First-Trimester Plasmatic microRNAs Are Associated with Fasting Glucose Levels in Late Second Trimester of Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. RNA Extraction and Library Preparation
2.3. Library Quality Control and Sequencing
2.4. Bioinformatics Analysis
2.5. Statistical Analysis
2.6. KEGG Pathway Analysis
3. Results
3.1. Participants’ Description
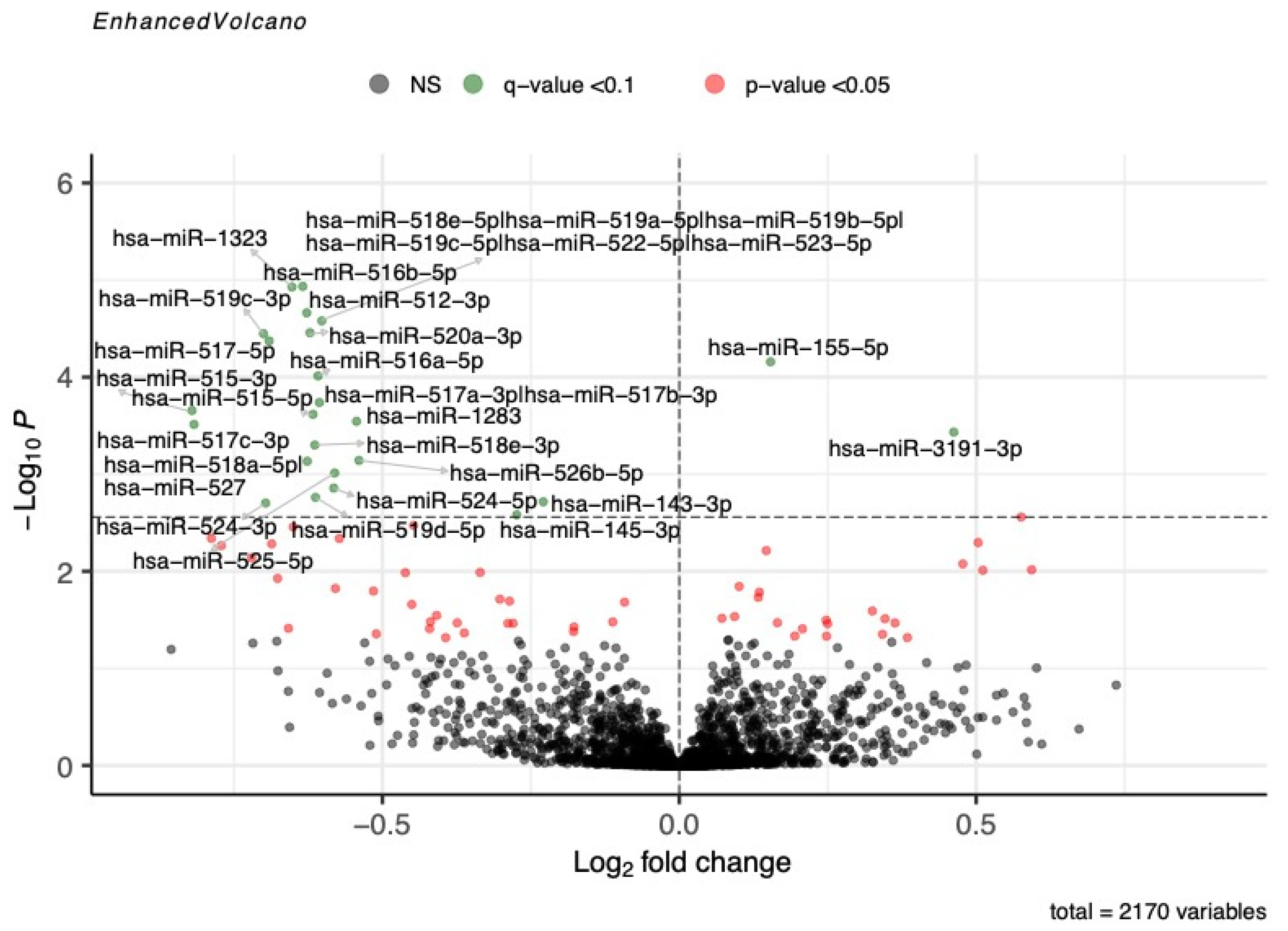
3.2. Associations between miRNA Levels and Fasting Blood Glucose during OGTT
3.3. Associations between miRNA Levels and 1 h and 2 h Post-OGTT Glucose Levels
3.4. Pathway Analysis of miRNA Associated with Fasting Glucose
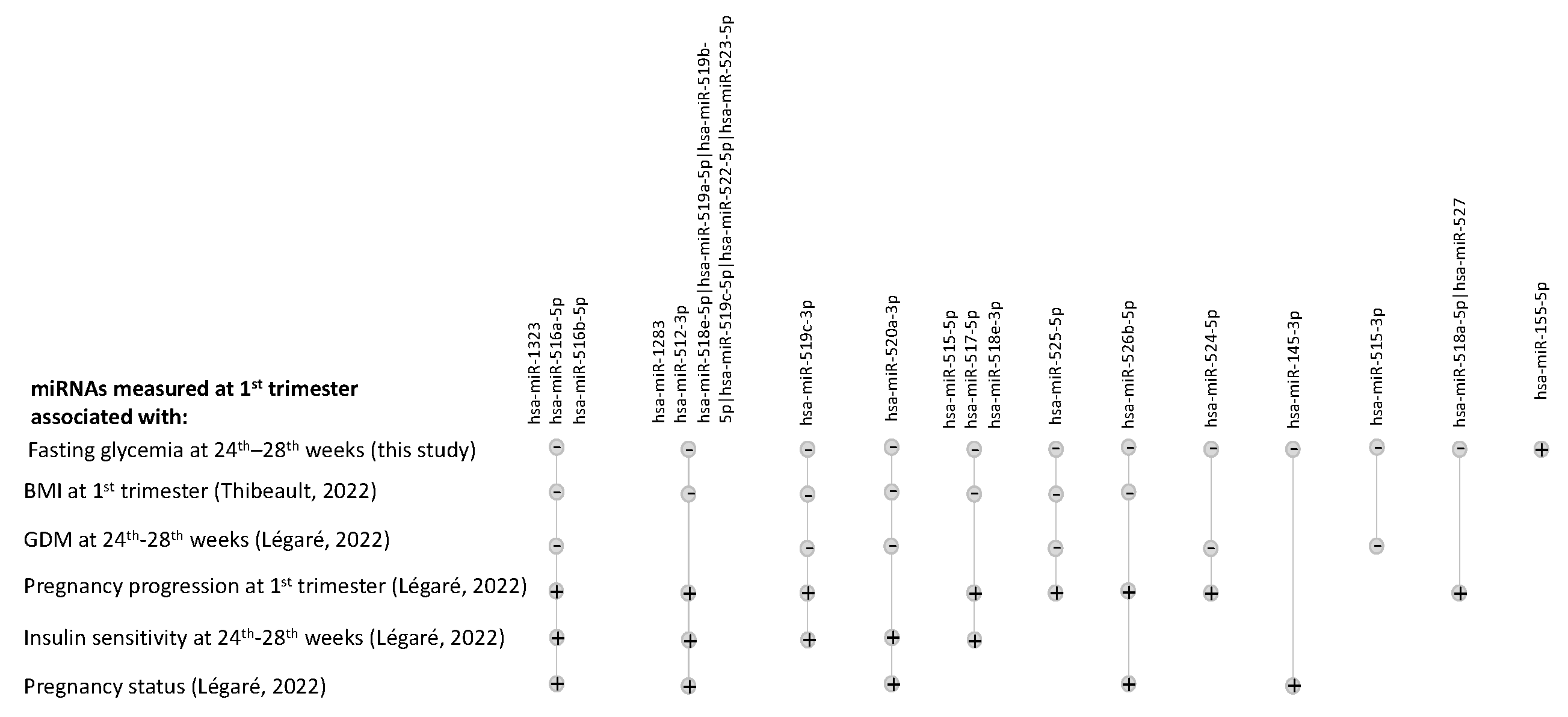
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes: The HAPO Study Cooperative Research Group. Obstet. Gynecol. Surv. 2008, 63, 615–616. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Poirier, C.; Desgagné, V.; Guérin, R.; Bouchard, L. MicroRNAs in Pregnancy and Gestational Diabetes Mellitus: Emerging Role in Maternal Metabolic Regulation. Curr. Diabetes Rep. 2017, 17, 35. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Ospina-Prieto, S.; Chaiwangyen, W.; Schoenleben, M.; Markert, U.R. Pregnancy-Associated miRNA-Clusters. J. Reprod. Immunol. 2013, 97, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A.; et al. Human Villous Trophoblasts Express and Secrete Placenta-Specific MicroRNAs into Maternal Circulation via Exosomes1. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Desgagné, V.; Poirier, C.; Thibeault, K.; White, F.; Clément, A.-A.; Scott, M.S.; Jacques, P.-É.; Perron, P.; Guérin, R.; et al. First Trimester Plasma microRNAs Levels Predict Matsuda Index-Estimated Insulin Sensitivity between 24th and 29th Week of Pregnancy. BMJ Open Diabetes Res. Care 2022, 10, e002703. [Google Scholar] [CrossRef]
- Thibeault, K.; Légaré, C.; Desgagné, V.; White, F.; Clément, A.-A.; Scott, M.S.; Jacques, P.-É.; Guérin, R.; Perron, P.; Hivert, M.-F.; et al. Maternal Body Mass Index Is Associated with Profile Variation in Circulating MicroRNAs at First Trimester of Pregnancy. Biomedicines 2022, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Desgagné, V.; Thibeault, K.; White, F.; Clément, A.-A.; Poirier, C.; Luo, Z.C.; Scott, M.S.; Jacques, P.-É.; Perron, P.; et al. First Trimester Plasma MicroRNA Levels Predict Risk of Developing Gestational Diabetes Mellitus. Front. Endocrinol. 2022, 13, 928508. [Google Scholar] [CrossRef]
- Légaré, C.; Clément, A.-A.; Desgagné, V.; Thibeault, K.; White, F.; Guay, S.-P.; Arsenault, B.J.; Scott, M.S.; Jacques, P.-É.; Perron, P.; et al. Human Plasma Pregnancy-Associated miRNAs and Their Temporal Variation within the First Trimester of Pregnancy. Reprod. Biol. Endocrinol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Guillemette, L.; Allard, C.; Lacroix, M.; Patenaude, J.; Battista, M.-C.; Doyon, M.; Moreau, J.; Ménard, J.; Bouchard, L.; Ardilouze, J.-L.; et al. Genetics of Glucose Regulation in Gestation and Growth (Gen3G): A Prospective Prebirth Cohort of Mother–Child Pairs in Sherbrooke, Canada. BMJ Open 2016, 6, e010031. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.D.; Shapiro, G.D.; Audibert, F.; Dubois, L.; Pasquier, J.; Julien, P.; Bérard, A.; Muckle, G.; Trasler, J.; Tremblay, R.E.; et al. 3D Cohort Study: The Integrated Research Network in Perinatology of Quebec and Eastern Ontario. Paediatr. Perinat. Epidemiol. 2016, 30, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.L.; Javaherian, A.; Bomprezzi, R.; Ghaffari, L.; Rhodes, S.; Courtright, A.; Tembe, W.; Kim, S.; Metpally, R.; Van Keuren-Jensen, K. Identification of Extracellular miRNA in Human Cerebrospinal Fluid by Next-Generation Sequencing. RNA 2013, 19, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Rozowsky, J.; Kitchen, R.R.; Park, J.J.; Galeev, T.R.; Diao, J.; Warrell, J.; Thistlethwaite, W.; Subramanian, S.L.; Milosavljevic, A.; Gerstein, M. exceRpt: A Comprehensive Analytic Platform for Extracellular RNA Profiling. Cell Syst. 2019, 8, 352–357.e3. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling 2021.
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA Function with Experimental Support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Paraskevopoulou, M.D.; Karagkouni, D.; Georgakilas, G.; Vergoulis, T.; Kanellos, I.; Anastasopoulos, I.-L.; Maniou, S.; Karathanou, K.; Kalfakakou, D.; et al. DIANA-TarBase v7.0: Indexing More than Half a Million Experimentally Supported miRNA:mRNA Interactions. Nucleic Acids Res. 2015, 43, D153–D159. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Okae, H.; Hiura, H.; Kubota, N.; Kobayashi, E.H.; Shibata, S.; Oike, A.; Hori, T.; Kikutake, C.; Hamada, H.; et al. The microRNA Cluster C19MC Confers Differentiation Potential into Trophoblast Lineages upon Human Pluripotent Stem Cells. Nat. Commun. 2022, 13, 3071. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Gambardella, J.; Sardu, C.; Lombardi, A.; Santulli, G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. ncRNA 2021, 7, 39. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The Extracellular Matrix and Insulin Resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; de Borja, R.; Tsao, M.-S.; McPherson, J.D. Robust Global microRNA Expression Profiling Using Next-Generation Sequencing Technologies. Lab. Investig. 2014, 94, 350–358. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Gen3G (n = 436) Mean ± SD (Range) | 3D (n = 106) Mean ± SD (Range) | p-Value a |
|---|---|---|---|
| 1st trimester variables | |||
| Gestational age (weeks) | 9.6 ± 2.3 (4.1–16.3) | 11.9 ± 1.6 (5.6–15.6) | <0.001 |
| Age (years) | 28.5 ± 4.3 (18.0–47.0) | 31.3 ± 4.4 (22.0–45.0) | <0.001 |
| Body mass index (kg/m2) | 25.9 ± 6.0 (16.1–54.1) | 26.4 ± 6.6 (18.1–47.2) | NS |
| 1 h post-GCT glycemia (mmol/L) b | 5.7 ± 1.4 (2.6–10.2) | NA | NA |
| 2nd trimester variables | |||
| GDM n (%) | 13 (56) | 45 (42) | NA |
| Gestational age (weeks) | 26.4 ± 1.0 (24.1–29.4) | 27.3 ± 1.4 (24.4–30.0) | <0.001 |
| Fasting OGTT glycemia (mmol/L) | 4.2 ± 0.4 (3.4–7.3) | 4.7 ± 0.5 (3.7–6.6) | <0.001 |
| 1 h post-OGTT glycemia (mmol/L) | 7.3 ± 1.7 (3.6–13.0) | 8.9 ± 1.6 (4.7–12.3) | <0.001 |
| 2 h post-OGTT glycemia (mmol/L) | 5.9 ± 1.4 (3.0–11.4) | 7.1 ± 1.4 (3.8–11.5) | <0.001 |
| miRNAs | Gen3G | 3D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Women with Detected miRNA | Normalized miRNA Levels Mean ± SD | L2FC | p-Value | q-Value | % Women with Detected miRNA | Normalized miRNA Levels Mean ± SD | L2FC | p-Value | q-Value | |
| hsa-miR-516b-5p a | 99.31 | 101.64 ± 97.46 | −0.634 | 1.16 × 10−5 | 0.005 | 100.00 | 280.99 ± 210.94 | −0.585 | 0.0019 | 0.02 |
| hsa-miR-1323 a | 99.77 | 146.42 ± 161.03 | −0.652 | 1.18 × 10−5 | 0.005 | 100.00 | 574.92 ± 449.09 | −0.631 | 0.0008 | 0.01 |
| hsa-miR-512-3p a | 100.00 | 287.03 ± 379.56 | −0.627 | 2.18 × 10−5 | 0.005 | 100.00 | 604.39 ± 470.70 | −0.679 | 0.0003 | 0.01 |
| hsa-miR-518e-5p|hsa-miR-519a-5p|hsa-miR-519b-5p|hsa-miR-519c-5p|hsa-miR-522-5p|hsa-miR-523-5p a | 98.62 | 44.04 ± 46.18 | −0.602 | 2.63 × 10−5 | 0.005 | 98.11 | 87.19 ± 101.65 | −0.788 | 0.0003 | 0.01 |
| hsa-miR-520a-3p a | 99.31 | 86.87 ± 103.00 | −0.622 | 3.49 × 10−5 | 0.005 | 99.06 | 172.45 ± 158.05 | −0.865 | 0.00002 | 0.003 |
| hsa-miR-519c-3p a | 89.68 | 11.08 ± 12.85 | −0.700 | 3.57 × 10−5 | 0.005 | 92.45 | 31.11 ± 30.30 | −0.817 | 0.0022 | 0.02 |
| hsa-miR-517-5p a | 93.12 | 16.90 ± 19.37 | −0.691 | 4.24 × 10−5 | 0.005 | 98.11 | 59.98 ± 51.93 | −0.648 | 0.0021 | 0.02 |
| hsa-miR-155-5p | 100.00 | 916.98 ± 229.65 | 0.154 | 6.95 × 10−5 | 0.008 | 100.00 | 648.33 ± 291.30 | 0.205 | 0.0218 | 0.09 |
| hsa-miR-516a-5p a | 96.56 | 31.30 ± 34.26 | −0.609 | 9.71 × 10−5 | 0.01 | 99.06 | 121.46 ± 111.14 | −0.626 | 0.0045 | 0.04 |
| hsa-miR-515-3p a | 63.53 | 3.13 ± 4.59 | −0.821 | 0.00022 | 0.02 | 82.08 | 13.07 ± 17.09 | −0.855 | 0.0063 | 0.05 |
| hsa-miR-515-5p a | 89.68 | 10.50 ± 12.77 | −0.617 | 0.00024 | 0.02 | 90.57 | 21.35 ± 19.31 | −0.623 | 0.0105 | 0.06 |
| hsa-miR-1283 a | 98.39 | 57.28 ± 59.87 | −0.544 | 0.00029 | 0.02 | 100.00 | 179.55 ± 178.70 | −0.811 | 0.0001 | 0.009 |
| hsa-miR-518e-3p a | 88.76 | 7.98 ± 9.25 | −0.614 | 0.00050 | 0.03 | 94.34 | 25.60 ± 27.94 | −0.883 | 0.0003 | 0.01 |
| hsa-miR-526b-5p a | 94.72 | 17.11 ± 17.00 | −0.540 | 0.00072 | 0.04 | 96.23 | 42.48 ± 45.67 | −0.712 | 0.0017 | 0.02 |
| hsa-miR-518a-5p|hsa-miR-527 a | 80.73 | 5.69 ± 7.14 | −0.627 | 0.00074 | 0.04 | 83.02 | 13.29 ± 15.58 | −1.197 | 0.00002 | 0.003 |
| hsa-miR-525-5p a | 88.99 | 9.10 ± 10.59 | −0.580 | 0.00097 | 0.05 | 96.23 | 67.12 ± 70.95 | −0.838 | 0.0002 | 0.009 |
| hsa-miR-524-5p a | 86.24 | 8.68 ± 9.93 | −0.582 | 0.00139 | 0.06 | 96.23 | 45.02 ± 48.01 | −0.893 | 0.00003 | 0.003 |
| hsa-miR-145-3p | 99.77 | 29.46 ± 18.44 | −0.274 | 0.00262 | 0.0991 | 98.11 | 86.92 ± 60.59 | −0.390 | 0.0136 | 0.07 |
| miRNAs | Gen3G | 3D | ||||||
|---|---|---|---|---|---|---|---|---|
| % Women with Detected miRNA | Normalized miRNA Levels Mean ± SD | L2FC | p-Value | % Women with Detected miRNA | Normalized miRNA Levels Mean ± SD | L2FC | p-Value | |
| hsa-miR-516b-5p a | 99.31 | 101.64± 97.46 | −0.361 | 0.02 | 100.00 | 280.99 ± 210.94 | −0.432 | 0.03 |
| hsa-miR-1323 a | 99.77 | 146.42 ± 161.03 | −0.393 | 0.01 | 100.00 | 574.92 ± 449.09 | −0.435 | 0.03 |
| hsa-miR-512-3p a | 100.00 | 287.03 ± 379.56 | −0.378 | 0.01 | 100.00 | 604.39 ± 470.70 | −0.527 | 0.01 |
| hsa-miR-518e-5p|hsa-miR-519a-5p|hsa-miR-519b-5p|hsa-miR-519c-5p|hsa-miR-522-5p|hsa-miR-523-5p a | 98.62 | 44.04 ± 46.18 | −0.348 | 0.02 | 98.11 | 87.19 ± 101.65 | −0.692 | 0.004 |
| hsa-miR-520a-3p a | 99.31 | 86.87 ± 103.00 | −0.371 | 0.02 | 99.06 | 172.45 ± 158.05 | −0.795 | 0.0005 |
| hsa-miR-519c-3p a | 89.68 | 11.08 ± 12.85 | −0.491 | 0.006 | 92.45 | 31.11 ± 30.30 | −0.697 | 0.02 |
| hsa-miR-517-5p a | 93.12 | 16.90± 19.37 | −0.466 | 0.009 | 98.11 | 59.98 ± 51.93 | −0.604 | 0.01 |
| hsa-miR-155-5p | 100.00 | 916.98± 229.65 | 0.113 | 0.006 | 100.00 | 648.33 ± 291.30 | 0.179 | 0.07 |
| hsa-miR-516a-5p a | 96.56 | 31.30 ± 34.26 | −0.336 | 0.04 | 99.06 | 121.46 ± 111.14 | −0.478 | 0.05 |
| hsa-miR-515-3p a | 63.53 | 3.13 ± 4.59 | −0.522 | 0.03 | 82.08 | 13.07 ± 17.09 | −0.745 | 0.03 |
| hsa-miR-515-5p a | 89.68 | 10.50 ± 12.77 | −0.419 | 0.02 | 90.57 | 21.35 ± 19.31 | −0.528 | 0.05 |
| hsa-miR-1283 a | 98.39 | 57.28 ± 59.87 | −0.282 | 0.07 | 100.00 | 179.55 ± 178.70 | −0.702 | 0.003 |
| hsa-miR-518e-3p a | 88.76 | 7.98 ± 9.25 | −0.296 | 0.1 | 94.34 | 25.60 ± 27.94 | −0.754 | 0.005 |
| hsa-miR-526b-5p a | 94.72 | 17.11 ± 17.00 | −0.293 | 0.08 | 96.23 | 42.48 ± 45.67 | −0.689 | 0.007 |
| hsa-miR-518a-5p|hsa-miR-527 a | 80.73 | 5.69 ± 7.14 | −0.444 | 0.02 | 83.02 | 13.29 ± 15.58 | −1.175 | 0.0002 |
| hsa-miR-525-5p a | 88.99 | 9.10 ± 10.59 | −0.301 | 0.1 | 96.23 | 67.12 ± 70.95 | −0.761 | 0.002 |
| hsa-miR-524-5p a | 86.24 | 8.68 ± 9.93 | −0.269 | 0.1 | 96.23 | 45.02 ± 48.01 | −0.929 | 0.0001 |
| hsa-miR-145-3p | 99.77 | 29.46 ± 18.44 | −0.180 | 0.06 | 98.11 | 86.92 ± 60.59 | −0.369 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Légaré, C.; Desgagné, V.; Thibeault, K.; White, F.; Clément, A.-A.; Poirier, C.; Luo, Z.-C.; Scott, M.S.; Jacques, P.-É.; Perron, P.; et al. First-Trimester Plasmatic microRNAs Are Associated with Fasting Glucose Levels in Late Second Trimester of Pregnancy. Biomedicines 2024, 12, 1285. https://doi.org/10.3390/biomedicines12061285
Légaré C, Desgagné V, Thibeault K, White F, Clément A-A, Poirier C, Luo Z-C, Scott MS, Jacques P-É, Perron P, et al. First-Trimester Plasmatic microRNAs Are Associated with Fasting Glucose Levels in Late Second Trimester of Pregnancy. Biomedicines. 2024; 12(6):1285. https://doi.org/10.3390/biomedicines12061285
Chicago/Turabian StyleLégaré, Cécilia, Véronique Desgagné, Kathrine Thibeault, Frédérique White, Andrée-Anne Clément, Cédrik Poirier, Zhong-Cheng Luo, Michelle S. Scott, Pierre-Étienne Jacques, Patrice Perron, and et al. 2024. "First-Trimester Plasmatic microRNAs Are Associated with Fasting Glucose Levels in Late Second Trimester of Pregnancy" Biomedicines 12, no. 6: 1285. https://doi.org/10.3390/biomedicines12061285





