Hemoglobin and Its Relationship with Fatigue in Long-COVID Patients Three to Six Months after SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Setting and Participants
2.2. Ethical Considerations
2.3. Variables in This Study
2.4. Statistical Analysis
3. Results
3.1. Hemoglobin Levels
3.2. Correlations between Hemoglobin and Inflammatory Biomarkers
3.3. Associations between Hemoglobin Levels and Fatigue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Sperling, S.; Bendstrup, E. A new paradigm is needed to explain long COVID. The Lancet. Respir. Med. 2023, 11, e12–e13. [Google Scholar]
- Wahlgren, C.; Forsberg, G.; Divanoglou, A.; Balkhed Å, Ö.; Niward, K.; Berg, S.; Levi, R. Two-year follow-up of patients with post-COVID-19 condition in Sweden: A prospective cohort study. Lancet Reg. Health–Eur. 2023, 28, 100595. [Google Scholar] [CrossRef] [PubMed]
- COVID, Gemelli Against, and Post-Acute Care Study Group. Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin. Exp. Res. 2020, 1, 1613–1620. [Google Scholar]
- NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 26, 374. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; Goërtz, Y.M.; Van Herck, M.; Machado, F.V.; Meys, R.; Delbressine, J.M.; Houben-Wilke, S.; Gaffron, S.; Maier, D.; Burtin, C.; et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021, 7, 00141-2021. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, N.; Maddox, T.; Humberstone, B.; Diamond, S.I.; Banerjee, A. Epidemiology of post-COVID syndrome following hospitalisation with coronavirus: A retrospective cohort study. MedRxiv 2021. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Montani, D.; Savale, L.; Noel, N.; Meyrignac, O.; Colle, R.; Gasnier, M.; Corruble, E.; Beurnier, A.; Jutant, E.M.; Pham, T.; et al. Post-acute COVID-19 syndrome. Eur. Respir. Rev. 2022, 31, 163. [Google Scholar] [CrossRef]
- Wong, J.J.M.; Abbas, Q.; Liauw, F.; Malisie, R.F.; Gan, C.S.; Abid, M.; Efar, P.; Gloriana, J.; Chuah, S.L.; Sultana, R.; et al. Development and validation of a clinical predictive model for severe and critical pediatric COVID-19 infection. PLoS ONE 2022, 17, e0275761. [Google Scholar] [CrossRef]
- Anai, M.; Akaike, K.; Iwagoe, H.; Akasaka, T.; Higuchi, T.; Miyazaki, A.; Naito, D.; Tajima, Y.; Takahashi, H.; Komatsu, T.; et al. Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID-19 patients with pneumonia. Respir. Investig. 2021, 59, 187–193. [Google Scholar] [CrossRef]
- Russo, A.; Tellone, E.; Barreca, D.; Ficarra, S.; Laganà, G. Implication of COVID-19 on Erythrocytes Functionality: Red Blood Cell Biochemical Implications and Morpho-Functional Aspects. Int. J. Mol. Sci. 2022, 23, 2171. [Google Scholar] [CrossRef]
- Lopes, D.V.; Neto, F.L.; Marques, L.C.; Lima, R.B.; Brandão, A.A.G.S. Methemoglobinemia and hemolytic anemia after COVID-19 infection without identifiable eliciting drug: A case-report. IDCases 2021, 23, e01013. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Restin, T.; Ferrari, M.; Quaresima, V. The role of methemoglobin and carboxyhemoglobin in COVID-19: A review. J. Clin. Med. 2020, 10, 50. [Google Scholar] [CrossRef]
- Lanser, L.; Burkert, F.R.; Bellmann-Weiler, R.; Schroll, A.; Wildner, S.; Fritsche, G.; Weiss, G. Dynamics in anemia development and dysregulation of iron homeostasis in hospitalized patients with COVID-19. Metabolites 2021, 11, 653. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Wöll, E.; Weiss, G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Parminder, S.S. Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Irene, M. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Vieth, J.T.; Lane, D.R. Anemia. Emerg. Med. Clin. 2014, 32, 613–628. [Google Scholar] [CrossRef]
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, pathophysiology, and treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller VB, B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and post-infective fatigue syndrome: A review. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021; Volume 8. [Google Scholar]
- Maisel, P.; Erika, B.; Norbert, D.-B. Fatigue as the Chief Complaint: Epidemiology, Causes, Diagnosis, and Treatment. Dtsch. Ärzteblatt Int. 2021, 118, 566. [Google Scholar]
- Pasini, E.; Corsetti, G.; Romano, C.; Scarabelli, T.M.; Chen-Scarabelli, C.; Saravolatz, L.; Dioguardi, F.S. Serum metabolic profile in patients with long-Covid (PASC) syndrome: Clinical implications. Front. Med. 2021, 1182, 714426. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Ryan-Murua, P.; Rodríguez-Jiménez, J.; Palacios-Ceña, M.; Arendt-Nielsen, L.; Torres-Macho, J. Serological biomarkers at hospital admission are not related to long-term post-COVID fatigue and dyspnea in COVID-19 survivors. Respiration 2022, 101, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Baalbaki, N.; Blankestijn, J.M.; Abdel-Aziz, M.I.; de Backer, J.; Bazdar, S.; Beekers, I.; Beijers, R.J.H.C.G.; Bergh, J.P.v.D.; Bloemsma, L.D.; Bogaard, H.J.; et al. Precision Medicine for More Oxygen (P4O2)—Study Design and First Results of the Long COVID-19 Extension. J. Pers. Med. 2023, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Nielsen, B.B.; Hvidman, L.; Nielsen, J.; Aaby, P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: Randomised double blind clinical trial. BMJ 2005, 331, 723. [Google Scholar]
- Atukunda, E.C.; Mugyenyi, G.R.; Obua, C.; Atuhumuza, E.B.; Musinguzi, N.; Tornes, Y.F.; Agaba, A.G.; Siedner, M.J. Measuring post-partum haemorrhage in low-resource settings: The diagnostic validity of weighed blood loss versus quantitative changes in hemoglobin. PLoS ONE 2016, 11, e0152408. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.F.; Wang, E.; Romanos, A.; O’Rourke, K.; Srinivas, S.K. Implementation of quantification of blood loss does not improve prediction of hemoglobin drop in deliveries with average blood loss. Am. J. Perinatol. 2018, 35, 134–139. [Google Scholar] [CrossRef]
- Jerković, A.; Proroković, A.; Matijaca, M.; Katić, A.Ć.; Košta, V.; Mihalj, M.; Dolić, K.; Đogaš, Z.; Vidaković, M.R. Validation of the fatigue severity scale in Croatian population of patients with multiple sclerosis disease: Factor structure, internal consistency, and correlates. Mult. Scler. Relat. Disord. 2022, 58, 103397. [Google Scholar] [CrossRef]
- Lerdal, A. Fatigue severity scale. Encyclopedia of Quality of Life and Well-Being Research; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–5. [Google Scholar]
- Samprathi, M.; Muralidharan, J. Biomarkers in COVID-19: An up-to-date review. Front. Pediatr. 2021, 8, 607647. [Google Scholar] [CrossRef]
- Budhiraja, S.; Indrayan, A.; Das, P.; Dewan, A.; Singh, O.; Nangia, V.; Singh, Y.P.; Pandey, R.; Gupta, A.K.; Gupta, M.; et al. Relative Importance of Various Inflammatory Markers and Their Critical Thresholds for COVID-19 Mortality. medRxiv 2021. [Google Scholar] [CrossRef]
- Alisik, M.; Erdogan, U.G.; Ates, M.; Sert, M.A.; Yis, O.M.; Bugdayci, G. Predictive value of immature granulocyte count and other inflammatory parameters for disease severity in COVID-19 patients. Int. J. Med. Biochem. 2021, 4, 143–149. [Google Scholar]
- Li, J.; Ji, L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 2005, 95, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-H.; Lu, Z.-S. Factors affecting the effective number of tests in genetic association studies: A comparative study of three PCA-based methods. J. Hum. 2011, 56, 6. [Google Scholar] [CrossRef]
- Textor, J.; Van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The influence of inflammation on anemia in CKD patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, G.; Kim, E.; Park, J.; Isobe, T.; Sakae, T.; Oh, S. The a body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int. J. Environ. Res. Public Health 2021, 18, 12874. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. eGFR Calculator. Available online: https://www.kidney.org/professionals/kdoqi/gfr_calculator (accessed on 27 November 2023).
- Abu-Ismail, L.; Taha MJ, J.; Abuawwad, M.T.; Al-Bustanji, Y.; Al-Shami, K.; Nashwan, A.; Yassin, M. COVID-19 and anemia: What do we know so far? Hemoglobin 2023, 47, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Carlos, M.M.; Salvatore, G.D.-S. Hematological alterations associated with long COVID-19. Front. Physiol. 2023, 14, 1203472. [Google Scholar] [CrossRef]
- Sonnweber, T.; Grubwieser, P.; Sahanic, S.; Böhm, A.K.; Pizzini, A.; Luger, A.; Schwabl, C.; Koppelstätter, S.; Kurz, K.; Puchner, B.; et al. The impact of iron dyshomeostasis and anaemia on long-term pulmonary recovery and persisting symptom burden after COVID-19: A prospective observational cohort study. Metabolites 2022, 12, 546. [Google Scholar] [CrossRef]
- Sobrero, A.; Puglisi, F.; Grossi, F. Fatigue: A main component of anemia symptomatology. In Seminars in Oncology; WB Saunders: Philadelphia, PE, USA, 2001; Volume 28. [Google Scholar]
- Prochaska, M.T.; Zhang, H.; Alavi, C.; Meltzer, D.O. Fatigability: A new perspective on and patient-centered outcome measure for patients with anemia. Am. J. Hematol. 2020, 95, E166. [Google Scholar] [CrossRef]
- Brownstein, C.G.; Daguenet, E.; Guyotat, D.; Millet, G.Y. Chronic fatigue in myelodysplastic syndromes: Looking beyond anemia. Crit. Rev. Oncol./Hematol. 2020, 154, 103067. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Hong, S.; Nelesen, R.; Dimsdale, J.E. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch. Intern. Med. 2005, 165, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Resnick, H.E.; Carter, E.A.; Aloia, M.; Phillips, B. Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: Results from the third national health and nutrition examination survey. J. Clin. Sleep Med. 2006, 2, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Dary, O.; Jefferds, M.E.; Pasricha, S.R. Diagnosing anemia: Challenges selecting methods, addressing underlying causes, and implementing actions at the public health level. Ann. N. Y. Acad. Sci. 2023, 1524, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tomas, G. Anemia of inflammation. Hematol./Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.L.; Mulè, M.P.; Ruffieux, H.; Mescia, F.; Bergamaschi, L.; Pelly, V.S.; Turner, L.; Kotagiri, P.; Göttgens, B.; Hess, C.; et al. Iron dysregulation and inflammatory stress erythropoiesis associates with long-term outcome of COVID-19. Nat. Immunol. 2024, 25, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Kaushik, A.; Kujawska, M.; Batiha, G.E.-S. Hemolytic anemia in COVID-19. Ann. Hematol. 2022, 101, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Onoja, A.; von Gerichten, J.; Lewis, H.-M.; Bailey, M.J.; Skene, D.J.; Geifman, N.; Spick, M. Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers. Int. J. Mol. Sci. 2023, 24, 14371. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtseva, N.V.; Ananyan, M.A. Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease 2019 (COVID-19) and its complications. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8585–8591. [Google Scholar]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The Influence of Oxidative Stress and Natural Antioxidants on Morphometric Parameters of Red Blood Cells, the Hemoglobin Oxygen Binding Capacity, and the Activity of Antioxidant Enzymes. BioMed Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef]
- Chudow, M.; Beatrice, A. ABC’s of vitamin supplementation in critical illness. J. Pharm. Pract. 2021, 34, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Atanasovska, E.; Petrusevska, M.; Zendelovska, D.; Spasovska, K.; Stevanovikj, M.; Kasapinova, K.; Gjorgjievska, K.; Labachevski, N. Vitamin D levels and oxidative stress markers in patients hospitalized with COVID-19. Redox Rep. 2021, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: A cohort study. Lancet Infect. Dis. 2024, 24, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, M.H.; Choi, M.G.; Park, J.H.; Chun, E.M. Hematologic abnormalities after COVID-19 vaccination: A large Korean population-based cohort study. medRxiv 2023. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
 exposure,
exposure,  outcome,
outcome,  ancestor of outcome,
ancestor of outcome,  ancestor of exposure and outcome,
ancestor of exposure and outcome,  (potentially) causal path,
(potentially) causal path,  biasing path.
biasing path.
 exposure,
exposure,  outcome,
outcome,  ancestor of outcome,
ancestor of outcome,  ancestor of exposure and outcome,
ancestor of exposure and outcome,  (potentially) causal path,
(potentially) causal path,  biasing path.
biasing path.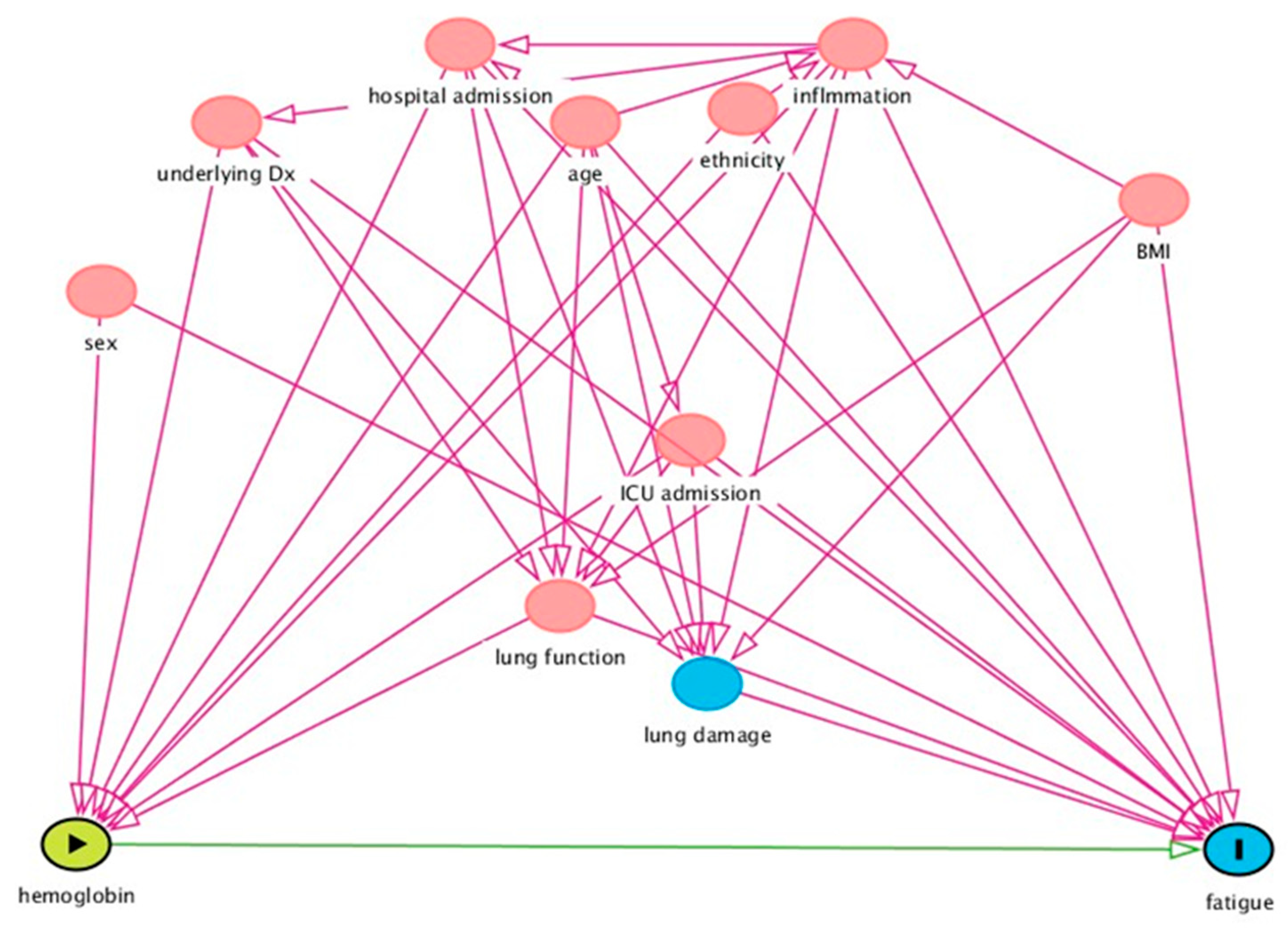

| Population Characteristics | Total (N = 95) | p-Value £ | Female (N = 47) | Male (N = 48) | ||
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (mean, SD) | 54.2 (6.2) | 0.765 | 54.3 (6.1) | 54.0 (6.3) | ||
| Ethnicity (N, %) | African | 8 (9.4%) | 0.077 | 5 (1.6%) | 3 (7.0%) | |
| Asian | 3 (3.5%) | 2 (4.7%) | 1 (2.3%) | |||
| Caucasian | 66 (76.7%) | 33 (76.7%) | 33 (76.7%) | |||
| Latin-American | 3 (3.5%) | 3 (7.0%) | 0 (0.0%) | |||
| Others | 6 (7.0%) | 0 (0.0%) | 6 (14.0%) | |||
| Clinical characteristics | ||||||
| Suffering from any comorbidities (N, %) | 85 (89.5%) | 0.170 | 40 (85.1%) | 45 (93.8%) | ||
| BMI (mean, SD) | 30.39 (5.3) | 0.002 | 32.15 (5.4%) | 28.85 (4.7) | ||
| Dominant SARS-CoV-2 variant at the time of infection (N, %) | Alpha | 43 (45.3%) | 0.278 | 23 (48.9%) | 20 (41.7%) | |
| Delta | 41 (43.2%) | 21 (44.7%) | 20 (41.7%) | |||
| Omicron | 11 (11.6%) | 3 (6.4%) | 8 (16.7%) | |||
| Hospitalized (N, %) | 85 (89.5%) | 0.170 | 40 (85.1%) | 45 (93.8%) | ||
| Hospital stay (days) (median, percentile 25–75) * | 8.00 (4.0–15.2) | 0.083 | 8.00 (3.0–13.0) | 10.00 (5.0–21.0) | ||
| ICU admission (N, %) | 27 (28.7%) | 0.40 | 9 (19.1%) | 18 (38.3%) | ||
| ICU stay (days) (median, percentile 25–75) * | 0.00 (0.0–4.0) | 0.044 | 0.00 (0.0–0.0) | 0.00 (0.0–8.0) | ||
| One dosage of SARS-CoV-2 vaccination | 66 (69.5%) | 0.771 | 32 (68.1%) | 34 (70.8%) | ||
| Two dosages of SARS-CoV-2 vaccination | 41 (43.2%) | 0.261 | 23 (48.9%) | 18 (37.5%) | ||
| WHO severity index (N, %) | Mild | 10 (10.8%) | 0.240 | 7 (15.2%) | 3 (6.4%) | |
| Moderate | 57 (61.3%) | 30 (65.2%) | 27 (57.4%) | |||
| Severe | 26 (28.0%) | 9 (19.6%) | 17 (36.2%) | |||
| Average FSS during the 3–6 months visit (median, percentile 25–75) ¥ | 5.6 (4.1–6.3) | 0.026 | 5.8 (4.7–6.4) | 5.3 (3.0–6.3) | ||
| Fatigue (Average FSS ≥ 4) during the 3–6 months visit (N, %) ¥ | 0.116 | |||||
| Yes | 66 (69.5%) | 35 (74.5%) | 31 (68.9%) | |||
| No | 21 (22.1%) | 7 (14.9%) | 14 (29.2%) | |||
| Missing | 8 (8.4%) | 5 (10.6%) | 3 (63%) | |||
| Hb and Anemia Status | Total | Female | Male | p-Value | Experiencing Fatigue 3–6 Months after Infection ¶ | No Fatigue 3–6 Months after Infection | p-Value π | |
|---|---|---|---|---|---|---|---|---|
| Total number of participants | 95 | 47 | 48 | --- | 66 | 21 | --- | |
| Hb ◊ level during the acute phase (mmol/L) (mean, SD) (n = 84) | 8.61 (0.99) | 8.35 (0.86) | 8.84 (1.05) | 0.023 | 8.53 (0.99) | 8.89 (1.06) | 0.171 | |
| Hb level during the 3–6 months visit (mmol/L) (mean, SD) (n = 68) | 8.70 (0.98) | 8.36 (0.87) | 9.04 (1.00) | 0.004 | 8.54 (0.99) | 9.24 (0.99) | 0.046 | |
| Hb level change (mmol/L) (mean, SD) ¥ (n = 59) | 0.30 (0.74) | 0.18 (0.80) | 0.42 (0.67) | 0.222 | 0.19 (0.73) | 0.68 (0.69) | 0.069 | |
| Clinically notable (10%) Hb change (N, %) | Hb decrease ‡ | 8 (8.4%) | 4 (10.50%) | 4 (9.50%) | 0.881 | 6 (10.90%) | 1 (5.90%) | 1.000 |
| Hb increase Ⱡ | 15 (23.7%) | 6 (15.80%) | 9 (21.40%) | 0.519 | 10 (18.20%) | 3 (17.6%) | 1.000 | |
| Hb level change µ (N, %) | Hb decrease * | 17 (28.8%) | 10 (34.50%) | 7 (23.30%) | 0.344 | 14 (32.20%) | 1 (10.00%) | 0.249 |
| Hb increase € | 39 (66.1%) | 19 (65.50%) | 20 (66.70%) | 0.926 | 26 (60.50%) | 9 (90.00%) | 0.137 | |
| Anemia during the acute phase (N, %) £ (n = 84) | 19 (22.61%) | 5 (12.80%) | 14 (31.10%) | 0.046 | 13 (23.2%) | 4 (20%) | 1.000 | |
| Anemia during the 3–6 months visit (N, %) £ (n = 68) | 11 (16.41%) | 5 (13.90%) | 6 (18.80%) | 0.587 | 10 (19.2%) | 1 (10%) | 0.674 | |
| Variable | Median (Percentile 25–75) | cc * with Hb Change | N Ⱡ | cc with Baseline Hb | N | cc with FU Hb | N |
|---|---|---|---|---|---|---|---|
| Baseline CRP | 91.00 (31.40–152.00) | 0.031 | 59 | 0.018 | 84 | −0.012 | 50 |
| FU CRP | 2.40 (1.85–6.30) | −0.002 | 49 | NA ‡ | 50 | −0.265 | 54 |
| Baseline LDH | 365.00 (272.00–516.00) | 0.082 | 46 | 0.012 | 64 | 0.089 | 46 |
| FU LDH | 200.00 (170.00–244.75) | 0.002 | 51 | NA | 51 | −0.126 | 55 |
| Baseline IG | 0.03 (0.01–0.08) | 0.072 | 30 | 0.108 | 36 | 0.331 | 30 |
| FU IG | 0.02 (0.01–0.03) | 0.075 | 25 | NA | 25 | −0.006 | 28 |
| Baseline CPK | 102.00 (57.00–298.00) | 0.070 | 34 | 0.053 | 52 | −0.003 | 34 |
| FU CPK | 72.00 (66.00–100.00) | 0.033 | 31 | NA | 31 | 0.084 | 34 |
| Baseline NLR | 5.05 (3.07–7.14) | 0.242 | 46 | −0.071 | 55 | 0.133 | 46 |
| FU NLR | 1.63 (1.29–2.13) | 0.294 | 36 | NA | 45 | −0.018 | 49 |
| Baseline D-dimer | 0.75 (0.54–2.20) | 0.206 | 55 | −0.093 | 80 | −0.030 | 55 |
| Unadjusted | Adjusted * | |
|---|---|---|
| OR ‡ (95% CI) | OR (95% CI) | |
| Baseline Hb levels | 0.676 (0.386–1.184) | 0.760 (0.430–1.342) |
| Follow-up Hb levels | 0.370 (0.141–0.969) | 0.350 (0.120–1.015) |
| Hb change | 0.380 (0.133–1.081) | 0.377 (0.130–1.091) |
| Baseline anemia | 1.209 (0.343–4.259) | 1.557 (0.420–5.779) |
| Follow-up anemia | 2.143 (0.243–18.919) | 2.246 (0.250–20.203) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazdar, S.; Bloemsma, L.D.; Baalbaki, N.; Blankestijn, J.M.; Cornelissen, M.E.B.; Beijers, R.J.H.C.G.; Sondermeijer, B.M.; van Wijck, Y.; Downward, G.S.; Maitland-van der Zee, A.H., on behalf of the P4O2 Consortium. Hemoglobin and Its Relationship with Fatigue in Long-COVID Patients Three to Six Months after SARS-CoV-2 Infection. Biomedicines 2024, 12, 1234. https://doi.org/10.3390/biomedicines12061234
Bazdar S, Bloemsma LD, Baalbaki N, Blankestijn JM, Cornelissen MEB, Beijers RJHCG, Sondermeijer BM, van Wijck Y, Downward GS, Maitland-van der Zee AH on behalf of the P4O2 Consortium. Hemoglobin and Its Relationship with Fatigue in Long-COVID Patients Three to Six Months after SARS-CoV-2 Infection. Biomedicines. 2024; 12(6):1234. https://doi.org/10.3390/biomedicines12061234
Chicago/Turabian StyleBazdar, Somayeh, Lizan D. Bloemsma, Nadia Baalbaki, Jelle M. Blankestijn, Merel E. B. Cornelissen, Rosanne J. H. C. G. Beijers, Brigitte M. Sondermeijer, Yolanda van Wijck, George S. Downward, and Anke H. Maitland-van der Zee on behalf of the P4O2 Consortium. 2024. "Hemoglobin and Its Relationship with Fatigue in Long-COVID Patients Three to Six Months after SARS-CoV-2 Infection" Biomedicines 12, no. 6: 1234. https://doi.org/10.3390/biomedicines12061234





