivatives and their utilization in the synthesis of coumarin, pyridine, thiazole and thiophene derivatives with antitumor activity. Molecules 2010, 16, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Dong, W. Aloe-emodin induces endoplasmic reticulum stress-dependent apoptosis in colorectal cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6331. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-L.; Lu, Y.-C.; Chung, J.-G.; Li, Y.-C.; Wang, S.-G.; Sue-Hwee, N.; Wu, C.-Y.; Su, H.-L.; Chen, S.-S. Aloe-emodin induces apoptosis of human nasopharyngeal carcinoma cells via caspase-8-mediated activation of the mitochondrial death pathway. Cancer Lett. 2010, 291, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Hu, Y.; Li, J.; Li, L.; Tian, Y.; Li, X.; Wu, Q.; Zou, Z. The Synthesis and Biological Evaluation of Aloe-Emodin-Coumarin Hybrids as Potential Antitumor Agents. Molecules 2022, 27, 6153. [Google Scholar] [CrossRef]
- Aaghaz, S.; Sharma, K.; Jain, R.; Kamal, A. β-Carbolines as potential anticancer agents. Eur. J. Med. Chem. 2021, 216, 113321. [Google Scholar] [CrossRef]
- Sathish, M.; Kavitha, B.; Nayak, V.L.; Tangella, Y.; Ajitha, A.; Nekkanti, S.; Alarifi, A.; Shankaraiah, N.; Nagesh, N.; Kamal, A. Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur. J. Med. Chem. 2018, 144, 557–571. [Google Scholar] [CrossRef]
- Xin, B.; Tang, W.; Wang, Y.; Lin, G.; Liu, H.; Jiao, Y.; Zhu, Y.; Yuan, H.; Chen, Y.; Lu, T. Design, synthesis and biological evaluation of β-carboline derivatives as novel inhibitors targeting B-Raf kinase. Bioorg. Med. Chem. Lett. 2012, 22, 4783–4786. [Google Scholar] [CrossRef]
- Ikeda, R.; Kurosawa, M.; Okabayashi, T.; Takei, A.; Yoshiwara, M.; Kumakura, T.; Sakai, N.; Funatsu, O.; Morita, A.; Ikekita, M. 3-(3-phenoxybenzyl) amino-β-carboline: A novel antitumor drug targeting α-tubulin. Bioorg.Med. Chem. Lett. 2011, 21, 4784–4787. [Google Scholar] [CrossRef]
- Pavić, K.; Beus, M.; Poje, G.; Uzelac, L.; Kralj, M.; Rajić, Z. Synthesis and biological evaluation of harmirins, novel harmine–coumarin hybrids as potential anticancer agents. Molecules 2021, 26, 6490. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Li, H.; Wang, H.; Wang, J.; Lin, Y.; Ma, Y. Synthesis of ergosterol peroxide conjugates as mitochondria targeting probes for enhanced anticancer activity. Molecules 2019, 24, 3307. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Batool, F.; Zahra Naqvi, S.A.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-H.; Wang, Z.; Xia, Y.; Ye, T.-H.; Deng, M.; Xu, Y.-Z.; Wei, Y.-Q.; Yu, L.-T. Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents. Molecules 2012, 17, 3933–3944. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.O.; Suggitt, M.; Swaine, D.J.; Bibby, M.C.; Stevens, M.F.; Bradshaw, T.D. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. Mol. Cancer Ther. 2004, 3, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Editorial: Hepatocellular carcinoma in type 2 diabetes: More than meets the eye. Am. Coll. Gastroenterol. ACG 2012, 107, 53–55. [Google Scholar] [CrossRef]
- Barbarossa, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosato, A.; Limongelli, F.; Carrieri, A.; Bonofiglio, D.; Sinicropi, M.S. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics 2023, 12, 1651. [Google Scholar] [CrossRef]
- Nagaraja, O.; Bodke, Y.D.; Thippeswamy, B.; Venkatesh, T.; Manjunatha, B. Synthesis, characterization and biological evaluation of heterocyclic compounds containing 4-methylumbelliferone. J. Mol. Struct. 2022, 1269, 133759. [Google Scholar]
- Kumar, M.; Nukala, S.K.; Thirukovela, N.S.; Sreerama, R.; Kamarajugadda, P.; Narsimha, S. Ramachary-Bressy-Wang [3+2]cycloaddition reaction: Synthesis of fully decorated 1,2,3-triazoles as potent anticancer and EGFR inhibitors. J. Mol. Struct. 2022, 1262, 132975. [Google Scholar]
- Ragab, F.A.; Eissa, A.A.; Fahim, S.H.; Salem, M.A.; Gamal, M.A.; Nissan, Y.M. Novel coumarin–pyrazoline hybrids: Synthesis, cytotoxicity evaluation and molecular dynamics study. New J. Chem. 2021, 45, 19043–19055. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Aljuhani, A.; Alsehli, M.; Rezki, N.; Alawi, A.; Aldhafiri, A.J.; Salama, S.A.; Ahmed, H.E.; Aouad, M.R. Discovery of triaromatic flexible agents bearing 1,2,3-Triazole with selective and potent anti-breast cancer activity and CDK9 inhibition supported by molecular dynamics. J. Mol. Struct. 2022, 1249, 131568. [Google Scholar] [CrossRef]
- Amin, K.M.; Eissa, A.A.; Abou-Seri, S.M.; Awadallah, F.M.; Hassan, G.S. Synthesis and biological evaluation of novel coumarin–pyrazoline hybrids endowed with phenylsulfonyl moiety as antitumor agents. Eur. J. Med. Chem. 2013, 60, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Latif, N.A.A.; Batran, R.Z.; Khedr, M.A.; Abdalla, M.M. 3-Substituted-4-hydroxycoumarin as a new scaffold with potent CDK inhibition and promising anticancer effect: Synthesis, molecular modeling and QSAR studies. Bioorg. Chem. 2016, 67, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Ejaz, S.A.; Ibrar, A.; Umar, M.I.; Lecka, J.; Sévigny, J.; Saeed, A. Expanding the Alkaline Phosphatase Inhibition, Cytotoxic and Proapoptotic Profile of Biscoumarin-Iminothiazole and Coumarin-Triazolothiadiazine Conjugates. ChemistrySelect 2018, 3, 13377–13386. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Alminderej, F.M.; Mounier, M.M.; Nossier, E.S.; Saleh, S.M.; Kassem, A.F. New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-triazolyl thioglycoside analogs targeting mitochondria apoptotic pathway: Synthesis, anticancer activity and docking simulation. Molecules 2022, 27, 5688. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.S.; Pooladanda, V.; Mahammad, G.S.; Jakkula, P.; Gatreddi, S.; Qureshi, I.A.; Alvala, R.; Godugu, C.; Alvala, M. Synthesis and biological evaluation of morpholines linked coumarin–triazole hybrids as anticancer agents. Chem. Biol. Drug Des. 2019, 94, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Awad, G.E.; Badria, F.A. Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur. J. Med. Chem. 2011, 46, 1505–1511. [Google Scholar] [CrossRef]
- Vaarla, K.; Karnewar, S.; Panuganti, D.; Peddi, S.R.; Vedula, R.R.; Manga, V.; Kotamraju, S. 3-(2-(5-Amino-3-aryl-1H-pyrazol-1-yl) thiazol-4-yl)-2H-chromen-2-ones as Potential Anticancer Agents: Synthesis, Anticancer Activity Evaluation and Molecular Docking Studies. ChemistrySelect 2019, 4, 4324–4330. [Google Scholar] [CrossRef]
- Shaikh, S.K.J.; Sannaikar, M.S.; Kumbar, M.N.; Bayannavar, P.K.; Kamble, R.R.; Inamdar, S.R.; Joshi, S.D. Microwave-Expedited Green Synthesis, Photophysical, Computational Studies of Coumarin-3-yl-thiazol-3-yl-1,2,4-triazolin-3-ones and Their Anticancer Activity. ChemistrySelect 2018, 3, 4448–4462. [Google Scholar] [CrossRef]
- Jashari, A.; Imeri, F.; Ballazhi, L.; Shabani, A.; Mikhova, B.; Dräger, G.; Popovski, E.; Huwiler, A. Synthesis and cellular characterization of novel isoxazolo-and thiazolohydrazinylidene-chroman-2,4-diones on cancer and non-cancer cell growth and death. Bioorg. Med. Chem. 2014, 22, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Toan, V.N.; Thanh, N.D. Novel thiazoline–coumarin hybrid compounds containing sugar moieties: Synthesis, biological evaluation and molecular docking study as antiproliferative agents. New J. Chem. 2021, 45, 10636–10653. [Google Scholar] [CrossRef]
- Dhawan, S.; Kerru, N.; Awolade, P.; Singh-Pillay, A.; Saha, S.T.; Kaur, M.; Jonnalagadda, S.B.; Singh, P. Synthesis, computational studies and antiproliferative activities of coumarin-tagged 1,3,4-oxadiazole conjugates against MDA-MB-231 and MCF-7 human breast cancer cells. Bioorg. Med. Chem. 2018, 26, 5612–5623. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Chen, X.-Y.; Huang, Y.-Q.; Feng, P.; Guo, Y.-L.; Yang, G.; Chen, Y. Hybrids of phenylsulfonylfuroxan and coumarin as potent antitumor agents. J. Med. Chem. 2014, 57, 9343–9356. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, N.; Wang, K.; Deng, Q.; Lei, Z.; Sun, J.; Chen, L. Design, synthesis and biological activity evaluation of novel scopoletin-NO donor derivatives against MCF-7 human breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2021, 224, 113701. [Google Scholar] [CrossRef] [PubMed]
- Zwergel, C.; Czepukojc, B.; Evain-Bana, E.; Xu, Z.; Stazi, G.; Mori, M.; Patsilinakos, A.; Mai, A.; Botta, B.; Ragno, R. Novel coumarin-and quinolinone-based polycycles as cell division cycle 25-A and-C phosphatases inhibitors induce proliferation arrest and apoptosis in cancer cells. Eur. J. Med. Chem. 2017, 134, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Alsaggaf, A.T.; Okasha, R.M.; Ahmed, H.E.; Bissessur, R.; Abdelghani, A.A.; Afifi, T.H. Antimicrobial and Antitumor Screening of Fluorescent 5,7-Dihydroxy-4-Propyl-2H-Chromen-2-One Derivatives with Docking Studies. ChemistrySelect 2016, 1, 5025–5033. [Google Scholar] [CrossRef]
- Yadagiri, B.; Holagunda, U.D.; Bantu, R.; Nagarapu, L.; Kumar, C.G.; Pombala, S.; Sridhar, B. Synthesis of novel building blocks of benzosuberone bearing coumarin moieties and their evaluation as potential anticancer agents. Eur. J. Med. Chem. 2014, 79, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Meydani, A.; Yousefi, S.; Gharibi, R.; Kazemi, S.; Teimouri, M.B. Synthesis of a New Series of Furopyranone-and Furocoumarin-Chromone Conjugates Followed by In–Vitro Cytotoxicity Activity Evaluation, and Molecular Docking Study. ChemistrySelect 2019, 4, 3315–3324. [Google Scholar] [CrossRef]
- Mira, A.; Alkhiary, W.; Zhu, Q.; Nakagawa, T.; Tran, H.B.; Amen, Y.M.; Shimizu, K. Improved biological activities of isoepoxypteryxin by biotransformation. Chem. Biodivers. 2016, 13, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.S.; Rodrigues, L.R.; Cerqueira, N.M.; Oliveira-Campos, A.M.; Rodrigues, L.M. Synthesis of novel benzofurocoumarin analogues and their anti-proliferative effect on human cancer cell lines. Eur. J. Med. Chem. 2012, 47, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.S.; Elbialy, E.E. Synthesis, Characterization, and Cytotoxicity Evaluation of Some New Benzo[f]coumarin Derivatives. J. Heterocycl. Chem. 2018, 55, 893–898. [Google Scholar] [CrossRef]
- Khoobi, M.; Foroumadi, A.; Emami, S.; Safavi, M.; Dehghan, G.; Alizadeh, B.H.; Ramazani, A.; Ardestani, S.K.; Shafiee, A. Coumarin-based bioactive compounds: Facile synthesis and biological evaluation of coumarin-fused 1,4-thiazepines. Chem. Biol. Drug Des. 2011, 78, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Motamedi, R.; Firuzi, O.; Meili, S.; Mehdipour, A.R.; Miri, R. Synthesis and cytotoxic activity of novel benzopyrano[3,2-c]chromene-6, 8-dione derivatives. Med. Chem. Res. 2011, 20, 466–474. [Google Scholar] [CrossRef]
- Wittine, K.; Ratkaj, I.; Benci, K.; Suhina, T.; Mandić, L.; Ilić, N.; Pavelić, S.K.; Pavelić, K.; Mintas, M. The novel coumarin[3,2-c]thiophene and its hydroxamic acid and ureido derivatives: Synthesis and cytostatic activity evaluations. Med. Chem. Res. 2016, 25, 728–737. [Google Scholar] [CrossRef]
- Salem, M.A.; Marzouk, M.I.; El-Kazak, A.M. Synthesis and characterization of some new coumarins with in vitro antitumor and antioxidant activity and high protective effects against DNA damage. Molecules 2016, 21, 249. [Google Scholar] [CrossRef] [PubMed]
- Lagunes, I.; Begines, P.; Silva, A.; Galán, A.R.; Puerta, A.; Fernandes, M.X.; Maya, I.; Fernández-Bolaños, J.G.; López, Ó.; Padrón, J.M. Selenocoumarins as new multitarget antiproliferative agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2019, 179, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Arsenyan, P.; Vasiljeva, J.; Domracheva, I.; Kanepe-Lapsa, I.; Gulbe, A. Selenopheno[2,3-f]coumarins: Novel scaffolds with antimetastatic activity against melanoma and breast cancer. New J. Chem. 2019, 43, 11851–11864. [Google Scholar] [CrossRef]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Sun, Y.; Liu, S.; Zhang, S.; Wang, L. Design, synthesis and preliminary biological evaluation of benzylsulfone coumarin derivatives as anti-cancer agents. Molecules 2019, 24, 4034. [Google Scholar] [CrossRef]
- Sabt, A.; Abdelhafez, O.M.; El-Haggar, R.S.; Madkour, H.M.; Eldehna, W.M.; El-Khrisy, E.E.-D.A.; Abdel-Rahman, M.A.; Rashed, L.A. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: Synthesis, in vitro biological evaluation, and QSAR studies. J. Enzym. Inhib. Med. Chem. 2018, 33, 1095–1107. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.; Li, G.; Chen, L.; Nie, M.; Wang, Z.; Ji, H. Coumarin sulfonamides and amides derivatives: Design, synthesis, and antitumor activity in vitro. Molecules 2021, 26, 786. [Google Scholar] [CrossRef] [PubMed]
- Daśko, M.; Przybyłowska, M.; Rachon, J.; Masłyk, M.; Kubiński, K.; Misiak, M.; Składanowski, A.; Demkowicz, S. Synthesis and biological evaluation of fluorinated N-benzoyl and N-phenylacetoyl derivatives of 3-(4-aminophenyl)-coumarin-7-O-sulfamate as steroid sulfatase inhibitors. Eur. J. Med. Chem. 2017, 128, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hng, Y.; Lin, M.-H.; Lin, T.-S.; Liu, I.-C.; Lin, I.-C.; Lu, Y.-L.; Chang, C.-N.; Chiu, P.-F.; Tsai, K.-C.; Chen, M.-J. Design and synthesis of 3-benzylaminocoumarin-7-O-sulfamate derivatives as steroid sulfatase inhibitors. Bioorg. Chem. 2020, 96, 103618. [Google Scholar] [CrossRef] [PubMed]
- Eker, Y.; Şenkuytu, E.; Ölçer, Z.; Yıldırım, T.; Çiftçi, G.Y. Novel coumarin cyclotriphosphazene derivatives: Synthesis, characterization, DNA binding analysis with automated biosensor and cytotoxicity. J. Mol. Struct. 2020, 1209, 127971. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Fan, Y.; Yang, Y.; Xu, M.; Shi, X. Synthesis and anticancer activity of cyclotriphosphazenes functionalized with 4-methyl-7-hydroxycoumarin. New J. Chem. 2019, 43, 18316–18321. [Google Scholar] [CrossRef]
- Ghany, L.M.A.; El-Dydamony, N.M.; Helwa, A.A.; Abdelraouf, S.M.; Abdelnaby, R.M. Coumarin-acetohydrazide derivatives as novel antiproliferative agents via VEGFR-2/AKT axis inhibition and apoptosis triggering. New J. Chem. 2022, 46, 17394–17409. [Google Scholar] [CrossRef]
- Vaseghi, S.; Yousefi, M.; Shokrzadeh, M.; Hossaini, Z.; Hosseini-Khah, Z.; Emami, S. Synthesis, computational study and cytotoxicity of 4-hydroxycoumarin-derived imines/enamines. Mol. Divers. 2021, 25, 1011–1024. [Google Scholar] [CrossRef]
- Baghdadi, M.A.; Al-Abbasi, F.A.; El-Halawany, A.M.; Aseeri, A.H.; Al-Abd, A.M. Anticancer profiling for coumarins and related O-naphthoquinones from mansonia gagei against solid tumor cells in vitro. Molecules 2018, 23, 1020. [Google Scholar] [CrossRef]
- Abdizadeh, T.; Kalani, M.R.; Abnous, K.; Tayarani-Najaran, Z.; Khashyarmanesh, B.Z.; Abdizadeh, R.; Ghodsi, R.; Hadizadeh, F. Design, synthesis and biological evaluation of novel coumarin-based benzamides as potent histone deacetylase inhibitors and anticancer agents. Eur. J. Med. Chem. 2017, 132, 42–62. [Google Scholar] [CrossRef]
- Durgapal, S.D.; Soni, R.; Umar, S.; Suresh, B.; Soman, S.S. Anticancer Activity and DNA Binding Studies of Novel 3,7-Disubstituted Benzopyrones. ChemistrySelect 2017, 2, 147–153. [Google Scholar] [CrossRef]
- Abd-El-Maksoud, M.A.; Maigali, S.S.; Soliman, F.M. Chemistry of phosphonium ylides. Part 39: Facile synthesis of aziridine, pyridine, pyrolotriazole chromenones and azaphosphinin chromenones as antitumor agents. J. Heterocycl. Chem. 2014, 51, 1830–1837. [Google Scholar] [CrossRef]
- Zhao, H.; Donnelly, A.C.; Kusuma, B.R.; Brandt, G.E.; Brown, D.; Rajewski, R.A.; Vielhauer, G.; Holzbeierlein, J.; Cohen, M.S.; Blagg, B.S. Engineering an antibiotic to fight cancer: Optimization of the novobiocin scaffold to produce anti-proliferative agents. J. Med. Chem. 2011, 54, 3839–3853. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Elsherief, H.A.; Hafez, H.M.; Alsaidan, O.A.; Alzarea, S.I.; AboulMagd, A.M. Synthesis, antiproliferative activity, and molecular modeling of novel 4-methylcoumarin derivatives and/or nitric oxide donor hybrids. Mol. Divers. 2022, 27, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Shylaja, R.; Loganathan, C.; Kabilan, S.; Vijayakumar, T.; Meganathan, C. Synthesis and evaluation of the antagonistic activity of 3-acetyl-2H-benzo[g]chromen-2-one against mutant Y537S estrogen receptor alpha via E-Pharmacophore modeling, molecular docking, molecular dynamics, and in-vitro cytotoxicity studies. J. Mol. Struct. 2021, 1224, 129289. [Google Scholar] [CrossRef]
- Reddy, N.S.; Gumireddy, K.; Mallireddigari, M.R.; Cosenza, S.C.; Venkatapuram, P.; Bell, S.C.; Reddy, E.P.; Reddy, M.R. Novel coumarin-3-(N-aryl) carboxamides arrest breast cancer cell growth by inhibiting ErbB-2 and ERK1. Bioorg. Med. Chem. 2005, 13, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, D.; Zhu, J.; Jarori, G.; Tanaka, J.; Higa, T.; Davisson, V.J.; Helquist, P. Synthesis of carbamate derivatives of iejimalides. Retention of normal antiproliferative activity and localization of binding in cancer cells. Bioorg. Med. Chem. 2007, 15, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Audisio, D.; Methy-Gonnot, D.; Radanyi, C.; Renoir, J.-M.; Denis, S.; Sauvage, F.; Vergnaud-Gauduchon, J.; Brion, J.-D.; Messaoudi, S.; Alami, M. Synthesis and antiproliferative activity of novobiocin analogues as potential hsp90 inhibitors. Eur. J. Med. Chem. 2014, 83, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.Y.; Latif, N.A.A.; El-Mansy, M.F.; Elserwy, W.S.; Abdelhafez, O.M. VEGFR-2 inhibiting effect and molecular modeling of newly synthesized coumarin derivatives as anti-breast cancer agents. Bioorg. Med. Chem. 2020, 28, 115328. [Google Scholar] [CrossRef]
- Pilli, G.; Dumala, N.; Sreeja, J.S.; John, R.; Sengupta, S.; Grover, P.; Prakash, M.J. Design, Synthesis and Pharmacological Evaluation of 4-Hydroxycoumarin Derivatives as Antiproliferative Agents. ChemistrySelect 2019, 4, 10805–10809. [Google Scholar] [CrossRef]
- Batran, R.Z.; Kassem, A.F.; Abbas, E.M.; Elseginy, S.A.; Mounier, M.M. Design, synthesis and molecular modeling of new 4-phenylcoumarin derivatives as tubulin polymerization inhibitors targeting MCF-7 breast cancer cells. Bioorg. Med. Chem. 2018, 26, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.C.; Zhao, H.; Kusuma, B.R.; Blagg, B.S. Cytotoxic sugar analogues of an optimized novobiocin scaffold. MedChemComm 2010, 1, 165–170. [Google Scholar] [CrossRef]
- Le Bras, G.; Radanyi, C.; Peyrat, J.-F.; Brion, J.-D.; Alami, M.; Marsaud, V.; Stella, B.; Renoir, J.-M. New novobiocin analogues as antiproliferative agents in breast cancer cells and potential inhibitors of heat shock protein 90. J. Med. Chem. 2007, 50, 6189–6200. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.O.; Abd El-Karim, S.S.; Anwar, M.M.; Gouda, R.H.; Zaghary, W.A.; Khedr, M.A. Discovery of new coumarin-based lead with potential anticancer, CDK4 inhibition and selective radiotheranostic effect: Synthesis, 2D & 3D QSAR, molecular dynamics, in vitro cytotoxicity, radioiodination, and biodistribution studies. Molecules 2021, 26, 2273. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Singh, K.; Kaur, K.; Singh, A.; Sharma, A.; Kaur, K.; Kaur, J.; Kaur, G.; Kaur, U.; Kaur, H.; et al. Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure–Activity Relationships. Biomedicines 2024, 12, 1192. https://doi.org/10.3390/biomedicines12061192
Singh A, Singh K, Kaur K, Singh A, Sharma A, Kaur K, Kaur J, Kaur G, Kaur U, Kaur H, et al. Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure–Activity Relationships. Biomedicines. 2024; 12(6):1192. https://doi.org/10.3390/biomedicines12061192
Chicago/Turabian StyleSingh, Atamjit, Karanvir Singh, Kamaljit Kaur, Amandeep Singh, Aman Sharma, Kirandeep Kaur, Jaskirat Kaur, Gurleen Kaur, Uttam Kaur, Harsimran Kaur, and et al. 2024. "Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure–Activity Relationships" Biomedicines 12, no. 6: 1192. https://doi.org/10.3390/biomedicines12061192






