Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Outcome Measures
2.3.1. EEG
2.3.2. Auditory Oddball Paradigm
2.3.3. Mini-Mental State Examination
2.4. Statistical Analysis
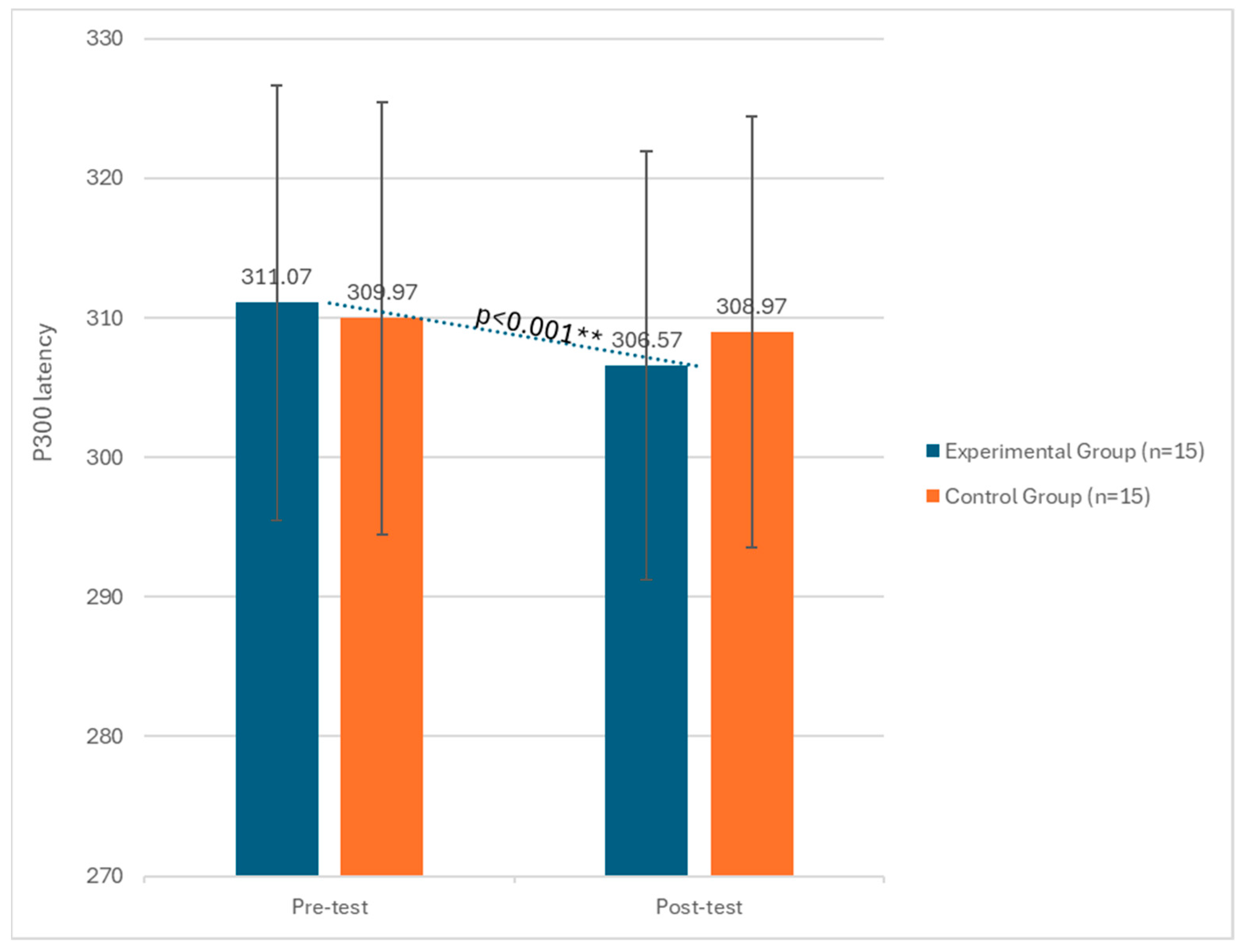
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhao, X.; Zhu, Z.; Zhang, L. Vascular dementia: A microglia’s perspective. Ageing Res. Rev. 2022, 81, 101734. [Google Scholar] [CrossRef] [PubMed]
- Nation, D.A. Vascular Disease, Including Vascular Dementia. 2023. Available online: https://journals.lww.com/continuum/abstract/2022/06000/vascular_cognitive_impairment_and_dementia.8.aspx (accessed on 7 February 2024).
- Zhang, Z.; Liu, J.; Guo, M.; Li, H. Panax Ginseng in the treatment of Alzheimer’s disease and vascular dementia. J. Ginseng Res. 2023, 47, 506–514. [Google Scholar]
- Ying, C.; Kang, P.; Binkley, M.M.; Ford, A.L.; Chen, Y.; Hassenstab, J.; Wang, Q.; Strain, J.; Morris, J.C.; Lee, J.-M.; et al. Neuroinflammation and amyloid deposition in the progression of mixed Alzheimer and vascular dementia. NeuroImage Clin. 2023, 38, 103373. [Google Scholar] [CrossRef] [PubMed]
- Weidung, B.; Lövheim, H.; Littbrand, H.; Wahlin, J.; Olofsson, B.; Gustafson, Y. Temporal Dementia and Cognitive Impairment Trends in the Very Old in the 21st Century. J. Alzheimers Dis. 2023, 93, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Price, R.S. Exploring what progress is being made in the development of health promotion material for vascular dementia: A systematic review of the evidence. Aging Med. 2023, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Yan, X.L.; Guo, Z.N.; Yang, Y. Pathological changes in neurovascular units: Lessons from cases of vascular dementia. CNS Neurosci. Ther. 2021, 27, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shue, F.; Bu, G.; Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Banchero, S.; Casciaro, M.; Tonacci, A.; Billeci, L.; Nencioni, A.; Pioggia, G.; Genovese, S.; Monacelli, F.; Gangemi, S. Potential predictors for cognitive decline in vascular dementia: A machine learning analysis. Processes 2022, 10, 2088. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-stroke cognitive impairment and dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Rundek, T.; Tolea, M.; Ariko, T.; Fagerli, E.A.; Camargo, C.J. Vascular cognitive impairment (VCI). Neurotherapeutics 2022, 19, 68–88. [Google Scholar] [CrossRef]
- Ferrer, J.I.; Travieso JC, F.; Núñez, Y.R.; Chávez, A.D.L.C.D. Dementia post ictus: Is policosanol a prevention therapy? Rev. CENIC. Cienc. Biológicas 2020, 51, 264–271. [Google Scholar]
- Akhter, F.; Persaud, A.; Zaokari, Y.; Zhao, Z.; Zhu, D. Vascular dementia and underlying sex differences. Front. Aging Neurosci. 2021, 13, 720715. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, C.A.; Theochari, C.A.; Zareifopoulos, N.; Arfaras-Melainis, A.; Giannakoulas, G.; Karamitsos, T.D.; Palaiodimos, L.; Ntaios, G.; Avgerinos, K.I.; Kapogiannis, D.; et al. Atrial fibrillation is associated with cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer’s disease: A systematic review and meta-analysis. J. Gen. Intern. Med. 2021, 36, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Bu, Y.; Li, M.; Han, R.; Zhang, N.; Hao, J.; Jiang, W. Remote ischemic conditioning improves cognition in patients with subcortical ischemic vascular dementia. BMC Neurol. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Beason-Held, L.L.; Kerley, C.I.; Chaganti, S.; Moghekar, A.; Thambisetty, M.; Ferrucci, L.; Resnick, S.M.; Landman, B.A. Health Conditions Associated with Alzheimer’s Disease and Vascular Dementia. Ann. Neurol. 2023, 93, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Grossberg, G.T. Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. Handb. Clin. Neurol. 2019, 165, 5–32. [Google Scholar] [PubMed]
- Isakov, R.I.; Borysenko, V.V.; Kazakov, O.A.; Kydon, P.V.; Fysun, Y.O.; Hryn, K.V.; Herasymenko, L.O. Psychosocial Maladjustment, Quality of Life and Social Functioning of Caregivers of Patients with Vascular Dementia and Alzheimer’s Disease. 2022. Available online: https://repository.pdmu.edu.ua/handle/123456789/19802 (accessed on 3 June 2024).
- Young, S.; Smith, M.; Shafique, S.; Piamjariyakul, U. You’re Not Who You Used to Be: A Case Report of a Family Living with Heart Failure and Vascular Dementia. Home Healthc. Now 2023, 41, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Zhou, Z.F.; Zhu, Y.G.; Wan, Z.K.; Yang, M.W.; Hong, F.F.; Yang, S.L. Pharmacological treatment of vascular dementia: A molecular mechanism perspective. Aging Dis. 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Linh TT, D.; Hsieh, Y.C.; Huang, L.K.; Hu, C.J. Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. Int. J. Mol. Sci. 2022, 23, 11067. [Google Scholar] [CrossRef]
- Perlett, L.; Smith, E.E. Treatment of Vascular and Neurodegenerative Forms of Cognitive Impairment and Dementias. Clin. Geriatr. Med. 2023, 39, 135–149. [Google Scholar] [CrossRef]
- Alexander, P.; Visagan, S.; Jawhar, S.; Kare, A.; Issa, N.; Issa, R.; Jawhar, A.; Thomas, S.; Gorantla, V. Antiplatelets and Vascular Dementia: A Systematic Review. J. Aging Res. 2022, 2022, 9780067. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mim, S.A.; Islam, R.; Parvez, A.; Islam, F.; Uddin, M.B.; Rahaman, S.; Shuvo, P.A.; Ahmed, M.; Greig, N.H.; et al. Exploring the recent trends in management of dementia and frailty: Focus on diagnosis and treatment. Curr. Med. Chem. 2022, 29, 5289. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Sun, C.; Kamari, R.; Bettermann, K. Current status and future prospects of pathophysiology-based neuroprotective drugs for the treatment of vascular dementia. Drug Discov. Today 2020, 25, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Super, N.; Epstein-Lubow, G.; Reuben, D.B.; Snyder, R.E.; Carmody, J.; Maglich, A. Payment for comprehensive dementia care: Five key recommendations. Health Aff. Forefr. 2023. [Google Scholar] [CrossRef]
- van de Schraaf, S.A.; Smit, M.F.; Muller, M.; Hertogh, C.M.; Rhodius-Meester, H.F.; Sizoo, E.M. Vascular cognitive impairment: When memory loss is not the biggest challenge. Dementia 2023, 23, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, R.V.; Goedecke, J.; Visser-Vandewalle, V.; Fink, G.R.; Onur, O.A. Brain Stimulation for the Treatment of Dementia. Fortschr. Neurol.-Psychiatr. 2022, 90, 336–342. [Google Scholar] [PubMed]
- Tseng, P.T.; Chen, Y.W.; Zeng, B.Y.; Zeng, B.S.; Hung, C.M.; Sun, C.K.; Li, C.T. The beneficial effect on cognition of noninvasive brain stimulation intervention in patients with dementia: A network meta-analysis of randomized controlled trials. Alzheimers Res. Ther. 2023, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Rasanan AH, H.; Rad, J.A.; Alavi, M.M.; Haghi, S.; Nitsche, M.A. Transcranial direct current stimulation (tDCS) alters the pattern of information processing in children with ADHD: Evidence from drift diffusion modeling. Neurophysiol. Clin. 2022, 52, 17–27. [Google Scholar] [CrossRef]
- Galimberti, A.; Tik, M.; Pellegrino, G.; Schuler, A.L. Effectiveness of rTMS and tDCS treatment for chronic TBI symptoms: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 128, 110863. [Google Scholar] [CrossRef]
- Jog, M.; Anderson, C.; Kubicki, A.; Boucher, M.; Leaver, A.; Hellemann, G.; Iacoboni, M.; Woods, R.; Narr, K. Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci. Rep. 2023, 13, 2841. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Cardenas-Rojas, A.; Thibaut, A.; Costa, B.; Ferreira, I.; Caumo, W.; Fregni, F. Methods and strategies of tDCS for the treatment of pain: Current status and future directions. Expert Rev. Med. Devices 2020, 17, 879–898. [Google Scholar] [CrossRef]
- Pergher, V.; Au, J.; Shalchy, M.A.; Santarnecchi, E.; Seitz, A.; Jaeggi, S.M.; Battelli, L. The benefits of simultaneous tDCS and working memory training on transfer outcomes: A systematic review and meta-analysis. Brain Stimul. 2022, 15, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Twiddy, J.; Dubljević, V. Present and emerging ethical issues with tDCS use: A summary and review. Neuroethics 2023, 16, 1. [Google Scholar] [CrossRef]
- Talar, K.; Vetrovsky, T.; van Haren, M.; Négyesi, J.; Granacher, U.; Váczi, M.; Martín-Arévalo, E.; Del Olmo, M.; Kałamacka, E.; Hortobágyi, T. The effects of aerobic exercise and transcranial direct current stimulation on cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101738. [Google Scholar] [CrossRef]
- Fabio, R.A.; Gangemi, A.; Semino, M.; Vignoli, A.; Canevini, M.P.; Priori, A.; Di Rosa, G.; Caprì, T. Effects of combined transcranial direct current stimulation with cognitive training in girls with rett syndrome. Brain Sci. 2020, 10, 276. [Google Scholar] [CrossRef]
- Fabio, R.A.; Suriano, R.; Gangemi, A. Effects of Transcranial Direct Current Stimulation on Potential P300-Related Events and Alpha-Beta EEG Band Rhythms in Parkinson’s Disease. J. Integr. Neurosci. 2024, 7, 23789. [Google Scholar] [CrossRef]
- Turnbull, C.; Boomsma, A.; Milte, R.; Stanton, T.R.; Hordacre, B. Safety and adverse events following non-invasive electrical brain stimulation in stroke: A systematic review. Top. Stroke Rehabil. 2023, 30, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Roncero, C.; Kniefel, H.; Service, E.; Thiel, A.; Probst, S.; Chertkow, H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 247–253. [Google Scholar] [CrossRef]
- Inagawa, T.; Yokoi, Y.; Narita, Z.; Maruo, K.; Okazaki, M.; Nakagome, K. Safety and feasibility of transcranial direct current stimulation for cognitive rehabilitation in patients with mild or major neurocognitive disorders: A randomized sham-controlled pilot study. Front. Hum. Neurosci. 2019, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.A.; De Souza, S. Safety of repeated neuromodulation by transcranial direct current stimulation (tDCS) in dementia: A narrative review. Eur. Psychiatry 2023, 66, S248. [Google Scholar] [CrossRef]
- Elder, G.J.; Taylor, J.P. Transcranial magnetic stimulation and transcranial direct current stimulation: Treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 2014, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Heinrich, S.; Kayser, F.; Menzler, K.; Kesselring, J.; Khader, P.H.; Lefaucheur, J.-P.; Mylius, V. At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J. Neurol. Sci. 2016, 369, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Song, M.K.; Kim, J.H.; Han, J.Y. A randomized controlled trial to evaluate the effectiveness and safety of electro acupuncture and transcranial direct current stimulation with computerized cognitive rehabilitation in patients with vascular cognitive impairment. Medicine 2020, 99, e21263. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Li, M.; Chen, Q.; Li, J.; Li, G.; Lin, W. The P300 event-related potential component and cognitive impairment in epilepsy: A systematic review and meta-analysis. Front. Neurol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Olichney, J.; Xia, J.; Church, K.J.; Moebius, H.J. Predictive power of cognitive biomarkers in neurodegenerative disease drug development: Utility of the P300 event-related potential. Neural Plast. 2022, 2022, 2104880. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Arakaki, X.; Bonanni, L.; Bujan, A.; Carrillo, M.C.; Del Percio, C.; Edelmayer, R.M.; Egan, G.; Elahh, F.M.; Evans, A.; et al. EEG measures for clinical research in major vascular cognitive impairment: Recommendations by an expert panel. Neurobiol. Aging 2021, 103, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, H.; Zhao, Y.; Zhang, L.; Zhang, Y. Application of the P300 potential in cognitive impairment assessments after transient ischemic attack or minor stroke. Neurol. Res. 2021, 43, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Poskotinova, L.; Khasanova, N.; Kharak, A.; Krivonogova, O.; Krivonogova, E. Parameters of Auditory Evoked Related Potentials P300 in Disorders of Different Cognitive Function Domains (Visuospatial/Executive and Memory) in Elderly Hypertensive Persons. Diagnostics 2023, 13, 1598. [Google Scholar] [CrossRef]
- Fabio, R.A.; Caprì, T.; Buzzai, C.; Pittalà, V.; Gangemi, A. Auditory and Visual Oddball Paradigm Evaluated Through P300 in Five Girls with Rett Syndrome. Neuroquantology 2019, 17, 40–49. [Google Scholar] [CrossRef]
- Ma, X.; Shen, J.; Sun, J.; Wang, L.; Wang, W.; He, K.; Chen, X.; Zhang, Q.; Jin, Y.; Gao, D.; et al. P300 Event-Related Potential Predicts Cognitive Dysfunction in Patients with Vestibular Disorders. Biomedicines 2023, 11, 2365. [Google Scholar] [CrossRef]
- Määattä, S.; Herrgard, E.; Saavalainen, P.; Pääkkönen, A.; Könönen, M.; Luoma, L.; Laukkanen, E.; Yppärilä, H.; Partanen, J. P3 amplitude and time-on-taskeffects in distractible adolescents. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2005, 116, 2175–2183. [Google Scholar]
- Hünerli, D.; Emek-Savaş, D.D.; Çavuşoğlu, B.; Çolakoğlu, B.D.; Ada, E.; Yener, G.G. Mild cognitive impairment in Parkinson’s disease is associated with decreased P300 amplitude and reduced putamen volume. Clin. Neurophysiol. 2019, 130, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Gongora, M.; Nicoliche, E.; Magalhaes, J.; Vicente, R.; Teixeira, S.; Bastos, V.H.; Bittencourt, J.; Cagy, M.; Basile, L.F.; Budde, H.; et al. Event-related potential (P300): The effects of levetiracetam in cognitive performance. Neurol. Sci. 2021, 42, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, T.H.; Hwang, W.J.; Lee, T.Y.; Kwon, J.S. Auditory P300 as a neurophysiological correlate of symptomatic improvement by transcranial direct current stimulation in patients with schizophrenia: A pilot study. Clin. EEG Neurosci. 2020, 51, 252–258. [Google Scholar] [CrossRef]
- Voegtle, A.; Reichert, C.; Hinrichs, H.; Sweeney-Reed, C.M. Repetitive Anodal TDCS to the Frontal Cortex Increases the P300 during Working Memory Processing. Brain Sci. 2022, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, P.; Kıyı, İ.; İşbitiren, Y.Ö.; Yener, G. Cognitive load associates prolonged P300 latency during target stimulus processing in individuals with mild cognitive impairment. Sci. Rep. 2023, 13, 15956. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.A.; Akiba, H.T.; Gomes, J.S.; Trevizol, A.P.; Lacerda AL, T.D.; Dias, Á.M. Transcranial direct current stimulation (tDCS) in elderly with mild cognitive impairment: A pilot study. Dement. Neuropsychol. 2019, 13, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Chen, Q.; Fu, Y.; Zheng, K. Transcranial direct current stimulation enhances cognitive function in patients with mild cognitive impairment and early/mid alzheimer’s disease: A systematic review and meta-analysis. Brain Sci. 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Saleh, O.; Assaf, M.; Alzoubi, A.; Anshase, A.; Tarkhan, H.; Ayoub, M.; Abuelazm, M. The effects of transcranial direct current stimulation on cognitive function for mild cognitive impairment: A systematic review and meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2023, 35, 2293–2306. [Google Scholar] [CrossRef]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250. [Google Scholar] [CrossRef]
- Jasper, H.H. The Ten-Twenty Electrode System of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Folstein, M.; Folstein, S.; McHugh, P. Mini-Mental State (MMSE). J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Helphrey, J.; Cabrera, H.; Neaves, S.; Ahmed, D.; Chiang, H.S.; Thakkar, V.; Peters, M.; McClintock, S.; Hart, J.; LoBue, C. A-22 Preliminary Outcomes on Depression Symptoms in a Randomized Controlled Trial of High-Definition Transcranial Direct Current Stimulation in Alzheimer’s Dementia. Arch. Clin. Neuropsychol. 2023, 38, 1184. [Google Scholar] [CrossRef]
- Figeys, M.; Zeeman, M.; Kim, E.S. Effects of transcranial direct current stimulation (tDCS) on cognitive performance and cerebral oxygen hemodynamics: A systematic review. Front. Hum. Neurosci. 2021, 15, 623315. [Google Scholar] [CrossRef] [PubMed]
- Aksu, S.; Uslu, A.; İşçen, P.; Tülay, E.E.; Barham, H.; Soyata, A.Z.; Demirtas-Tatlidede, A.; Yıldız, G.B.; Bilgiç, B.; Hanağası, H.; et al. Does transcranial direct current stimulation enhance cognitive performance in Parkinson’s disease mild cognitive impairment? An event-related potentials and neuropsychological assessment study. Neurol. Sci. 2022, 43, 4029–4044. [Google Scholar] [CrossRef] [PubMed]
- Iannizzotto, G.; Nucita, A.; Fabio, R.A.; Caprì, T.; Bello, L.L. Remote eye-tracking for cognitive telerehabilitation and interactive school tasks in times of COVID-19. Information 2020, 11, 296. [Google Scholar] [CrossRef]
- Gangemi, A.; Suriano, R.; Fabio, R.A. Longitudinal Exploration of Cortical Brain Activity in Cognitive Fog: An EEG Study in Patients with and Without Anosmia. J. Integr. Neurosci. 2024, 23, 105. [Google Scholar] [CrossRef]


| Pre-Test | Post-Test | ||||
|---|---|---|---|---|---|
| M (±SD) | M (±SD) | t | p | d | |
| Experimental Group | |||||
| MMSE | 20.35 (±2.67) | 23.83 (±1.88) | 7.22 | 0.001 ** | 0.78 |
| P300 Latency | 311.07 (±2.43) | 306.57 (±2.37) | 6.81 | 0.001 ** | 0.79 |
| P300 Amplitude | 14.53 (±2.51) | 20.28 (±2.21) | 12.05 | 0.001 ** | 0.85 |
| Control Group | |||||
| MMSE | 21.09 (±3.98) | 22.99 (±2.56) | 1.25 | 0.23 | 0.45 |
| P300 Latency | 309.87 (±3.11) | 308.99 (±3.21) | 1.06 | 0.43 | 0.39 |
| P300 Amplitude | 14.77 (±3.65) | 19.77 (±3.18) | 0.98 | 0.45 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangemi, A.; Fabio, R.A.; Suriano, R.; De Luca, R.; Marra, A.; Tomo, M.; Quartarone, A.; Calabrò, R.S. Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study. Biomedicines 2024, 12, 1290. https://doi.org/10.3390/biomedicines12061290
Gangemi A, Fabio RA, Suriano R, De Luca R, Marra A, Tomo M, Quartarone A, Calabrò RS. Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study. Biomedicines. 2024; 12(6):1290. https://doi.org/10.3390/biomedicines12061290
Chicago/Turabian StyleGangemi, Antonio, Rosa Angela Fabio, Rossella Suriano, Rosaria De Luca, Angela Marra, Mariangela Tomo, Angelo Quartarone, and Rocco Salvatore Calabrò. 2024. "Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study" Biomedicines 12, no. 6: 1290. https://doi.org/10.3390/biomedicines12061290
APA StyleGangemi, A., Fabio, R. A., Suriano, R., De Luca, R., Marra, A., Tomo, M., Quartarone, A., & Calabrò, R. S. (2024). Does Transcranial Direct Current Stimulation Affect Potential P300-Related Events in Vascular Dementia? Considerations from a Pilot Study. Biomedicines, 12(6), 1290. https://doi.org/10.3390/biomedicines12061290









