Three-Dimensional Hepatocyte Spheroids: Model for Assessing Chemotherapy in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Liver Perfusion
2.2. Cell Culture and Spheroid Formation
2.3. Spheroid Imaging
2.4. Flow Cytometry
2.5. EV Collection and Characterization
2.6. Protein Analysis
2.7. RNA Analysis
2.8. Statistics
3. Results
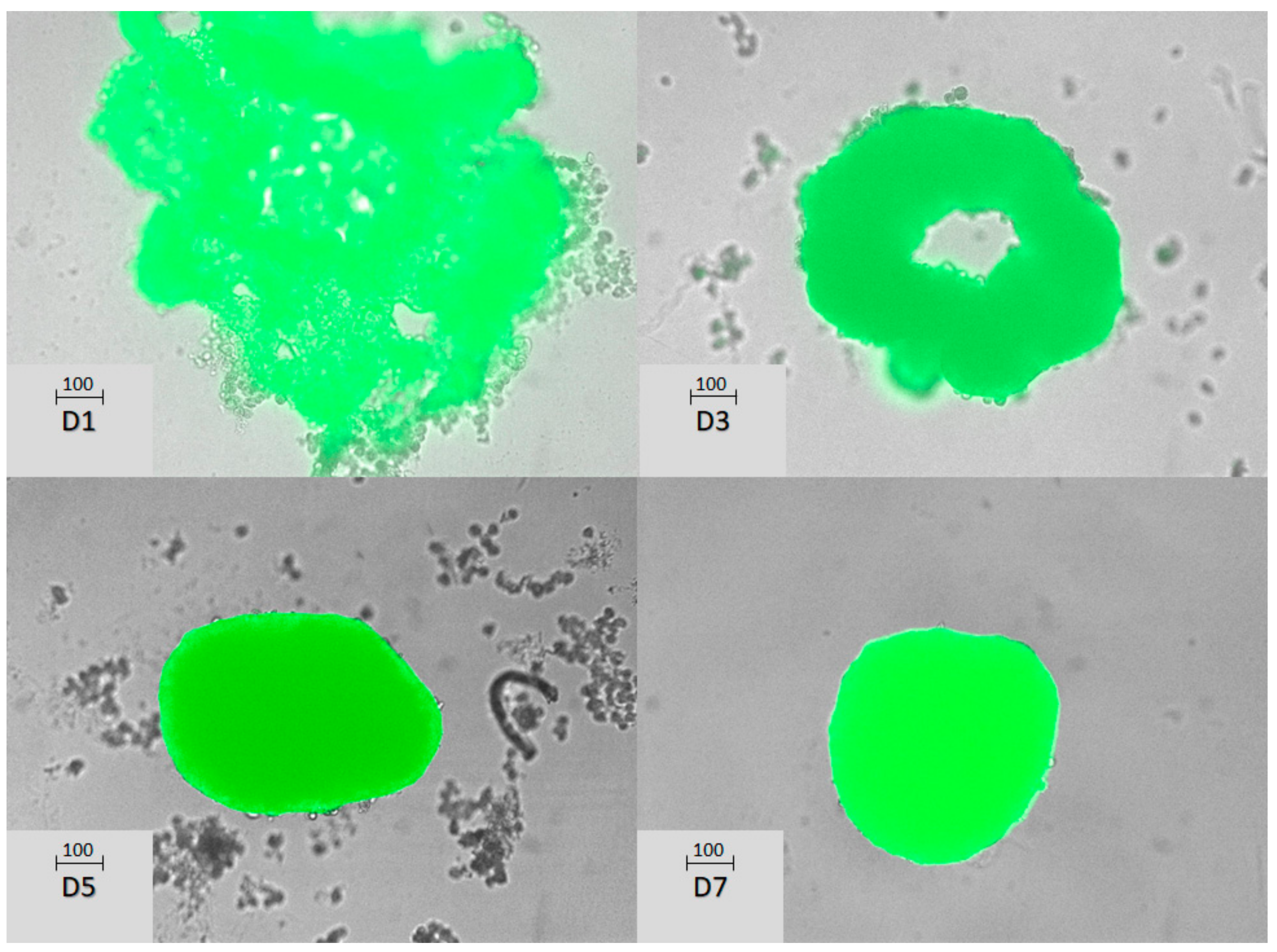
3.1. Characterization of Mice Hepatocyte Spheroids
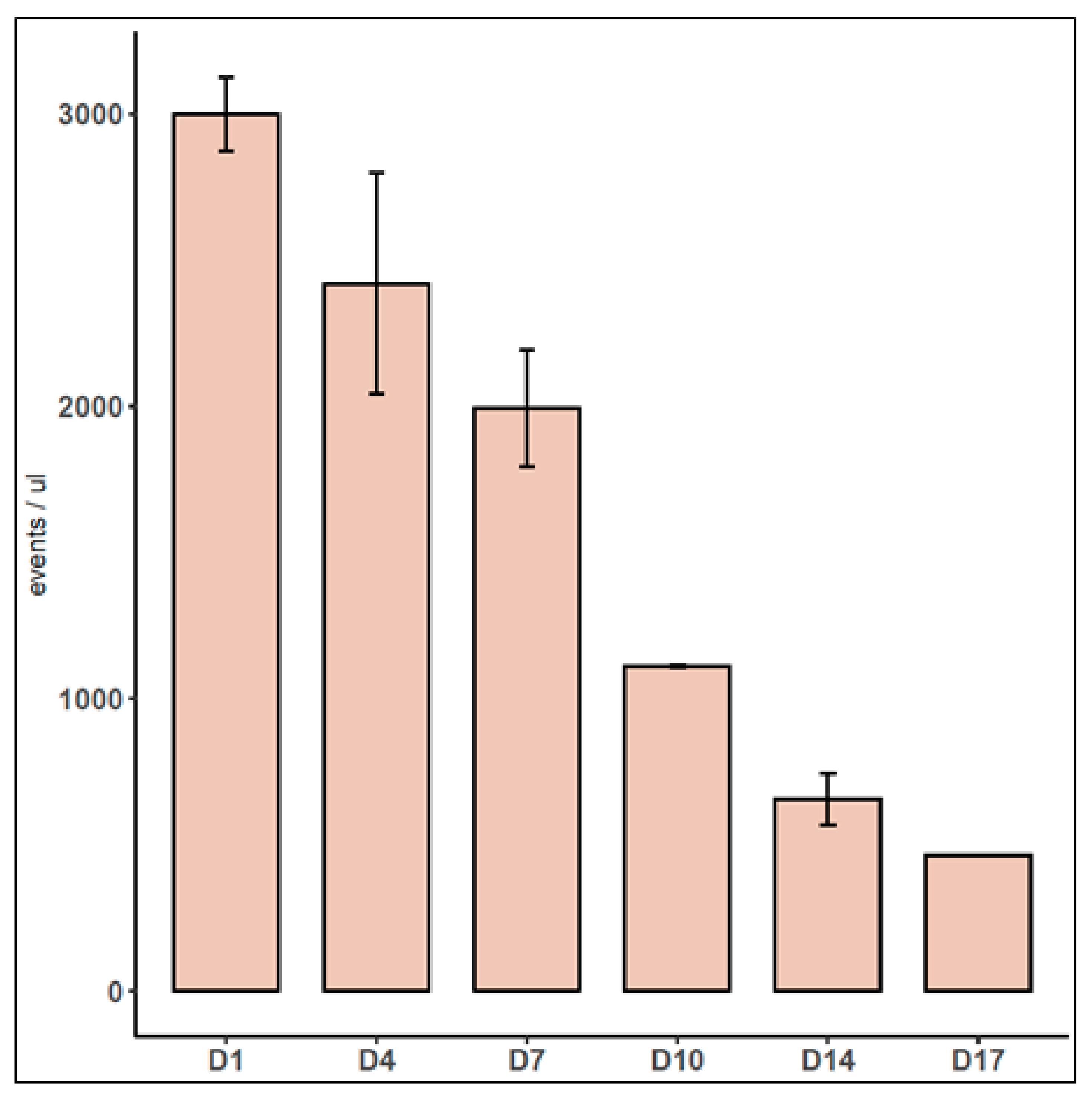
3.2. Characterization of EVs Released by Spheroids
3.3. Characterization of Mixed Spheroids
3.4. Antitumoral Treatment of Mixed Spheroids
4. Discussion and Conclusions
4.1. Culture Conditions and Spheroid Formation
4.2. Release of EVs
4.3. Tumoral Cells Assayed
4.4. Chemotherapeutic Agents Used
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Guohao, D.; Hongyan, Z.; Seung-Schik, Y. Generation of 3-D glioblastoma-vascular niche using 3-D bioprinting. In Proceedings of the 2015 41st Annual Northeast Biomedical Engineering Conference, Troy, NY, USA, 17–19 April 2015; pp. 1–2. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Schooley, A.M.; Andrews, N.M.; Zhao, H.; Addison, C.L. β1 integrin is required for anchorage-independent growth and invasion of tumor cells in a context dependent manner. Cancer Lett. 2012, 316, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Hendriks, D.F.G.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Fredriksson Puigvert, L.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef]
- Hendriks, D.F.G.; Vorrink, S.U.; Smutny, T.; Sim, S.C.; Nordling, Å.; Ullah, S.; Kumondai, M.; Jones, B.C.; Johansson, I.; Andersson, T.B.; et al. Clinically Relevant Cytochrome P450 3A4 Induction Mechanisms and Drug Screening in Three-Dimensional Spheroid Cultures of Primary Human Hepatocytes. Clin. Pharmacol. Ther. 2020, 108, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Vorrink, S.U.; Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Prediction of Drug-Induced Hepatotoxicity Using Long-Term Stable Primary Hepatic 3D Spheroid Cultures in Chemically Defined Conditions. Toxicol. Sci. 2018, 163, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; He, C.-X.; Cheng, J.; Wen, J.; Li, P.-Y.; Wang, N.; Li, G.; Zeng, X.-R.; Cao, J.-M.; Yang, Y. Sodium Tanshinone II-A Sulfonate (DS-201) Induces Vasorelaxation of Rat Mesenteric Arteries via Inhibition of L-Type Ca2+ Channel. Front. Pharmacol. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef] [PubMed]
- Friemel, J.; Rechsteiner, M.; Frick, L.; Böhm, F.; Struckmann, K.; Egger, M.; Moch, H.; Heikenwalder, M.; Weber, A. Intratumor Heterogeneity in Hepatocellular Carcinoma. Clin. Cancer Res. 2015, 21, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Nuciforo, S.; Heim, M.H. Organoids to model liver disease. JHEP Rep. 2021, 3, 100198. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Riffle, S.; Pandey, R.N.; Albert, M.; Hegde, R.S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer 2017, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Duliban, A.; Brodaczewska, K.; Kajdasz, A.; Kieda, C. Spheroid Culture Differentially Affects Cancer Cell Sensitivity to Drugs in Melanoma and RCC Models. Int. J. Mol. Sci. 2022, 23, 1166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; He, Z.; Ma, Y.; Uchiyama, K.; Lin, J.-M. A novel approach for precisely controlled multiple cell patterning in microfluidic chips by inkjet printing and the detection of drug metabolism and diffusion. Analyst 2016, 141, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-G.; Jenabi, J.M.; Zhang, J.; Keshelava, N.; Shimada, H.; May, W.A.; Ng, T.; Reynolds, C.P.; Triche, T.J.; Sorensen, P.H.B. E-Cadherin Cell-Cell Adhesion in Ewing Tumor Cells Mediates Suppression of Anoikis through Activation of the ErbB4 Tyrosine Kinase. Cancer Res. 2007, 67, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tan, O.L.; Loessner, D.; Stephens, C.; Walpole, C.; Boyle, G.M.; Parsons, P.G.; Clements, J.A. Kallikrein-Related Peptidase 7 Promotes Multicellular Aggregation via the α5β1 Integrin Pathway and Paclitaxel Chemoresistance in Serous Epithelial Ovarian Carcinoma. Cancer Res. 2010, 70, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Bordanaba-Florit, G.; Madarieta, I.; Olalde, B.; Falcón-Pérez, J.M.; Royo, F. 3D Cell Cultures as Prospective Models to Study Extracellular Vesicles in Cancer. Cancers 2021, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Kyykallio, H.; Faria, A.V.S.; Hartman, R.; Capra, J.; Rilla, K.; Siljander, P.R.M. A quick pipeline for the isolation of 3D cell culture-derived extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12273. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Ryabinin, A.E.; Nemoto, S.; Kubota, T.; Ohno, H. Metabolic differences and differentially expressed genes between C57BL/6J and C57BL/6N mice substrains. PLoS ONE 2022, 17, e0271651. [Google Scholar] [CrossRef] [PubMed]
- Siendones, E.; JimÉNez-GÓMez, Y.; Montero, J.L.; GÓMez-DÍAz, C.; Villalba, J.M.; MuntanÉ, J. PGE1 abolishes the mitochondrial-independent cell death pathway induced by D-galactosamine in primary culture of rat hepatocytes. J. Gastroenterol. Hepatol. 2009, 20, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-c.; Meng, F.-d.; Qu, K.; Wang, Z.-x.; Wu, Q.-f.; Zhang, L.-q.; Pang, Q.; Liu, C. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol. Sin. 2015, 36, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Etebari, M.; Jafarian-Dehkordi, A.; Lame, V. Evaluation of protective effect of amifostine on dacarbazine induced genotoxicity. Res. Pharm. Sci. 2015, 10, 68–74. [Google Scholar] [PubMed]
- Elsawy, H.; Algefare, A.I.; Alfwuaires, M.; Khalil, M.; Elmenshawy, O.M.; Sedky, A.; Abdel-Moneim, A.M. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci. Rep. 2020, 40, BSR20193686. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, E.; Hammer, H.S.; Potz, O.; Ingelman-Sundberg, M.; Stage, T.B. 3D Spheroid Primary Human Hepatocytes for Prediction of Cytochrome P450 and Drug Transporter Induction. Clin. Pharmacol. Ther. 2023, 113, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.A.; Rink, A.; Beattie, C.W.; Hu, W.-S. Differential gene expression analysis during porcine hepatocyte spheroid formation. Mamm. Genome 2002, 13, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef] [PubMed]
- Baze, A.; Parmentier, C.; Hendriks, D.F.G.; Hurrell, T.; Heyd, B.; Bachellier, P.; Schuster, C.; Ingelman-Sundberg, M.; Richert, L. Three-Dimensional Spheroid Primary Human Hepatocytes in Monoculture and Coculture with Nonparenchymal Cells. Tissue Eng. Part. C Methods 2018, 24, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, T.; Chen, D.; Wang, Q.; Zhang, L.W. Three-dimensional liver models: State of the art and their application for hepatotoxicity evaluation. Crit. Rev. Toxicol. 2020, 50, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Cameron, S. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay. Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Bouwmeester, M.C.; Tao, Y.; Proenca, S.; van Steenbeek, F.G.; Samsom, R.A.; Nijmeijer, S.M.; Sinnige, T.; van der Laan, L.J.W.; Legler, J.; Schneeberger, K.; et al. Drug Metabolism of Hepatocyte-like Organoids and Their Applicability in In Vitro Toxicity Testing. Molecules 2023, 28, 621. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, X.; Zhong, Z.; Qiu, Y.; Liu, S.; Wu, H.; Tang, X.; Chen, C.; Fu, Y.; Chen, Q.; et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J. Nanobiotechnol. 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Flores-Bellver, M.; Pan, J.; Benito-Martin, A.; Shi, C.; Onwumere, O.; Mighty, J.; Qian, J.; Zhong, X.; Hogue, T.; et al. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci. Rep. 2021, 11, 21128. [Google Scholar] [CrossRef]
- Giusti, I.; Poppa, G.; D’Ascenzo, S.; Esposito, L.; Vitale, A.R.; Calvisi, G.; Dolo, V. Cancer Three-Dimensional Spheroids Mimic In Vivo Tumor Features, Displaying “Inner” Extracellular Vesicles and Vasculogenic Mimicry. Int. J. Mol. Sci. 2022, 23, 11782. [Google Scholar] [CrossRef]
- Palomo, L.; Mleczko, J.E.; Azkargorta, M.; Conde-Vancells, J.; González, E.; Elortza, F.; Royo, F.; Falcon-Perez, J.M. Abundance of Cytochromes in Hepatic Extracellular Vesicles Is Altered by Drugs Related With Drug-Induced Liver Injury. Hepatol. Commun. 2018, 2, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Schlangen, K.; Palomo, L.; Gonzalez, E.; Conde-Vancells, J.; Berisa, A.; Aransay, A.M.; Falcon-Perez, J.M. Transcriptome of Extracellular Vesicles Released by Hepatocytes. PLoS ONE 2013, 8, e68693. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Momen-Heravi, F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 455–466. [Google Scholar] [CrossRef]
- Hellmold, D.; Kubelt, C.; Daunke, T.; Beckinger, S.; Janssen, O.; Hauck, M.; Schütt, F.; Adelung, R.; Lucius, R.; Haag, J.; et al. Sequential Treatment with Temozolomide Plus Naturally Derived AT101 as an Alternative Therapeutic Strategy: Insights into Chemoresistance Mechanisms of Surviving Glioblastoma Cells. Int. J. Mol. Sci. 2023, 24, 9075. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Amirmostofian, M.; Kobarfard, F.; Shahraki, J. Biological reactive intermediates that mediate dacarbazine cytotoxicity. Cancer Chemother. Pharmacol. 2009, 65, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Marrazzo, P.; Pizzuti, V.; Marchionni, C.; Rossi, M.; Michelotti, M.; Petrovic, B.; Ciani, E.; Simonazzi, G.; Pession, A.; et al. Characterization of Perinatal Stem Cell Spheroids for the Development of Cell Therapy Strategy. Bioengineering 2023, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Heffelfinger, S.C.; Hawkins, H.H.; Barrish, J.; Taylor, L.; Darlington, G.J. SK HEP-1: A human cell line of endothelial origin. In Vitro Cell Dev. Biol. Anim. 1992, 28, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, H.; Chhim, S.; Ding, Y.; Jiang, W.; Queen, C.; Kim, K.J. A Novel Monoclonal Antibody to Fibroblast Growth Factor 2 Effectively Inhibits Growth of Hepatocellular Carcinoma Xenografts. Mol. Cancer Ther. 2012, 11, 864–872. [Google Scholar] [CrossRef]
- Liu, P.; Menon, K.; Alvarez, E.; Lu, K.; Teicher, B.A. Transforming growth factor-beta and response to anticancer therapies in human liver and gastric tumors in vitro and in vivo. Int. J. Oncol. 2000, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.E.; Colman, R.W. Fibronectin synthesized by a human hepatoma cell line. Cancer Res. 1984, 44, 3022–3028. [Google Scholar] [PubMed]
- Gonzalez-Molina, J.; Mendonça da Silva, J.; Fuller, B.; Selden, C. The extracellular fluid macromolecular composition differentially affects cell-substrate adhesion and cell morphology. Sci. Rep. 2019, 9, 8505. [Google Scholar] [CrossRef] [PubMed]
- Nagata, J.; Guerra, M.T.; Shugrue, C.A.; Gomes, D.A.; Nagata, N.; Nathanson, M.H. Lipid Rafts Establish Calcium Waves in Hepatocytes. Gastroenterology 2007, 133, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jimenez, F.; Benedicto, I.; Dao Thi, V.L.; Gondar, V.; Lavillette, D.; Marin, J.J.; Briz, O.; Moreno-Otero, R.; Aldabe, R.; Baumert, T.F.; et al. Matrigel-embedded 3D culture of Huh-7 cells as a hepatocyte-like polarized system to study hepatitis C virus cycle. Virology 2012, 425, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Moriwaki, K.; Terao, N.; Nakagawa, T.; Miyamoto, Y.; Kamada, Y.; Miyoshi, E. Analysis of Polarized Secretion of Fucosylated Alpha-Fetoprotein in HepG2 Cells. J. Proteome Res. 2012, 11, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Huerta, E.P.; Torres-Martinez, A.; Charles-Nino, C.; Rivas-Estilla, A.M.; Paez, A.; Fortoul, T.I.; Montano, L.F. Pegylated interferon-alpha2b and ribavirin decrease claudin-1 and E-cadherin expression in HepG2 and Huh-7.5 cells. Ann. Hepatol. 2013, 12, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, X.; Lyu, L.; Jiang, H.; Zhu, H.-J. Comparison of protein expression between human livers and the hepatic cell lines HepG2, Hep3B, and Huh7 using SWATH and MRM-HR proteomics: Focusing on drug-metabolizing enzymes. Drug Metab. Pharmacokinet. 2018, 33, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Schicht, G.; Seidemann, L.; Haensel, R.; Seehofer, D.; Damm, G. Critical Investigation of the Usability of Hepatoma Cell Lines HepG2 and Huh7 as Models for the Metabolic Representation of Resectable Hepatocellular Carcinoma. Cancers 2022, 14, 4227. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, M.A.; Chapresto-Garzón, R.; Cadenas, M.; Navarro-Villarán, E.; Negrete, M.; Gómez-Bravo, M.A.; Victor, V.M.; Padillo, F.J.; Muntané, J. Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells. Cell Death Dis. 2020, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef]
- Juratli, M.A.; Zhou, H.; Oppermann, E.; Bechstein, W.O.; Pascher, A.; Chun, F.K.H.; Juengel, E.; Rutz, J.; Blaheta, R.A. Integrin α2 and β1 Cross-Communication with mTOR/AKT and the CDK-Cyclin Axis in Hepatocellular Carcinoma Cells. Cancers 2022, 14, 2430. [Google Scholar] [CrossRef]
- Muenzner, J.K.; Kunze, P.; Lindner, P.; Polaschek, S.; Menke, K.; Eckstein, M.; Geppert, C.I.; Chanvorachote, P.; Baeuerle, T.; Hartmann, A.; et al. Generation and characterization of hepatocellular carcinoma cell lines with enhanced cancer stem cell potential. J. Cell Mol. Med. 2018, 22, 6238–6248. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Pramfalk, C.; Morten, K.J.; Hodson, L. From whole body to cellular models of hepatic triglyceride metabolism: Man has got to know his limitations. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1–E20. [Google Scholar] [CrossRef]
- Guo, L.; Dial, S.; Shi, L.; Branham, W.; Liu, J.; Fang, J.-L.; Green, B.; Deng, H.; Kaput, J.; Ning, B. Similarities and Differences in the Expression of Drug-Metabolizing Enzymes between Human Hepatic Cell Lines and Primary Human Hepatocytes. Drug Metab. Dispos. 2011, 39, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Mu, H. Identification of biomarkers for hepatocellular carcinoma by semiquantitative immunocytochemistry. World J. Gastroenterol. 2014, 20, 5826. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Zen, Y.; Fujii, T.; Sato, Y.; Ohta, T.; Aoyagi, Y.; Nakanuma, Y. Characterization of CD133+parenchymal cells in the liver: Histology and culture. World J. Gastroenterol. 2009, 15, 4896. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Tanaka, Y.; Hayashido, Y.; Toratani, S.; Kan, M.; Kitamoto, M.; Nakanishi, T.; Kajiyama, G.; Chayama, K.; Okamoto, T. Expression of Fibroblast Growth Factor Receptor Genes in Human Hepatoma-Derived Cell Lines. In Vitro Cell Dev. Biol. Anim. 2003, 39, 321. [Google Scholar]
- Saraswati, S.; Alhaider, A.; Abdelgadir, A.M.; Tanwer, P.; Korashy, H.M. Phloretin attenuates STAT-3 activity and overcomes sorafenib resistance targeting SHP-1–mediated inhibition of STAT3 and Akt/VEGFR2 pathway in hepatocellular carcinoma. Cell Commun. Signal. 2019, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Lang, L. FDA Approves Sorafenib for Patients With Inoperable Liver Cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Chien, D.-S. BAY 43-9006: Preclinical Data. Curr. Pharm. Des. 2002, 8, 2255–2257. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Stadler, W. Sorafenib. Curr. Opin. Oncol. 2006, 18, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, H.; Sun, L.; Chen, X.; Wei, H.; Suo, C.; Feng, J.; Yuan, M.; Shen, S.; Jia, W.; et al. Metformin sensitises hepatocarcinoma cells to methotrexate by targeting dihydrofolate reductase. Cell Death Dis. 2021, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Sanada, M.; Hidaka, M.; Takagi, Y.; Takano, T.Y.; Nakatsu, Y.; Tsuzuki, T.; Sekiguchi, M. Modes of actions of two types of anti-neoplastic drugs, dacarbazine and ACNU, to induce apoptosis. Carcinogenesis 2007, 28, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Kaufmann, Y.; LoBianco, F.V.; Schleiff, M.A.; Aykin-Burns, N.; Miller, G.P. The Role of Cytochrome P450 3A4-Mediated Metabolism in Sorafenib and Lapatinib Hepatotoxicity. Livers 2023, 3, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, K.; Hosono, M.; Kojima, H.; Ogihara, T. Establishment of a primary human hepatocyte spheroid system for evaluating metabolic toxicity using dacarbazine under conditions of CYP1A2 induction. Drug Metab. Pharmacokinet. 2020, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, V.; Verhoeven, D.W.; Verhoeven, F.; Aubin, F.; Avouac, J.; Vuitton, L.; Lioté, F.; Thévenot, T.; Wendling, D. Busting the myth of methotrexate chronic hepatotoxicity. Nat. Rev. Rheumatol. 2022, 19, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, G.; El-Orfali, Y. Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals 2024, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Morganroth, J.; Shah, D.R. Hepatotoxicity of Tyrosine Kinase Inhibitors: Clinical and Regulatory Perspectives. Drug Saf. 2013, 36, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Paech, F.; Bouitbir, J.; Krähenbühl, S. Hepatocellular Toxicity Associated with Tyrosine Kinase Inhibitors: Mitochondrial Damage and Inhibition of Glycolysis. Front. Pharmacol. 2017, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, S.; Heo, J.; Shum, D.; Lee, S.Y.; Lee, M.; Kim, A.R.; Seo, H.R. Identification of hepatic fibrosis inhibitors through morphometry analysis of a hepatic multicellular spheroids model. Sci. Rep. 2021, 11, 10931. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, D.A.; Sargenti, A.; Bonetti, S.; Musmeci, F.; Delprete, C.; Bacchi, F.; Pasqua, S.; Cavallo, C.; Bonsi, L.; Alviano, F.; et al. A Reliable Flow-Based Method for the Accurate Measure of Mass Density, Size and Weight of Live 3D Tumor Spheroids. Micromachines 2020, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Myungjin Lee, J.; Mhawech-Fauceglia, P.; Lee, N.; Cristina Parsanian, L.; Gail Lin, Y.; Andrew Gayther, S.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royo, F.; Garcia-Vallicrosa, C.; Azparren-Angulo, M.; Bordanaba-Florit, G.; Lopez-Sarrio, S.; Falcon-Perez, J.M. Three-Dimensional Hepatocyte Spheroids: Model for Assessing Chemotherapy in Hepatocellular Carcinoma. Biomedicines 2024, 12, 1200. https://doi.org/10.3390/biomedicines12061200
Royo F, Garcia-Vallicrosa C, Azparren-Angulo M, Bordanaba-Florit G, Lopez-Sarrio S, Falcon-Perez JM. Three-Dimensional Hepatocyte Spheroids: Model for Assessing Chemotherapy in Hepatocellular Carcinoma. Biomedicines. 2024; 12(6):1200. https://doi.org/10.3390/biomedicines12061200
Chicago/Turabian StyleRoyo, Felix, Clara Garcia-Vallicrosa, Maria Azparren-Angulo, Guillermo Bordanaba-Florit, Silvia Lopez-Sarrio, and Juan Manuel Falcon-Perez. 2024. "Three-Dimensional Hepatocyte Spheroids: Model for Assessing Chemotherapy in Hepatocellular Carcinoma" Biomedicines 12, no. 6: 1200. https://doi.org/10.3390/biomedicines12061200





