Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review
Abstract
:1. Introduction
2. Anatomy and Pathophysiology of the Ocular Surface
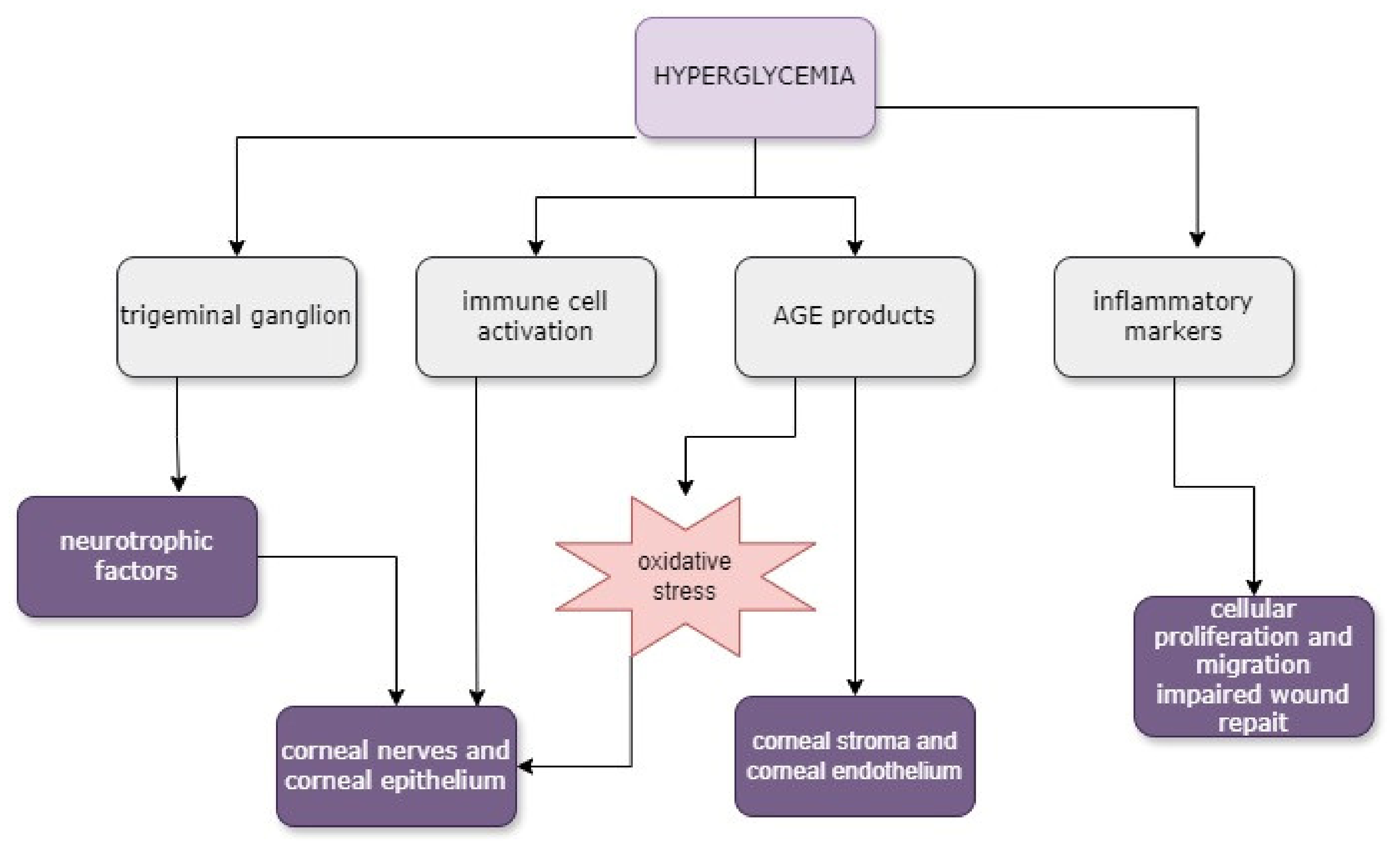
3. Corneal Alterations and Pathogenesis in Diabetes Mellitus
3.1. Corneal Epithelial Alterations
3.2. Corneal Stromal Alterations
3.3. Corneal Endothelial Alterations
3.4. Tear Film Alterations
3.5. Diabetic Corneal Neuropathy
4. Novel Diagnostic Approaches
5. Updates on Treatment Options
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas Report, 10th ed.; IDF Diabetes Atlas: Brussels, Belgium, 2021. [Google Scholar]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic Investigations of Diabetic Ocular Surface Diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, R.E.; Azar, N.S.; Mousa, H.M.; Quiroga-Garza, M.E.; Komai, S.; Wheelock-Gutierrez, L.; Cartes, C.; Perez, V.L. Ocular Surface Disease: A Known yet Overlooked Side Effect of Topical Glaucoma Therapy. Front. Toxicol. 2023, 5, 1067942. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.; Magdum, R.; Ahuja, A.; Kaul, S.; Johnson, S.; Mishra, A.; Patil, M.; Dhore, D.N.; Alapati, A. Ocular Surface Diseases in Patients with Diabetes. Cureus 2022, 14, e23401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, L.; Deng, S.; Sun, X.; Wang, N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J. Ophthalmol. 2016, 2016, 8201053. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Elgstøen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, Ø.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef]
- Knop, N.; Knop, E. Conjunctiva-Associated Lymphoid Tissue in the Human Eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1270–1279. [Google Scholar]
- Knop, E.; Knop, N. Lacrimal Drainage-Associated Lymphoid Tissue (LDALT): A Part of the Human Mucosal Immune System. Investig. Ophthalmol. Vis. Sci. 2001, 42, 566–574. [Google Scholar]
- Steven, P.; Rupp, J.; Hüttmann, G.; Koop, N.; Lensing, C.; Laqua, H.; Gebert, A. Experimental Induction and Three-Dimensional Two-Photon Imaging of Conjunctiva-Associated Lymphoid Tissue. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1512. [Google Scholar] [CrossRef] [PubMed]
- Siebelmann, S.; Gehlsen, U.; Hüttmann, G.; Koop, N.; Bölke, T.; Gebert, A.; Stern, M.E.; Niederkorn, J.Y.; Steven, P. Development, Alteration and Real Time Dynamics of Conjunctiva-Associated Lymphoid Tissue. PLoS ONE 2013, 8, e82355. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Schwab, S.; Kiesewetter, A.; Saban, D.R.; Stern, M.E.; Gehlsen, U. Disease-Specific Expression of Conjunctiva Associated Lymphoid Tissue (CALT) in Mouse Models of Dry Eye Disease and Ocular Allergy. Int. J. Mol. Sci. 2020, 21, 7514. [Google Scholar] [CrossRef]
- Septimiu-Radu, S.; Gadela, T.; Gabriela, D.; Oancea, C.; Rosca, O.; Lazureanu, V.E.; Fericean, R.M.; Bratosin, F.; Dumitrescu, A.; Stoicescu, E.R.; et al. A Systematic Review of Lung Autopsy Findings in Elderly Patients after SARS-CoV-2 Infection. J. Clin. Med. 2023, 12, 2070. [Google Scholar] [CrossRef] [PubMed]
- Puro, D.G. Role of Ion Channels in the Functional Response of Conjunctival Goblet Cells to Dry Eye. Am. J. Physiol.-Cell Physiol. 2018, 315, C236–C246. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Tukler Henriksson, J.; Barbosa, F.L.; De Paiva, C.S.; Pflugfelder, S.C. Interferon-γ–Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjögren Syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; De Paiva, C.S.; Yu, Z.; De Souza, R.G.; Li, D.-Q.; Pflugfelder, S.C. Goblet Cell-Produced Retinoic Acid Suppresses CD86 Expression and IL-12 Production in Bone Marrow-Derived Cells. Int. Immunol. 2018, 30, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z. Resident Innate Immune Cells in the Cornea. Front. Immunol. 2021, 12, 620284. [Google Scholar] [CrossRef] [PubMed]
- Loi, J.K.; Alexandre, Y.O.; Senthil, K.; Schienstock, D.; Sandford, S.; Devi, S.; Christo, S.N.; Mackay, L.K.; Chinnery, H.R.; Osborne, P.B.; et al. Corneal Tissue-Resident Memory T Cells Form a Unique Immune Compartment at the Ocular Surface. Cell Rep. 2022, 39, 110852. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, C.; Wang, H.; Xue, Y.; Dong, D.; Lin, C.; Song, F.; Fu, T.; Wang, Z.; Chen, J.; et al. Local Group 2 Innate Lymphoid Cells Promote Corneal Regeneration after Epithelial Abrasion. Am. J. Pathol. 2017, 187, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.C.; Lam, K.S.-L.; Tong, L. A Systematic Review on the Impact of Diabetes Mellitus on the Ocular Surface. Nutr. Diabetes 2017, 7, e251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Ren, Y.-R.; Chen, B.-H. Corneal Alteration and Pathogenesis in Diabetes Mellitus. Int. J. Ophthalmol. 2019, 12, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The Impact of Diabetes on Corneal Nerve Morphology and Ocular Surface Integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells–Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-Y.; Carrel, H.; Huang, J.-S.; Wang, I.-J.; Hou, Y.-C.; Chen, W.-L.; Wang, J.-Y.; Hu, F.-R. Decreased Density of Corneal Basal Epithelium and Subbasal Corneal Nerve Bundle Changes in Patients with Diabetic Retinopathy. Am. J. Ophthalmol. 2006, 142, 488–490.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Titone, R.; Robertson, D.M. The Impact of Hyperglycemia on the Corneal Epithelium: Molecular Mechanisms and Insight. Ocul. Surf. 2019, 17, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Caixinha, M.; Oliveira, P.; Aires, I.D.; Ambrósio, A.F.; Santiago, A.R.; Santos, M.; Santos, J. In Vivo Characterization of Corneal Changes in a Type 1 Diabetic Animal Model. Ultrasound Med. Biol. 2019, 45, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Richardson, A.; Pandzic, E.; Lobo, E.P.; Whan, R.; Watson, S.L.; Lyons, J.G.; Wakefield, D.; Di Girolamo, N. Visualizing the Contribution of Keratin-14+ Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Rep. 2019, 12, 14–28. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, L.; Montorio, D.; Concilio, M.; Giordano, M.; Cennamo, G.; Costagliola, C. Anterior segment-optical coherence tomography and diabetic retinopathy: Could it be an early biomarker? Photodiagn. Photodyn. Ther. 2022, 39, 102995. [Google Scholar] [CrossRef] [PubMed]
- Yusufoğlu, E.; Güngör Kobat, S.; Keser, S. Evaluation of Central Corneal Epithelial Thickness with Anterior Segment OCT in Patients with Type 2 Diabetes Mellitus. Int. Ophthalmol. 2022, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V. Diabetic Complications in the Cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Kwok, S.S.; Chan, Y.K.; Lo, A.C.-Y.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Experimental Modeling of Cornea Wound Healing in Diabetes: Clinical Applications and Beyond. BMJ Open Diabetes Res. Care 2019, 7, e000779. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Lee, P.S.Y.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The Impact of Sensory Neuropathy and Inflammation on Epithelial Wound Healing in Diabetic Corneas. Prog. Retin. Eye Res. 2022, 89, 101039. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Zhang, W.; Zhu, J.; Huang, H.; Mo, K.; Guo, H.; Zhu, L.; Liu, J.; Li, M.; Wang, L.; et al. dsRNA Induced IFNβ-MMP13 Axis Drives Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2022, 63, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Sohn, E.; Jeong, I.-H.; Kim, H.; Kim, J.S. Involvement of Advanced Glycation End Products, Oxidative Stress and Nuclear Factor-kappaB in the Development of Diabetic Keratopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Del Buey, M.A.; Casas, P.; Caramello, C.; López, N.; De La Rica, M.; Subirón, A.B.; Lanchares, E.; Huerva, V.; Grzybowski, A.; Ascaso, F.J. An Update on Corneal Biomechanics and Architecture in Diabetes. J. Ophthalmol. 2019, 2019, 7645352. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of Collagen Crosslinking in Diabetes and Keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Ladea, L.; Zemba, M.; Calancea, M.I.; Călțaru, M.V.; Dragosloveanu, C.D.M.; Coroleucă, R.; Catrina, E.L.; Brezean, I.; Dinu, V. Corneal Epithelial Changes in Diabetic Patients: A Review. Int. J. Mol. Sci. 2024, 25, 3471. [Google Scholar] [CrossRef]
- Lee, J.S.; Oum, B.S.; Choi, H.Y.; Lee, J.E.; Cho, B.M. Differences in Corneal Thickness and Corneal Endothelium Related to Duration in Diabetes. Eye 2006, 20, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, N.G.M.; Dubbelman, M.; Kostense, P.J.; Ringens, P.J.; Polak, B.C.P. The Influence of Chronic Diabetes Mellitus on the Thickness and the Shape of the Anterior and Posterior Surface of the Cornea. Cornea 2007, 26, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Busted, N.; Olsen, T.; Schmitz, O. Clinical Observations on the Corneal Thickness and the Corneal Endothelium in Diabetes Mellitus. Br. J. Ophthalmol. 1981, 65, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pop-Busui, R.; Musch, D.C.; Reed, D.M.; Momont, A.C.; Hussain, M.; Raval, N.; Moroi, S.E.; Shtein, R. Central Corneal Thickness Increase Due to Stromal Thickening with Diabetic Peripheral Neuropathy Severity. Cornea 2018, 37, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Aldrich, B.T.; Burckart, K.A.; Schmidt, G.A.; Zimmerman, M.B.; Reed, C.R.; Greiner, M.A.; Sander, E.A. Descemet Membrane Adhesion Strength Is Greater in Diabetics with Advanced Disease Compared to Healthy Donor Corneas. Exp. Eye Res. 2016, 153, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; McKay, T.B.; Sarker-Nag, A.; Allegood, J.; Chalfant, C.; Ma, J.-X.; Karamichos, D. Complete Metabolome and Lipidome Analysis Reveals Novel Biomarkers in the Human Diabetic Corneal Stroma. Exp. Eye Res. 2016, 153, 90–100. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, A.; Alsubaie, S. Corneal Endothelium and Central Corneal Thickness Changes in Type 2 Diabetes Mellitus. Clin. Ophthalmol. 2017, 11, 481–486. [Google Scholar] [CrossRef]
- Larsson, L.-I.; Bourne, W.M.; Pach, J.M.; Brubaker, R.F. Structure and Function of the Corneal Endothelium in Diabetes Mellitus Type I and Type II. Arch. Ophthalmol. 1996, 114, 9. [Google Scholar] [CrossRef] [PubMed]
- Liaboe, C.A.; Aldrich, B.T.; Carter, P.C.; Skeie, J.M.; Burckart, K.A.; Schmidt, G.A.; Reed, C.R.; Zimmerman, M.B.; Greiner, M.A. Assessing the Impact of Diabetes Mellitus on Donor Corneal Endothelial Cell Density. Cornea 2017, 36, 561–566. [Google Scholar] [CrossRef] [PubMed]
- dell’Omo, R.; Cifariello, F.; De Turris, S.; Romano, V.; Di Renzo, F.; Di Taranto, D.; Coclite, G.; Agnifili, L.; Mastropasqua, L.; Costagliola, C. Confocal Microscopy of Corneal Nerve Plexus as an Early Marker of Eye Involvement in Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2018, 142, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, T.G. The Effects of Type 2 Diabetes Mellitus on the Corneal Endothelium and Central Corneal Thickness. Sci. Rep. 2021, 11, 8324. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Liao, N.; Wen, F. Assessment of Biomarkers Using Multiplex Assays in Aqueous Humor of Patients with Diabetic Retinopathy. BMC Ophthalmol. 2017, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, Z.; Liu, Y.; Lu, L.; Fu, Y. Assessment of Ocular Surface Damage during the Course of Type 2 Diabetes Mellitus. J. Ophthalmol. 2018, 2018, 1206808. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, E.W.; Hussain, A.; Rashid, W.; Qazi, I.A. Prevalence of Dry Eye and Tear Film Changes in Diabetic Population: Experience at Our Tertiary Care Centre. Int. J. Res. Med. Sci. 2023, 11, 961–966. [Google Scholar] [CrossRef]
- Kuo, Y.-K.; Shao, S.-C.; Lin, E.-T.; Pan, L.-Y.; Yeung, L.; Sun, C.-C. Tear Function in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 1036002. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Ahoor, M.H.; Ghorbani Haghjo, A.; Tabei, E.; Taheri, N. Assessment of Tear Inflammatory Cytokines Concentration in Patients with Diabetes with Varying Severity of Involvement. Exp. Eye Res. 2022, 224, 109233. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, K. Diabetes and dry eye. Int. J. Ocul. Oncol. Oculoplasty 2017, 3, 40–42. [Google Scholar]
- Sarkar, K.C.; Bhattacharyya, S.; Sarkar, P.; Maitra, A.; Mandal, R. An observational study on the prevalence of dry eyes in type 2 diabetes mellitus patients and its relation to the duration and severity of disease. J. Med. Sci. Health 2021, 7, 68–72. [Google Scholar] [CrossRef]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M.R. Prevalence of Dry Eye Syndrome and Diabetic Retinopathy in Type 2 Diabetic Patients. BMC Ophthalmol. 2008, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E. The Practical Detection of MMP-9 Diagnoses Ocular Surface Disease and May Help Prevent Its Complications. Cornea 2013, 32, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cancarini, A.; Fostinelli, J.; Napoli, L.; Gilberti, M.E.; Apostoli, P.; Semeraro, F. Trace Elements and Diabetes: Assessment of Levels in Tears and Serum. Exp. Eye Res. 2017, 154, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Fan, J.; Zhang, X.; Jiang, Y.; Zeng, S.; Pan, X.; Sheng, M.; Chen, Y. Sirt1 Attenuates Diabetic Keratopathy by Regulating the Endoplasmic Reticulum Stress Pathway. Life Sci. 2021, 265, 118789. [Google Scholar] [CrossRef]
- Alhalwani, A.Y.; Abudawood, K.; Qadizadah, A.B.E.A.; Jambi, S.; Sannan, N.S. Immunoglobulin A Levels and Its Correlation with Neutrophil-to-Lymphocyte Ratio as Inflammatory Biomarkers for Dry Eye Disease in Type 2 Diabetes: A Retrospective Study. Front. Immunol. 2023, 14, 1184862. [Google Scholar] [CrossRef] [PubMed]
- Efron, N. The Glenn A. Fry Award Lecture 2010: Ophthalmic Markers of Diabetic Neuropathy. Optometry Vis. Sci. 2011, 88, 661–683. [Google Scholar] [CrossRef] [PubMed]
- Dănilă, A.-I.; Ghenciu, L.A.; Stoicescu, E.R.; Bolintineanu, S.L.; Iacob, R.; Săndesc, M.-A.; Faur, A.C. Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review. Biomedicines 2024, 12, 747. [Google Scholar] [CrossRef]
- Ziegler, D.; Papanas, N.; Zhivov, A.; Allgeier, S.; Winter, K.; Ziegler, I.; Brüggemann, J.; Strom, A.; Peschel, S.; Köhler, B.; et al. Early Detection of Nerve Fiber Loss by Corneal Confocal Microscopy and Skin Biopsy in Recently Diagnosed Type 2 Diabetes. Diabetes 2014, 63, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Wang, R.; Jones, M.; Karson, N.; Jussel, A.; Smith, J.; Richdale, K.; Harrison, W. The Relationship between Corneal Nerve Density and Hemoglobin A1c in Patients with Prediabetes and Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Pritchard, N.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Utility of Corneal Confocal Microscopy for Assessing Mild Diabetic Neuropathy: Baseline Findings of the LANDMark Study. Clin. Exp. Optometry 2012, 95, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-S.; Yuan, Y.; Gu, Z.-X.; Zhuang, S.-L. Corneal Confocal Microscopy for Assessment of Diabetic Peripheral Neuropathy: A Meta-Analysis. Br. J. Ophthalmol. 2016, 100, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Craig, J.P.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Ellyett, K.; Kilfoyle, D.; Braatvedt, G.D. In Vivo Confocal Microscopy of Corneal Nerves: An Ocular Biomarker for Peripheral and Cardiac Autonomic Neuropathy in Type 1 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5060. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, N.; Edwards, K.; Dehghani, C.; Fadavi, H.; Jeziorska, M.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Russell, A.W.; Sampson, G.P.; et al. Longitudinal Assessment of Neuropathy in Type 1 Diabetes Using Novel Ophthalmic Markers (LANDMark): Study Design and Baseline Characteristics. Diabetes Res. Clin. Pract. 2014, 104, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Spektor, T.M.; Weisenberger, D.J.; Ding, H.; Patil, R.; Amador, C.; Song, X.-Y.; Chun, S.T.; Inzalaco, J.; Turjman, S.; et al. Reversal of Dual Epigenetic Repression of Non-Canonical Wnt-5a Normalises Diabetic Corneal Epithelial Wound Healing and Stem Cells. Diabetologia 2023, 66, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Kramerov, A.; Shah, R.; Ding, H.; Saghizadeh, M.; Ljubimova, J.; Ljubimov, A. Validation of nanoconjugates targeting various diabetes-associated protein markers for gene therapy of diabetic keratopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 757. [Google Scholar]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, L.; Li, Y.; Sun, D.; Chen, R.; Dou, S.; Liu, T.; Zhang, S.; Zhou, Q.; Xie, L. Interference of Sympathetic Overactivation Restores Limbal Stem/Progenitor Cells Function and Accelerates Corneal Epithelial Wound Healing in Diabetic Mice. Biomed. Pharmacother. 2023, 161, 114523. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Bastion, M.-L.C. Use of Commercially Available Sodium Hyaluronate 0.18% Eye Drops for Corneal Epithelial Healing in Diabetic Patients. Int. Ophthalmol. 2019, 39, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Tan, H.C.; Lin, M.T.-Y.; Mehta, J.S.; Liu, Y.-C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.; Petropoulos, I.N.; Khan, A.; Ponirakis, G.; MacDonald, R.; Alam, U.; Malik, R.A. Corneal Confocal Microscopy for the Diagnosis of Diabetic Peripheral Neuropathy: A Systematic Review and Meta-analysis. J. Diabetes Investig. 2022, 13, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.T.; Grosen, K.; Tankisi, H.; Charles, M.; Andersen, N.T.; Andersen, H.; Petropoulos, I.N.; Malik, R.A.; Jensen, T.S.; Karlsson, P. Corneal Confocal Microscopy as a Tool for Detecting Diabetic Polyneuropathy in a Cohort with Screen-Detected Type 2 Diabetes: ADDITION-Denmark. J. Diabetes Its Complicat. 2018, 32, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, F.; Khansari, M.; Berendschot, T.T.J.M.; Xu, X.; Dashtbozorg, B.; Sun, Y.; Zhang, J.; Tan, T. Automatic Corneal Nerve Fiber Segmentation and Geometric Biomarker Quantification. Eur. Phys. J. Plus 2020, 135, 266. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Nubile, M.; Lanzini, M.; Calienno, R.; Dua, H.S. In Vivo Microscopic and Optical Coherence Tomography Classification of Neurotrophic Keratopathy. J. Cell. Physiol. 2019, 234, 6108–6115. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha Dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior Segment Optical Coherence Tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Schlatter, A.; Chua, J.; Tan, B.; Garhöfer, G.; Schmetterer, L. Novel Approaches for Imaging-Based Diagnosis of Ocular Surface Disease. Diagnostics 2020, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Segev, F.; Geffen, N.; Galor, A.; Cohen, Y.; Gefen, R.; Belkin, A.; Arieli, Y.; Epshtein, S.; Oren, A.; Harris, A. Dynamic Assessment of the Tear Film Muco-Aqueous and Lipid Layers Using a Novel Tear Film Imager (TFI). Br. J. Ophthalmol. 2020, 104, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Blackie, C.A.; Solomon, J.D.; Scaffidi, R.C.; Greiner, J.V.; Lemp, M.A.; Korb, D.R. The Relationship between Dry Eye Symptoms and Lipid Layer Thickness. Cornea 2009, 28, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Duong, T.B.; Lin, M.; Papas, E. Imaging the Tear Film: A Comparison between the Subjective Keeler Tearscope-PlusTM and the Objective Oculus® Keratograph 5M and LipiView® Interferometer. Curr. Eye Res. 2018, 43, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Rosu, L.M.; Prodan-Bărbulescu, C.; Maghiari, A.L.; Bernad, E.S.; Bernad, R.L.; Iacob, R.; Stoicescu, E.R.; Borozan, F.; Ghenciu, L.A. Current Trends in Diagnosis and Treatment Approach of Diabetic Retinopathy during Pregnancy: A Narrative Review. Diagnostics 2024, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Kamrul-Hasan, A.B.M.; Mondal, S.; Nagendra, L.; Yadav, A.; Aalpona, F.T.Z.; Dutta, D. Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, M.; Wang, X.; Zhang, H.; Yao, C.; Sun, S.; Liu, Q.; Pan, H.; Liu, S.; Huan, Y.; et al. Glutazumab, a Novel Long-Lasting GLP-1/Anti-GLP-1R Antibody Fusion Protein, Exerts Anti-Diabetic Effects through Targeting Dual Receptor Binding Sites. Biochem. Pharmacol. 2018, 150, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Wong, E. Afrezza (Insulin Human) Inhalation Powder: A New Inhaled Insulin for the Management of Type-1 or Type-2 Diabetes Mellitus. Pharm. Ther. 2015, 40, 735–741. [Google Scholar]
- Abdul-Hamid, M.; Moustafa, N. Amelioration of Alloxan-Induced Diabetic Keratopathy by Beta-Carotene. Exp. Toxicol. Pathol. 2014, 66, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Bikbov, M.; Yamamoto, S. Diabetic corneal neuropathy: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 981–987. [Google Scholar] [PubMed]
- Sosne, G.; Kleinman, H.K.; Springs, C.; Gross, R.H.; Sung, J.; Kang, S. 0.1% RGN-259 (Thymosin β4) Ophthalmic Solution Promotes Healing and Improves Comfort in Neurotrophic Keratopathy Patients in a Randomized, Placebo-Controlled, Double-Masked Phase III Clinical Trial. Int. J. Mol. Sci. 2023, 24, 554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Q.; Luo, Y.; Nguyen, T.; Rosenblatt, M.I.; Guaiquil, V.H. Semaphorin3A Induces Nerve Regeneration in the Adult Cornea-a Switch from Its Repulsive Role in Development. PLoS ONE 2018, 13, e0191962. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-Loaded Silicone Hydrogels as Contact Lenses to Address Diabetic-Eye Complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P Promotes Diabetic Corneal Epithelial Wound Healing through Molecular Mechanisms Mediated via the Neurokinin-1 Receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, P.; Di, G.; Zhang, Y.; Wang, Y.; Qi, X.; Duan, H.; Xie, L. Ciliary Neurotrophic Factor Promotes the Activation of Corneal Epithelial Stem/Progenitor Cells and Accelerates Corneal Epithelial Wound Healing. Stem Cells 2015, 33, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Morishige, N.; Uemura, A.; Morita, Y.; Nishida, T. Promotion of Corneal Epithelial Wound Healing in Diabetic Rats by the Fibronectin-Derived Peptide PHSRN. Cornea 2017, 36, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Di, G.; Du, X.; Qi, X.; Zhao, X.; Duan, H.; Li, S.; Xie, L.; Zhou, Q. Mesenchymal Stem Cells Promote Diabetic Corneal Epithelial Wound Healing through TSG-6–Dependent Stem Cell Activation and Macrophage Switch. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4344. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from Normal and Diabetic Human Corneolimbal Keratocytes Differentially Regulate Migration, Proliferation and Marker Expression of Limbal Epithelial Cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, X.; Kan, T. MiR-34c Participates in Diabetic Corneal Neuropathy via Regulation of Autophagy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 16. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Leszczynska, A.; Wei, G.; Winkler, M.A.; Tang, J.; Funari, V.A.; Deng, N.; Liu, Z.; Punj, V.; Deng, S.X.; et al. Genome-Wide Analysis Suggests a Differential microRNA Signature Associated with Normal and Diabetic Human Corneal Limbus. Sci. Rep. 2017, 7, 3448. [Google Scholar] [CrossRef] [PubMed]
- Herencia-Bueno, K.E.; Aldrovani, M.; Crivelaro, R.M.; Thiesen, R.; Barros-Sobrinho, A.A.F.; Claros-Chacaltana, F.D.Y.; Padua, I.R.M.; Santos, D.M.; Laus, J.L. Reduction in Histone H3 Acetylation and Chromatin Remodeling in Corneas of Alloxan-Induced Diabetic Rats. Cornea 2018, 37, 624–632. [Google Scholar] [CrossRef]
- Lin, J.B.; Shen, X.; Pfeifer, C.W.; Shiau, F.; Santeford, A.; Ruzycki, P.A.; Clark, B.S.; Liu, Q.; Huang, A.J.W.; Apte, R.S. Dry eye disease in mice activates adaptive corneal epithelial regeneration distinct from constitutive renewal in homeostasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2204134120. [Google Scholar] [CrossRef]
- Chen, D.; Wang, L.; Guo, X.; Zhang, Z.; Xu, X.; Jin, Z.; Liang, Q. Evaluation of Limbal Stem Cells in Patients with Type 2 Diabetes: An In Vivo Confocal Microscopy Study. Cornea 2024, 43, 67–75. [Google Scholar] [CrossRef]
- Nureen, L.; Di Girolamo, N. Limbal Epithelial Stem Cells in the Diabetic Cornea. Cells 2023, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Wang, H.; Li, M.; Wang, X.; Liu, R.; Zhang, D.; Xu, W. Corneal Regeneration Strategies: From Stem Cell Therapy to Tissue Engineered Stem Cell Scaffolds. Biomed. Pharmacother. 2023, 165, 115206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; Backman, L.J.; Zhou, Q.; Danielson, P. Ciliary Neurotrophic Factor Promotes the Migration of Corneal Epithelial Stem/Progenitor Cells by Up-Regulation of MMPs through the Phosphorylation of Akt. Sci. Rep. 2016, 6, 25870. [Google Scholar] [CrossRef] [PubMed]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Venkateswaran, N. The Role of KPI-121 0.25% in the Treatment of Dry Eye Disease: Penetrating the Mucus Barrier to Treat Periodic Flares. Ophthalmol. Eye Dis. 2021, 13, 251584142110127. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Shtein, R.M.; Sugar, A.; Soong, H.K.; Woodward, M.A.; DeLoss, K.; Mian, S.I. Long-Term Use of Autologous Serum 50% Eye Drops for the Treatment of Dry Eye Disease. Cornea 2014, 33, 1245–1251. [Google Scholar] [CrossRef]
- Ghenciu, L.A.; Faur, A.C.; Bolintineanu, S.L.; Salavat, M.C.; Maghiari, A.L. Recent Advances in Diagnosis and Treatment Approaches in Fungal Keratitis: A Narrative Review. Microorganisms 2024, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Bonini, S.; Lambiase, A.; Rama, P.; et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Suri, K.; Amouzegar, A.; Li, M.; Katikireddy, K.R.; Mittal, S.K.; Chauhan, S.K. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol. Ther. 2017, 25, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Patel, D.V.; McGhee, C.N.; Alany, R.G. New Therapeutic Approaches in the Treatment of Diabetic Keratopathy: A Review: Treatment of Neurotrophic Corneal Ulcers. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Raîche-Marcoux, G.; Maranda, C.; Bertrand, N.; Boisselier, E. Animal Models in Eye Research: Focus on Corneal Pathologies. Int. J. Mol. Sci. 2023, 24, 16661. [Google Scholar] [CrossRef]

| Ocular Structure | Diabetic Alteration |
|---|---|
| Epithelium | Increased susceptibility to damage, delayed wound healing, epithelial defects |
| Stroma | Corneal edema, decreased sensitivity, alterations in collagen structure |
| Endothelium | Endothelial dysfunction, decreased cell density, increased risk of corneal edema |
| Tear film | Reduced tear secretion, altered composition (e.g., increased osmolarity) |
| Treatment | Route of Administration | Current State of Development |
|---|---|---|
| Teplizumab [87] | intravenous infusion | FDA approved (2022) |
| Glutazumab [88] | intravenous injection | clinical trials, approved in a few countries |
| Canaglifolzin [91] | oral | FDA approved (2013) |
| Dapaglifolzin [91] | oral | FDA approved (2014) |
| Technosphere insulin [89] | subcutaneous bolus | FDA approved (2014) |
| Beta-carotene [90] | systemic | human clinical trials |
| Thymosin beta 4 [92] | eye drops | phase III clinical trial |
| Semaphorins [93] | intrastromal injection | clinical trials |
| Nerve growth factor [94] | eye drops | clinical trials |
| Epalerestat [95] | silicon hydrogel contact lens | animal trials |
| Substance P [96] | eye drops | animal trials |
| Ciliary neurotrophic factor [97] | subconjunctival injection | animal trials |
| Fibronectin-derived peptide PHSRN [98] | eye drops | animal trials |
| Study | Genetic/Epigenetic Target | Mechanisms |
|---|---|---|
| Leszczynska et al. [100] | limbal stromal cell-derived exosomes | When primary limbal epithelial cells are cultured with exosomes, their proliferation rate is noticeably higher than that of untreated cells. Wound healing is importantly increased. |
| Shah et al. [70] | Wnt-5a | Improved the healing process while also increasing the levels of limbal epithelial stem cells. |
| Hu et al. [101] | miR-34c | By directly interacting with antigen4B, miR-34c influences both the development of trigeminal sensory neurons and the healing of diabetic corneal nerve endings. Subconjunctival injection may promote corneal epithelial healing. |
| Kramerov [71] | miR-203a antagomir inhibitor | Improved Wnt5a expression and accelerated the healing process in limbal epithelial cells. |
| Bikbova [91] | recombinant adenovirus (rAV)-driven small hairpin RNA (rAV-sh) | Improved wound healing, corneal epithelial and limbal stem cells. Even more effective when in a combined treatment with the overexpression of C-met. |
| Kulkarni et al. [102] | miR-10b | Regulated limbal epithelial stem cells’ early proliferation state during renewal and division, improved stem cell function and corneal homeostasis. |
| Herencia-Bueno et al. [103] | histone H3 | Reduced chromatin compaction changes in both epithelial and stromal cells, as well as a decrease in histone H3 acetylation, were seen in diabetic rats’ corneal sensitivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghenciu, L.A.; Hațegan, O.A.; Bolintineanu, S.L.; Dănilă, A.-I.; Faur, A.C.; Prodan-Bărbulescu, C.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review. Biomedicines 2024, 12, 1303. https://doi.org/10.3390/biomedicines12061303
Ghenciu LA, Hațegan OA, Bolintineanu SL, Dănilă A-I, Faur AC, Prodan-Bărbulescu C, Stoicescu ER, Iacob R, Șișu AM. Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review. Biomedicines. 2024; 12(6):1303. https://doi.org/10.3390/biomedicines12061303
Chicago/Turabian StyleGhenciu, Laura Andreea, Ovidiu Alin Hațegan, Sorin Lucian Bolintineanu, Alexandra-Ioana Dănilă, Alexandra Corina Faur, Cătălin Prodan-Bărbulescu, Emil Robert Stoicescu, Roxana Iacob, and Alina Maria Șișu. 2024. "Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review" Biomedicines 12, no. 6: 1303. https://doi.org/10.3390/biomedicines12061303







