Retrospective Evaluation of Bone Turnover Markers in Serum for the Prediction of Metastases Development in Breast Cancer Patients: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants and Clinicopathologic Data
2.3. Serum Samples and Markers Analysis
2.4. Statistical Analysis
2.4.1. cBioPortal–TCGA Bioinformatic Analysis
2.4.2. Statistical Analysis of Original Data
3. Results
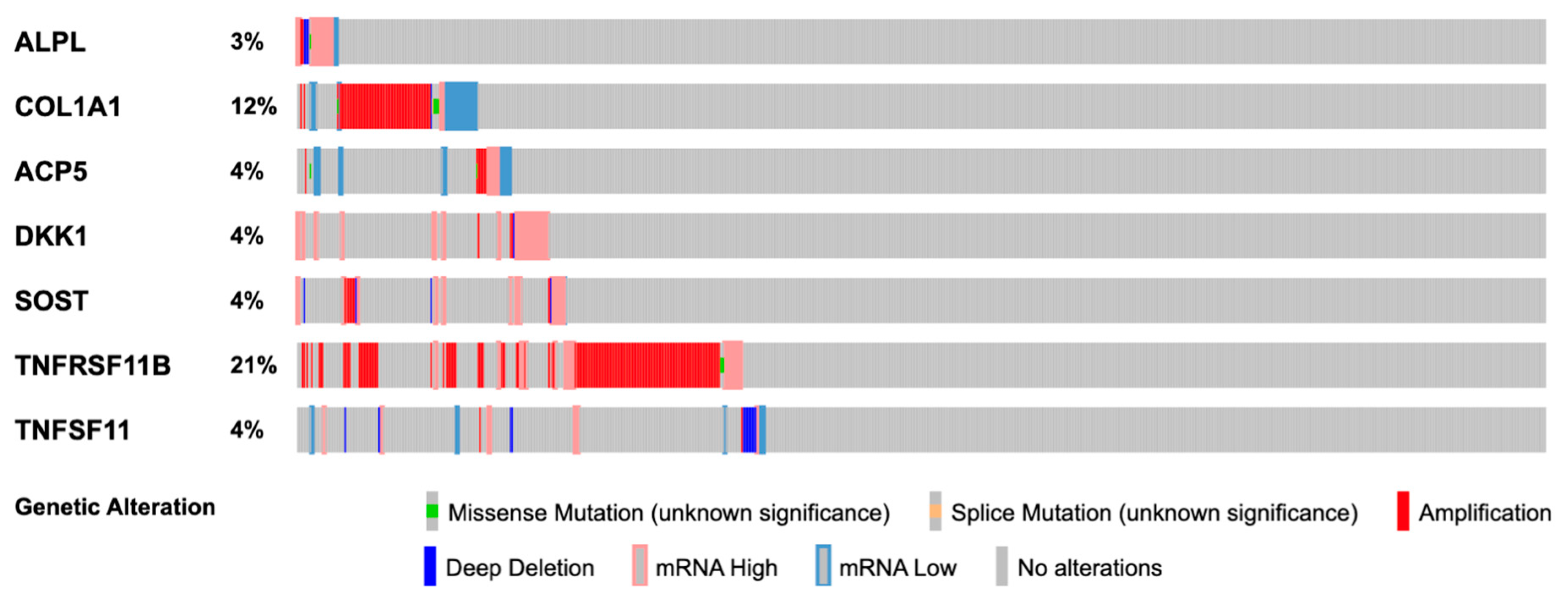
3.1. In Silico Gene Alteration Analysis
3.2. Baseline Characteristics
3.3. Subgroup Differences
3.4. Univariable Cox Proportional Hazards Model
3.5. Receiver Operating Curves (ROCs)
3.5.1. All Types of Metastases (Figure 2)
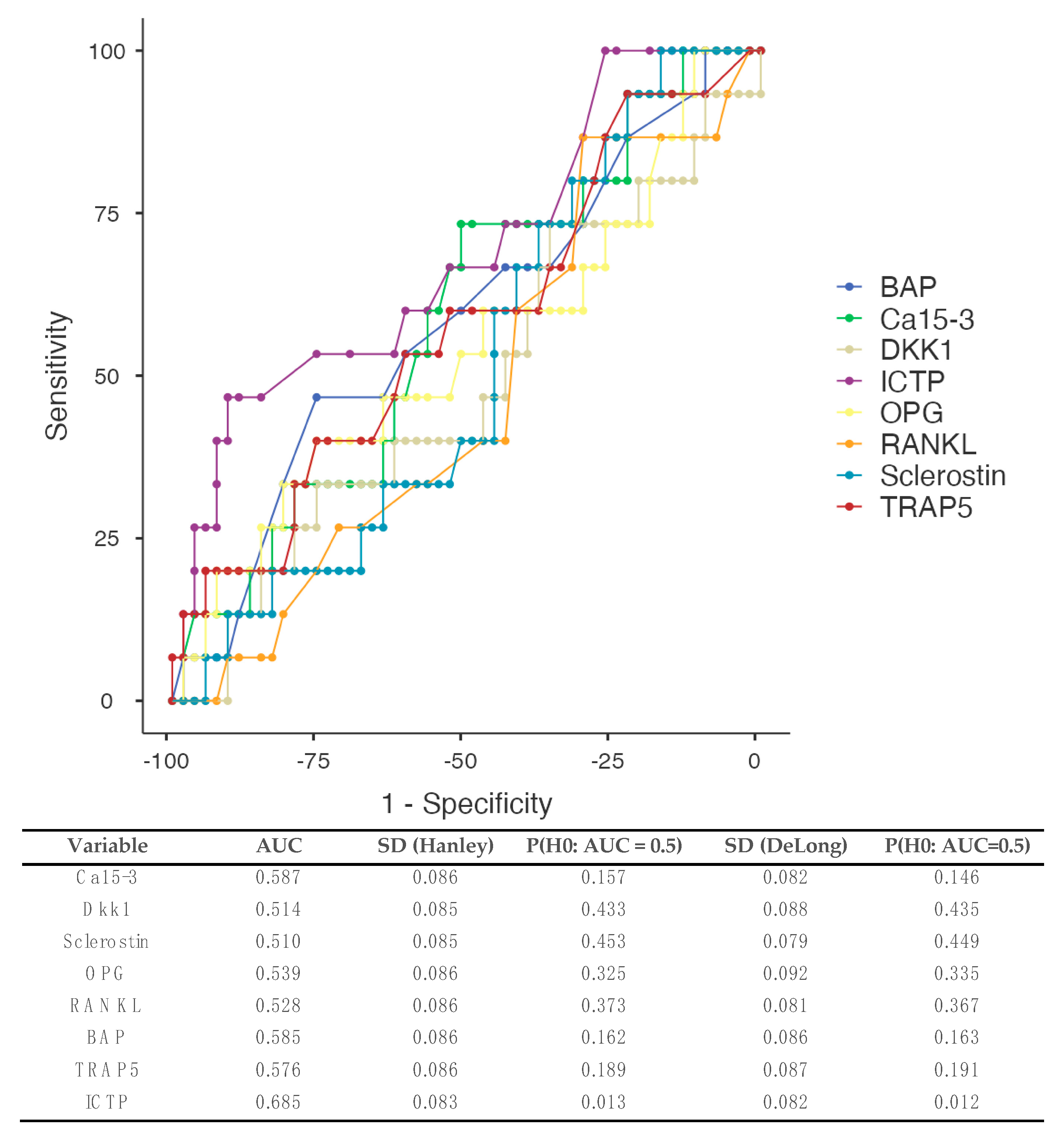
3.5.2. Patients Developing Bone Metastases (Figure 3)
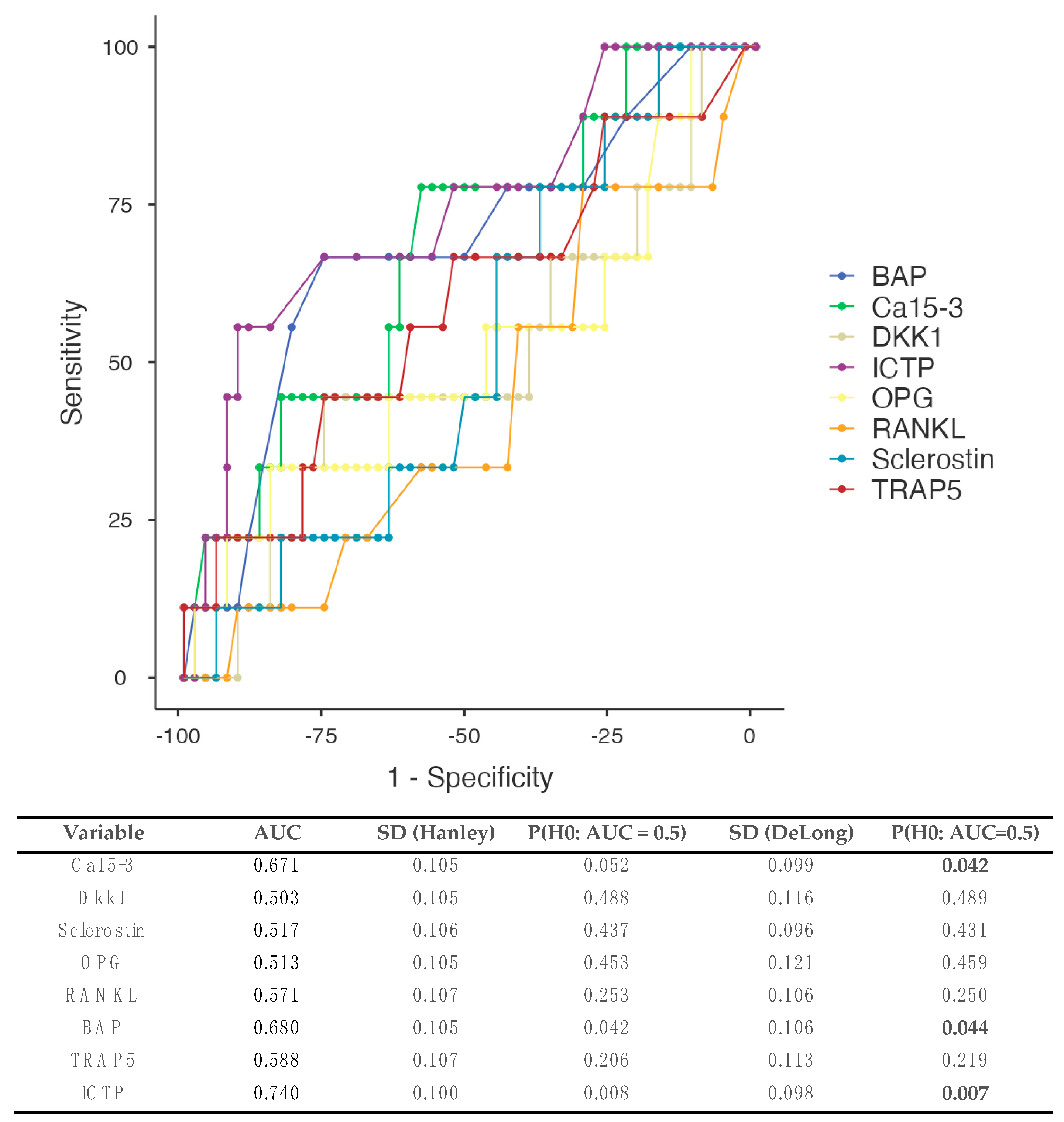
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Iuliani, M.; Simonetti, S.; Ribelli, G.; Napolitano, A.; Pantano, F.; Vincenzi, B.; Tonini, G.; Santini, D. Current and Emerging Biomarkers Predicting Bone Metastasis Development. Front. Oncol. 2020, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Mercatali, L.; La Manna, F.; Miserocchi, G.; Liverani, C.; De Vita, A.; Spadazzi, C.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ghetti, M.; et al. Tumor-Stroma Crosstalk in Bone Tissue: The Osteoclastogenic Potential of a Breast Cancer Cell Line in a Co-Culture System and the Role of EGFR Inhibition. Int. J. Mol. Sci. 2017, 18, 1655. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Ferreira, A.; Alho, I.; Casimiro, S.; Costa, L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. BoneKEy Rep. 2015, 4, 668. [Google Scholar] [CrossRef]
- Lumachi, F.; Basso, S.M.; Camozzi, V.; Tozzoli, R.; Spaziante, R.; Ermani, M. Bone turnover markers in women with early stage breast cancer who developed bone metastases. A prospective study with multivariate logistic regression analysis of accuracy. Clin. Chim. Acta 2016, 460, 227–230. [Google Scholar] [CrossRef]
- Brown, J.; Rathbone, E.; Hinsley, S.; Gregory, W.; Gossiel, F.; Marshall, H.; Burkinshaw, R.; Shulver, H.; Thandar, H.; Bertelli, G.; et al. Associations between Serum Bone Biomarkers in Early Breast Cancer and Development of Bone Metastasis: Results from the AZURE (BIG01/04) Trial. J. Natl. Cancer Inst. 2018, 110, 871–879. [Google Scholar] [CrossRef]
- Shimoda, M.; Sato, Y.; Abe, K.; Masunaga, N.; Tsukabe, M.; Yoshinami, T.; Sota, Y.; Miyake, T.; Tanei, T.; Shimazu, K. Prognostic value of serum tartrate-resistant acid phosphatase-5b for bone metastasis in patients with resectable breast cancer. Oncol. Lett. Oncol. Lett. 2024, 27, 250. [Google Scholar] [CrossRef] [PubMed]
- S3-Leitlinie Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. 2021. Available online: https://register.awmf.org/de/leitlinien/detail/032-045OL (accessed on 14 April 2024).
- Biomedica Protocol Booklet: DKK-1. Available online: https://www.bmgrp.com/wp-content/uploads/2022/05/BI-20413-DKK-1-ELISA-IFU-220524.pdf (accessed on 30 March 2024).
- Biomedica Protocol Booklet: Sclerostin. Available online: https://www.bmgrp.com/wp-content/uploads/2022/05/BI-20492-Sclerostin-ELISA-IFU-220524.pdf (accessed on 30 March 2024).
- Biomedica Protocol Booklet: Osteoprotegerin. Available online: https://www.bmgrp.com/wp-content/uploads/2023/04/BI-20403-OPG-ELISA-IFU-230323.pdf (accessed on 30 March 2024).
- Biomedica Protocol Booklet: Free soluble RANKL. Available online: https://www.bmgrp.com/wp-content/uploads/2022/05/BI-20462-free-soluble-RANKL-ELISA-IFU-220524.pdf (accessed on 30 March 2024).
- Home—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 30 March 2024).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.; Li, C. Targeting type I collagen for cancer treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.-X.; Wu, H.-T.; Li, X.-L.; Wen, X.-F.; Du, C.-W.; Zhang, G.-J. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov. Med. 2018, 25, 211–223. [Google Scholar]
- Jääskeläinen, A.; Jukkola, A.; Risteli, J.; Haapasaari, K.-M.; Karihtala, P. Elevated preoperative serum levels of collagen I carboxyterminal telopeptide predict better outcome in early-stage luminal-B-like (HER2-negative) and triple-negative subtypes of breast cancer. Tumor Biol. 2019, 41. [Google Scholar] [CrossRef]
- Imamura, M.; Nishimukai, A.; Higuchi, T.; Ozawa, H.; Yanai, A.; Miyagawa, Y.; Murase, K.; Sakita, I.; Hatada, T.; Takatsuka, Y.; et al. High levels at baseline of serum pyridinoline crosslinked carboxyterminal telopeptide of type I collagen are associated with worse prognosis for breast cancer patients. Breast Cancer Res. Treat. 2015, 154, 521–531. [Google Scholar] [CrossRef]
- Zuo, C.-T.; Yin, D.-C.; Fan, H.-X.; Lin, M.; Meng, Z.; Xin, G.-W.; Zhang, Y.-C.; Cheng, L. Study on diagnostic value of P1NP and β-CTX in bone metastasis of patients with breast cancer and the correlation between them. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5277–5284. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Lin, X.; Zhang, K.M.; Wu, Q.; Luo, M.; Zhou, J. Predictive and prognostic biomarkers of bone metastasis in breast cancer: Current status and future directions. Cell Biosci. 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Massaccesi, L.; de Benedettis, E.; Longhi, E.; de Toma, D.; Romanelli, M.M.C.; Banfi, G. Longitudinal evaluation of Wnt inhibitors and comparison with others serum osteoimmunological biomarkers in osteolytic bone metastasis. J. Leukoc. Biol. 2020, 108, 697–704. [Google Scholar] [CrossRef] [PubMed]
- D’oronzo, S.; Cives, M.; Lauricella, E.; Stucci, S.; Centonza, A.; Gentile, M.; Ostuni, C.; Porta, C. Assessment of bone turnover markers and DXA parameters to predict bone metastasis progression during zoledronate treatment: A single-center experience. Clin. Exp. Med. 2024, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Ricci, M.; Scarpi, E.; Bongiovanni, A.; Ricci, R.; Riva, N.; Liverani, C.; De Vita, A.; La Manna, F.; Oboldi, D.; et al. RANKL: A promising circulating marker for bone metastasis response. Oncol. Lett. 2016, 12, 2970–2975. [Google Scholar] [CrossRef] [PubMed]
- Dincel, A.S.; Jørgensen, N.R. New Emerging Biomarkers for Bone Disease: Sclerostin and Dickkopf-1 (DKK1). Calcif. Tissue Int. 2022, 112, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, R.I.; Zakhary, M.M.; Maximous, D.W.; A Mokhtar, A.; El Dosoky, M.I. Circulating osteocyte-related biomarkers (vitamin D, sclerostin, dickkopf-1), hepcidin, and oxidative stress markers in early breast cancer: Their impact in disease progression and outcome. J. Steroid Biochem. Mol. Biol. 2020, 204, 105773. [Google Scholar] [CrossRef] [PubMed]
- Mariz, K.; Ingolf, J.-B.; Daniel, H.; Teresa, N.J.; Erich-Franz, S. The Wnt inhibitor dickkopf-1: A link between breast cancer and bone metastases. Clin. Exp. Metastasis 2015, 32, 857–866. [Google Scholar] [CrossRef]
- Kasoha, M.; Bohle, R.M.; Seibold, A.; Gerlinger, C.; Juhasz-Böss, I.; Solomayer, E.-F. Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin. Exp. Metastasis 2018, 35, 763–775. [Google Scholar]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.C.; Lacroix-Triki, M.; Savage, K.; Arnedos, M.; Lambros, M.B.; MacKay, A.; Natrajan, R.; Reis-Filho, J.S. Β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011, 24, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Q.; Dong, D.; Ren, L. The role of TPS, CA125, CA15-3 and CEA in prediction of distant metastasis of breast cancer. Clin. Chim. Acta 2021, 523, 19–25. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value | Missing Data |
|---|---|---|
| Age (years) | 62.4 (46.1–84.2) | - |
| BMI (kg/m2) | 25.7 (18.7–32.7) | - |
| Pre-/perimenopausal | 16 (20%) | - |
| Histological subtypes | - | |
| Ductal/lobular | 59 (73.8%)/21 (26.3%) | |
| Molecular subtypes | - | |
| Luminal A | 39 (48.8%) | |
| Luminal B | 30 (37.5%) | |
| Her2 positive | 5 (6.3%) | |
| Triple negative | 6 (7.5%) | |
| T-Stage | - | |
| T1/T2/T3-4 | 50 (62.5 %)/27 (33.8 %)/3 (3.8 %) | |
| N-Stage | - | |
| N0/N1/N2-3 | 54 (67.5%)/22 (27.5%)/4 (5%) | |
| Presence of metastases | 4 (5%) | - |
| G-Stage | - | |
| G1/G2/G3 | 6 (7.5%)/62 (77.5%)/12 (15%) | |
| Ki-67 (%) | 10 (2–70) | 1 (1.3%) |
| Follow-up (months) | 79.4 (15.5–161) | |
| Ca15-3 (U/mL) | 19.3 (6.30–151) | 8 (10%) |
| Dkk1 (pg/mL) | 1249 (419–2727) | - |
| Sclerostin (pg/L) | 709 (182–3818) | - |
| OPG (pg/mL) | 84.4 (15.2–202) | - |
| RANKL (pg/mL) | 2.4 (0.4–7.6) | - |
| OPG/RANKL | 35.5 (4.84–305) | - |
| BAP (μg/L) | 16. (6.2–33.0) | - |
| TRAP5 (U/L) | 2.95 (1.5–21.0) | - |
| ICTP (μg/L) | 3.0 (1.1–17.0) | - |
| Variable | Luminal A (n = 39) | Luminal B (n = 30) | TNBC (n = 6) | Her2neu Positive (n = 5) | p-Value 1 |
|---|---|---|---|---|---|
| Age (years) | 58.7 (46.1–80.2) | 67.7 (49.6–82.7) | 66.2 (49.5–84.2) | 69.1 (51.1–77.1) | 0.03 |
| BMI (kg/m2) | 25.6 (18.7–31.9) | 26.3 (21.1–32.7) | 24.9 (19.8–27.8) | 24.7 (23.0–27.5) | 0.24 |
| Ki67 (%) | 10 (2–15) | 25 (15–50) | 40 (15–70) | 10 (10–20) | <0.001 |
| Ca15-3 (U/mL) | 17.8 (7.2–54.0) | 22.6 (7.6–37.6) | 28.5 (13.0–37.8) | 21.4 (6.3–151) | 0.43 |
| Dkk1 (pg/mL) | 1311 (419–2727) | 1151 (460–2113) | 1063 (652–1935) | 1335 (1110–1679) | 0.74 |
| Sclerostin (pg/L) | 655 (182–1834) | 761 (189–3818) | 770 (468–2043) | 784 (607–952) | 0.79 |
| OPG (pg/mL) | 86.0 (15.2–177) | 76.1 (23.4–187) | 107 (83.8–202) | 75.4 (24.0–144) | 0.11 |
| RANKL (pg/mL) | 2.4 (0.6–7.6) | 2.3 (0.4–7.0) | 3.0 (0.6–3.4) | 2.0 (0.8–4.6) | 0.98 |
| OPG/RANKL | 39.7 (4.8–177) | 35.1 (7.1–305) | 46.0 (25.2–200) | 33.2 (10.9–180) | 0.77 |
| BAP (μg/L) | 14.0 (6.2–27.0) | 17.0 (8.2–33.0) | 19.0 (12.0–32.0) | 19.0 (14.0–25.0) | 0.09 |
| TRAP5 (U/L) | 2.8 (1.5–6.0) | 3.0 (1.6–21.0) | 4.1 (1.7–6.7) | 2.9 (2.0–5.7) | 0.32 |
| ICTP (μg/L) | 2.9 (1.2–17.0) | 3.1 (1.1–10.0) | 3.4 (2.8–11.0) | 3.6 (3.4–4.3) | 0.08 |
| Variable | Ca15-3 | p | Dkk1 | p | Sclerostin | p | OPG | p | RANKL | p | OPG/RANKL | p | BAP | p | TRAP5 | p | ICTP | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.06 | 0.61 | 0.56 | 0.64 | 0.45 | 0.49 | 0.60 | 0.94 | 0.90 | |||||||||
| ≤55 years old | 17.8 (6.3–54.0) | 1254 (553–2429) | 789 (189–1434) | 83.0 (24.0–130) | 2.6 (0.6–7.0) | 28.1 (4.8–130) | 16.0 (6.2–27.0) | 3.1 (1.5–5.5) | 3.2 (1.1–4.7) | |||||||||
| >55 years old | 21.4 (7.2–151) | 1235 (419–2727) | 695 (182–3818) | 86.0 (15.2–202) | 2.4 (0.4–7.6) | 35.8 (4.8–305) | 16.0 (8.1–33.0) | 2.9 (1.6–21.0) | 3.0 (1.2–17.0) | |||||||||
| Menopausal status | 0.05 | 0.22 | 0.17 | 0.93 | 0.63 | 0.77 | 0.48 | 0.51 | 0.19 | |||||||||
| Pre-/perimenopausal | 17.8 (6.3–54.0) | 1379 (553–2429) | 819 (314–1225) | 83.4 (24.0–130) | 2.4 (0.6–7.0) | 37.1 (4.8–130) | 17.0 (8.9–23.0) | 2.8 (1.7–5.5) | 3.4 (1.5–17.0) | |||||||||
| Postmenopausal | 19.6 (7.2–151) | 1219 (419–2727) | 670 (182–3818) | 85.0 (15.2–202) | 2.4 (0.4–7.6) | 35.5 (5.1–305) | 16.0 (6.2–33.0) | 3.0 (1.5–21.0) | 3.0 (1.1–11.0) | |||||||||
| Histology | 0.66 | 0.02 | 0.09 | 0.14 | 0.41 | 0.35 | 0.29 | 0.71 | 0.005 | |||||||||
| Invasive ductal | 19.2 (7.2–54.0) | 1120 (419–2727) | 655 (182–3818) | 83.4 (15.2–202) | 2.4 (0.6–7.6) | 35.8 (4.8–233) | 16.0 (6.2–33.0) | 3.0 (1.5–6.7) | 2.9 (1.1–11.0) | |||||||||
| Invasive lobular | 22.6 (6.3–151) | 1424 (672–2565) | 751 (339–1823) | 86.2 (24.0–176) | 2.4 (0.4–4.6) | 34.8 (10.9–305) | 16.0 (8.2–33.0) | 2.8 (1.7–21.0) | 3.5 (1.8–17.0) | |||||||||
| T-Stage | 0.08 | 0.68 | 0.05 | 0.87 | 0.43 | 0.92 | 0.07 | 0.14 | 0.28 | |||||||||
| T1 | 17.8 (7.2–37.8) | 1294 (423–2727) | 652 (182–2043) | 85.0 (25.4–202) | 2.5 (0.6–7.6) | 39.4 (4.84–294) | 14.5 (6.2–33.0) | 2.9 (1.5–6.7) | 3.0 (1.1–17.0) | |||||||||
| T2 | 20.2 (6.30–151) | 1102 (419–2113) | 859 (198–3818) | 83.0 (15.2–187) | 2.0 (0.4–5.6) | 34.6 (5.07–305) | 19.0 (9.0–33.0) | 3.1 (1.6–21.0) | 3.2 (2.0–10.0) | |||||||||
| T3-4 | 30.6 (29.5–31.8) | 1341 (881–1638) | 345 (339–827) | 86.2 (46.0–103) | 3.4 (1.2–5.6) | 30.4 (8.21–71.8) | 16.0 (12.0–17.0) | 4.1 (3.6–5.8) | 3.1 (2.5–6.7) | |||||||||
| N-Stage | 0.07 | 0.87 | 0.50 | 0.65 | 0.15 | 0.29 | 0.40 | 0.60 | 0.04 | |||||||||
| N negative | 18.7 (6.3–37.8) | 1271 (423–2727) | 701 (182–2043) | 85.4 (23.4–202) | 2.5 (0.6–7.6) | 34.3 (4.8–294) | 16.0 (8.1–33.0) | 2.9 (1.5–6.7) | 2.9 (1.1–11.0) | |||||||||
| N positive | 21.4 (12.8–151) | 1208 (419–2113) | 711 (198–3818) | 83.4 (15.2–187) | 2.0 (0.4–5.6) | 35.6 (5.1–305) | 16.5 (6.2–33.0) | 3.2 (1.6–21) | 3.4 (1.9–17.0) | |||||||||
| G-Status | 0.55 | 0.36 | 0.37 | 0.62 | 0.53 | 0.41 | 0.28 | 0.76 | 0.60 | |||||||||
| G1 | 17.8 (12.3–28.7) | 1336 (423–2727) | 853 (314–1595) | 67.0 (25.4–177) | 3.1 (1.0–7.6) | 18.4 (4.8–177) | 12.5 (8.9–17.0) | 2.9 (2.1–4.2) | 3.2 (2.0–4.5) | |||||||||
| G2 | 19.2 (6.30–54.0) | 1248 (419–2565) | 748 (182–3818) | 85.0 (15.2–202) | 2.4 (0.4–7.0) | 38.4 (4.8–305) | 16.0 (6.2–33.0) | 3.0 (1.5–21.0) | 2.9 (1.1–17.0) | |||||||||
| G3 | 22.1 (8.00–151) | 935 (512–1708) | 651 (332–859) | 84.8 (23.4–120) | 2.2 (0.6–6.6) | 31.6 (10.2–200) | 19.0 (8.1–25.0) | 3.0 (1.6–5.8) | 3.3 (2.4–4.5) | |||||||||
| Development of metastases 1 | 0.31 | 0.65 | 0.98 | 0.76 | 0.82 | 0.86 | 0.30 | 0.15 | 0.053 | |||||||||
| No metastases | 18.1 (6.3–54.0) | 1308 (423–2727) | 718 (182–3818) | 85.0 (23.4–202) | 2.4 (0.4–7.6) | 35.8 (4.8–305) | 16.0 (6.2–33.0) | 2.9 (1.5–6.7) | 2.9 (1.1–17.0) | |||||||||
| Developed metastases | 20.2 (10.7–38.9) | 1181 (419–2016) | 709 (225–1823) | 85.8 (15.2–187) | 2.0 (0.6–5.6) | 37.6 (5.1–233) | 17.0 (9.9–33.0) | 3.4 (1.7–21.0) | 3.20 (2.4–10.0) | |||||||||
| Development of bone metastases 2 | 0.08 | 0.74 | 0.61 | 1.00 | 0.23 | 0.42 | 0.07 | 0.89 | 0.44 | |||||||||
| Bone | 18.9 (10.7–28.5) | 1173 (419–1935) | 681 (225–1430) | 94.5 (15.2–135) | 2.7 (1.6–4.0) | 31.3 (5.1–61.6) | 13.5 (9.9–20.0) | 3.3 (2.3–6.0) | 3.1 (2.4–6.8) | |||||||||
| Other than bone | 22.9 (13.0–38.9) | 1181 (652–2016) | 714 (345–1823) | 83.8 (46.0–187) | 2.0 (0.6–5.6) | 39.1 (8.2–233) | 21.0 (11.0–33.0) | 3.4 (1.7–21.0) | 3.9 (2.4–10) | |||||||||
| Variable | Progression | Death | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Histology | ||||
| Ductal | - | - | - | - |
| Lobular | 0.68 (0.22–2.07) | p = 0.50 | 0.94 (0.33–2.70) | p = 0.91 |
| T-Stage | ||||
| T1 | - | - | - | - |
| T2 | 2.47 (0.95–6.41) | p = 0.06 | 2.20 (0.80–6.08) | p = 0.13 |
| T3–4 | 6.61 (1.39–31.37) | p = 0.02 | 5.66 (1.17–27.33) | p = 0.03 |
| N-Stage | ||||
| N negative | - | - | - | - |
| N positive | 4.39 (1.73–11.16) | p = 0.002 | 2.60 (1.00–6.73) | p = 0.05 |
| Ki67 | ||||
| Ki67 < 15% | - | - | - | - |
| Ki67 ≥ 15% | 3.00 (1.11–8.09) | p = 0.03 | 3.06 (1.06–8.87) | p = 0.04 |
| Ca15-3 (median 26.4 U/mL) | ||||
| <26.4 U/mL | - | - | - | - |
| ≥26.4 U/mL | 1.27 (0.45–3.62) | p = 0.65 | 1.38 (0.47–4.04) | p = 0.56 |
| Dkk1 (median 1249 pg/mL) | ||||
| <1249 pg/mL | - | - | - | - |
| ≥1249 pg/mL | 0.58 (0.23–1.47) | p = 0.25 | 0.74 (0.28–1.95) | p = 0.54 |
| Sclerostin (median 709 pg/L) | ||||
| <709 pg/L | - | - | ||
| ≥709 pg/L | 0.80 (0.32–1.97) | p = 0.63 | 1.43 (0.54–3.76) | p = 0.47 |
| OPG (median 84.4 pg/mL) | ||||
| <84.4 pg/L | - | - | - | - |
| ≥84.4 pg/L | 1.28 (0.51–3.18) | p = 0.60 | 1.48 (0.56–3.90) | p = 0.43 |
| RANKL (median 2.4 pg/mL) | ||||
| <2.4 pg/mL | - | - | - | - |
| ≥2.4 pg/mL | 1.11 (0.44–2.82) | p = 0.83 | 1.31 (0.46–3.72) | p = 0.62 |
| BAP (median 16 μg/L) | ||||
| <16 μg/L | - | - | - | - |
| ≥16 μg/L | 1.14 (0.46–2.82) | p = 0.79 | 0.90 (0.35–2.34) | p = 0.83 |
| TRAP5 (median 2.95 U/L) | ||||
| <2.95 U/L | - | - | - | - |
| ≥2.95 U/L | 1.59 (0.62–4.03) | p = 0.33 | 1.42 (0.54–3.73) | p = 0.48 |
| ICTP (median 3.0 μg/L) | ||||
| <3.0 μg/L | - | - | - | - |
| ≥3.0 μg/L | 2.02 (0.77–5.31) | 0.15 | 1.58 (0.58–4.27) | p = 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasoha, M.; Findeklee, S.; Nigdelis, M.P.; Schmidt, G.; Solomayer, E.-F.; Haj Hamoud, B. Retrospective Evaluation of Bone Turnover Markers in Serum for the Prediction of Metastases Development in Breast Cancer Patients: A Cohort Study. Biomedicines 2024, 12, 1201. https://doi.org/10.3390/biomedicines12061201
Kasoha M, Findeklee S, Nigdelis MP, Schmidt G, Solomayer E-F, Haj Hamoud B. Retrospective Evaluation of Bone Turnover Markers in Serum for the Prediction of Metastases Development in Breast Cancer Patients: A Cohort Study. Biomedicines. 2024; 12(6):1201. https://doi.org/10.3390/biomedicines12061201
Chicago/Turabian StyleKasoha, Mariz, Sebastian Findeklee, Meletios P. Nigdelis, Gilda Schmidt, Erich-Franz Solomayer, and Bashar Haj Hamoud. 2024. "Retrospective Evaluation of Bone Turnover Markers in Serum for the Prediction of Metastases Development in Breast Cancer Patients: A Cohort Study" Biomedicines 12, no. 6: 1201. https://doi.org/10.3390/biomedicines12061201
APA StyleKasoha, M., Findeklee, S., Nigdelis, M. P., Schmidt, G., Solomayer, E.-F., & Haj Hamoud, B. (2024). Retrospective Evaluation of Bone Turnover Markers in Serum for the Prediction of Metastases Development in Breast Cancer Patients: A Cohort Study. Biomedicines, 12(6), 1201. https://doi.org/10.3390/biomedicines12061201






