Effect of 5-Aminolevulinic Acid (5-ALA) in “ALADENT” Gel Formulation and Photodynamic Therapy (PDT) against Human Oral and Pancreatic Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Culture
2.3. Light Source and Irradiation Parameters
2.4. Cell Treatment
2.5. Cytotoxicity and Cell-Viability Assay (MTS Assay)
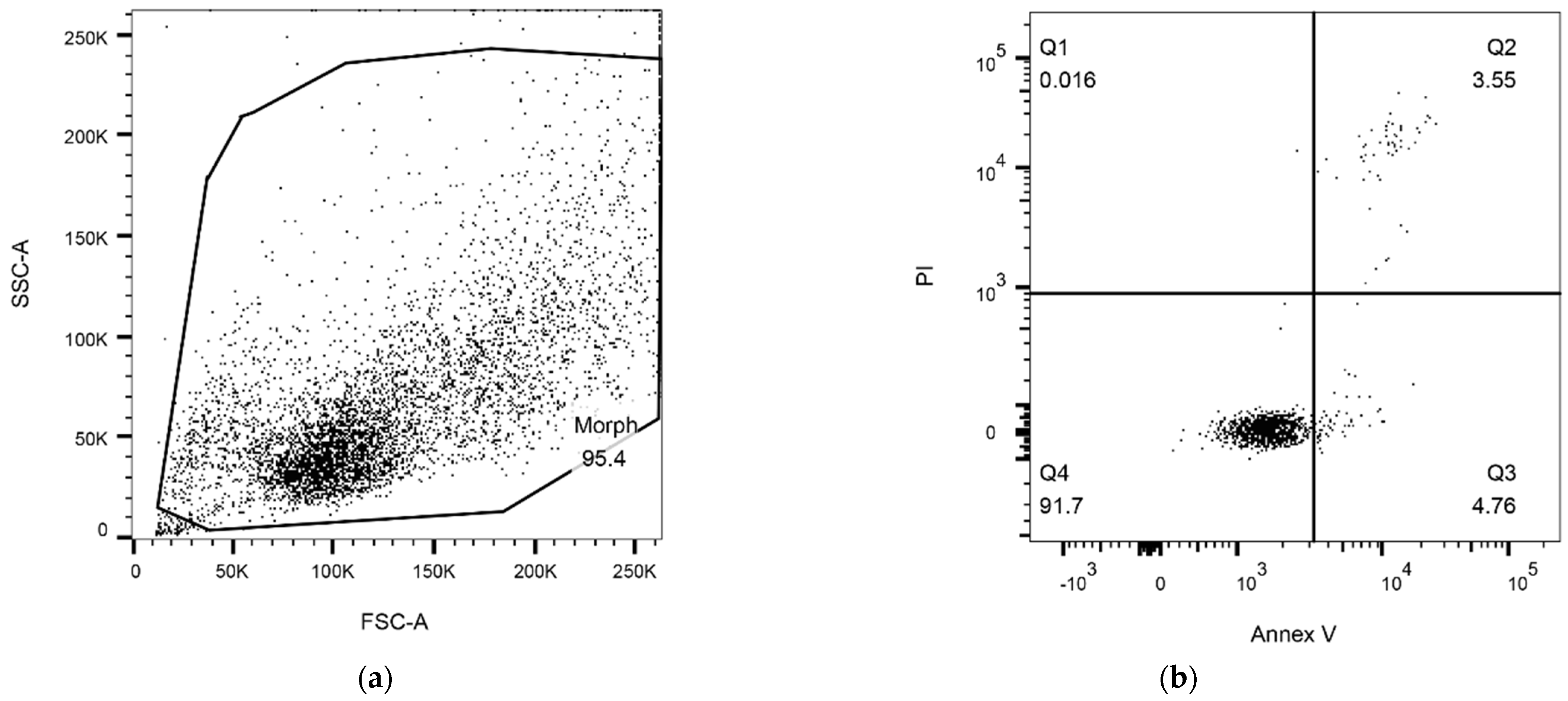
2.6. Flow Cytometry Analysis
2.6.1. Detection of Apoptosis
2.6.2. Cell-Cycle Analysis
2.6.3. Reactive Oxygen Species (ROS) Levels
2.7. PpIX Fluorescence Measurements
2.8. Statistical Analysis
3. Results
3.1. Cytotoxicity
3.2. Apoptosis Rates and Cell-Cycle Analysis
3.3. ROS-Level Variation
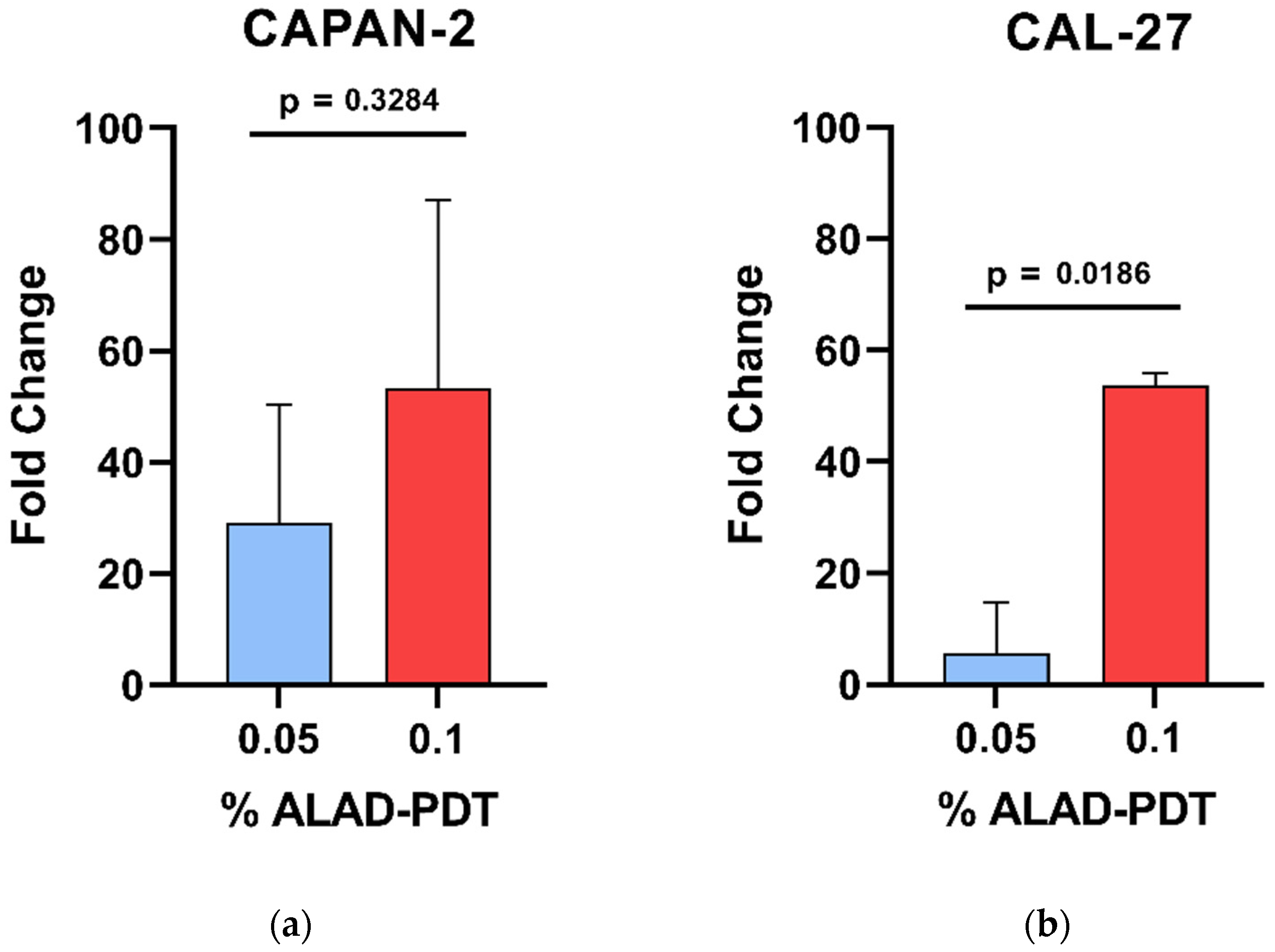
3.4. Generation of Intracellular PpIX
4. Discussion
Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | Pancreatic cancer |
| OSCC | Oral squamous-cell carcinoma |
| 5-ALA | 5-aminolevulinic acid |
| PpIX | Protoporphyrin IX |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| ALAD | ALADENT |
| HGF-1 | Human gingival fibroblast |
| FECH | Ferrochelatase |
| HClO4 | Perchloric acid |
| Control | CTRL |
| MED | Minimum effective dose |
| PL | Poloxamer |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| PI | Propidium iodide |
| FITC | Fluorescein-isothiocyanate |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| TLR | Toll-like receptor |
| AlGaAs | Aluminium gallium arsenide |
| FHWM | Full width half maximum |
| CM-H2DCFDA | 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester |
References
- International Agency for Research on Cancer (IARC). Globocan 2020. Available online: https://gco.iarc.fr/today/data/fact-sheets/cancers/13-Pancreas-fact-sheet.pdf (accessed on 12 May 2024).
- Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 12 May 2024).
- Cancer Stat Facts: Oral Cavity and Pharynx Cancer. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html (accessed on 12 May 2024).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Baek, B.; Lee, H. Prediction of survival and recurrence in patients with pancreatic cancer by integrating multi-omics data. Sci. Rep. 2020, 10, 18951. [Google Scholar] [CrossRef] [PubMed]
- Graboyes, E.M.; Kompelli, A.R.; Neskey, D.M.; Brennan, E.; Nguyen, S.; Sterba, K.R.; Warren, G.W.; Hughes-Halbert, C.; Nussenbaum, B.; Day, T.A. Association of Treatment Delays with Survival for Patients with Head and Neck Cancer: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Noronha, V.; Joshi, A.; Abhyankar, A.; Menon, N.; Dhumal, S.; Prabhash, K. Beyond conventional chemotherapy, targeted therapy and immunotherapy in squamous cell cancer of the oral cavity. Oral. Oncol. 2020, 105, 104673. [Google Scholar] [CrossRef]
- Parmar, K.; Mohamed, A.; Vaish, E.; Thawani, R.; Cetnar, J.; Thein, K.Z. Immunotherapy in head and neck squamous cell carcinoma: An updated review. Cancer Treat. Res. Commun. 2022, 33, 100649. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Zheng, S.; He, Y. Photodynamic therapy in the treatment of oral leukoplakia: A systematic review. Photodiagnosis Photodyn. Ther. 2019, 25, 17–22. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, D.; Basilion, J.P. Photodynamic Therapy: Targeting Cancer Biomarkers for the Treatment of Cancers. Cancers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Markina, Y.V.; Markin, A.M.; Kirichenko, T.V.; Tolstik, T.V.; Cherednichenko, V.R.; Kiseleva, D.G.; Orekhov, A.N. Effect of 5-aminolevulinic Acid on Mitochondrial Activity. Front. Biosci. 2023, 15, 17. [Google Scholar] [CrossRef]
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic Therapy—A Non-invasive Treatment Modality for Precancerous Lesions. J. Lasers Med. Sci. 2016, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Q.; Luo, Y.; He, Z.; Tian, X.; Battaglia, G. Thermosensitive nanocomposite gel for intra-tumoral two-photon photodynamic therapy. J. Control. Release 2019, 298, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Cannarozzo, G.; et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. Ital. J. Dermatol. Venereol. 2021, 156, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Peng, Q.; Gurny, R.; Lange, N. Thermosetting gel for the delivery of 5-aminolevulinic acid esters to the cervix. J. Pharm. Sci. 2008, 97, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.M.; Cannizzaro, M.V.; Dattola, A.; Garofalo, V.; Del Duca, E.; Ventura, A.; Milani, M.; Campione, E.; Bianchi, L. The efficacy and tolerability of 5-aminolevulinic acid 5% thermosetting gel photodynamic therapy (PDT) in the treatment of mild-to-moderate acne vulgaris. A two-center, prospective assessor-blinded, proof-of-concept study. J. Cosmet. Dermatol. 2019, 18, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Scarabello, A.; Pulvirenti, C.; Adebanjo, G.A.R.; Parisella, F.R.; Chello, C.; Tammaro, A. Photodynamic therapy with 5 aminolaevulinic acid: A promising therapeutic option for the treatment of Hailey-Hailey disease. Photodiagnosis Photodyn. Ther. 2022, 38, 102794. [Google Scholar] [CrossRef] [PubMed]
- Bourre, L.; Thibaut, S.; Briffaud, A.; Lajat, Y.; Patrice, T. Potential efficacy of a delta 5-aminolevulinic acid thermosetting gel formulation for use in photodynamic therapy of lesions of the gastrointestinal tract. Pharmacol. Res. 2002, 45, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a novel gel containing 5-aminolevulinic acid and red LED against bacteria involved in peri-implantitis and other oral infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef]
- Pignatelli, P.; Umme, S.; D’Antonio, D.L.; Piattelli, A.; Curia, M.C. Reactive Oxygen Species Produced by 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Cancer. Int. J. Mol. Sci. 2023, 24, 8964. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, Y.K.; Hu, C.S.; Xiao, L.Y.; Huang, W.L.; Chi, T.C.; Cheng, K.H.; Wang, Y.M.; Yuan, S.F. MAL-PDT inhibits oral precancerous cells and lesions via autophagic cell death. Oral. Dis. 2019, 25, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Li, Q.; Luo, Y.; Liu, Y.; Liu, D.; Li, B.; Wang, T. FECH Expression Correlates with the Prognosis and Tumor Immune Microenvironment in Clear Cell Renal Cell Carcinoma. J. Oncol. 2022, 2022, 8943643. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Tatsumi, Y.; Hori, S.; Morizawa, Y.; Iida, K.; Onishi, K.; Miyake, M.; Oda, Y.; Owari, T.; Fujii, T.; et al. 5-Aminolevurinic acid inhibits the proliferation of bladder cancer cells by activating heme synthesis. Oncol. Rep. 2022, 48, 186. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Murayama, Y.; Kamada, Y.; Arita, T.; Kosuga, T.; Konishi, H.; Morimura, R.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; et al. Radiosensitizing effect of 5-aminolevulinic acid in colorectal cancer. Oncol. Lett. 2019, 17, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Kraus, D.; Mansi, M.; Braun, A.; Howley, R.; Myers, K.A.; Chen, B. Ferrochelatase Deficiency Abrogated the Enhancement of Aminolevulinic Acid-mediated Protoporphyrin IX by Iron Chelator Deferoxamine. Photochem. Photobiol. 2019, 95, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, H.; Fu, L.; Cui, J.; Yang, Y. Global Trends and Research Progress of Photodynamic Therapy in Skin Cancer: A Bibliometric Analysis and Literature Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 479–498. [Google Scholar] [CrossRef]
- Tanaka, Y.; Murayama, Y.; Matsumoto, T.; Kubo, H.; Harada, K.; Matsuo, H.; Kubota, T.; Okamoto, K.; Otsuji, E. Efficacy of 5-aminolevulinic acid-mediated photodynamic therapy in a mouse model of esophageal cancer. Oncol. Lett. 2020, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.; Bedia, C.; Diao, D.; Mosteiro, A.; Ferrés, A.; Stanzani, E.; Martínez-Soler, F.; Tortosa, A.; Pineda, E.; Aldecoa, I.; et al. Preclinical Studies with Glioblastoma Brain Organoid Co-Cultures Show Efficient 5-ALA Photodynamic Therapy. Cells 2023, 12, 1125. [Google Scholar] [CrossRef]
- Kamanlı, A.F.; Yıldız, M.Z.; Özyol, E.; Deveci Ozkan, A.; Sozen Kucukkara, E.; Guney Eskiler, G. Investigation of LED-based photodynamic therapy efficiency on breast cancer cells. Lasers Med. Sci. 2021, 36, 563–569. [Google Scholar] [CrossRef]
- Sansaloni-Pastor, S.; Lange, N. Unleashing the potential of 5-Aminolevulinic acid: Unveiling a promising target for cancer diagnosis and treatment beyond photodynamic therapy. J. Photochem. Photobiol. B 2023, 247, 112771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pecquenard, F.; Baydoun, M.; Quilbé, A.; Moralès, O.; Leroux, B.; Aoudjehane, L.; Conti, F.; Boleslawski, E.; Delhem, N. An Efficient 5-Aminolevulinic Acid Photodynamic Therapy Treatment for Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 426. [Google Scholar] [CrossRef] [PubMed]
- Gondivkar, S.M.; Gadbail, A.R.; Choudhary, M.G.; Vedpathak, P.R.; Likhitkar, M.S. Photodynamic treatment outcomes of potentially-malignant lesions and malignancies of the head and neck region: A systematic review. J. Investig. Clin. Dent. 2018, 9, e12270. [Google Scholar] [CrossRef] [PubMed]
- Kelloniemi, E.; Järvinen, R.; Hellström, P.; Rintala, E.; Aaltomaa, S.; Isotalo, T.; Innos, K.; Kaasinen, E. Repeated 5-aminolevulinic Acid Instillations During Follow-up in Non-muscle-invasive Bladder Cancer: A Randomized Study. In Vivo 2021, 35, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Kohoutova, D.; Haidry, R.; Banks, M.; Butt, M.A.; Dunn, J.; Thorpe, S.; Lovat, L. Long-term outcomes of the randomized controlled trial comparing 5-aminolaevulinic acid and Photofrin photodynamic therapy for Barrett’s oesophagus related neoplasia. Scand. J. Gastroenterol. 2018, 53, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Y.; Tong, Y.; Zhang, L.; Li, P.; Zhang, H.; Zhang, X.; Tang, Y.; Qin, L.; Shen, Y.; et al. Effect and rational application of topical photodynamic therapy (PDT) with 5-aminolevulinic acid (5-ALA) for treatment of cervical intraepithelial neoplasia with vaginal intraepithelial neoplasia. Photodiagnosis Photodyn. Ther. 2022, 37, 102634. [Google Scholar] [CrossRef] [PubMed]
- Bai-Habelski, J.C.; Ko, A.; Ortland, C.; Stocker, M.; Ebeling, A.; Reinhold, U. 5-ALA loaded self-adhesive patch-PDT is effective and safe in the treatment of actinic keratoses on hands and arms. Exp. Dermatol. 2022, 31, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chelakkot, V.S.; Newhook, N.; Tucker, S.; Hirasawa, K. Inflammatory cell death induced by 5-aminolevulinic acid-photodynamic therapy initiates anticancer immunity. Front. Oncol. 2023, 13, 1156763. [Google Scholar] [CrossRef]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef]
- Regula, J.; Ravi, B.; Bedwell, J.; MacRobert, A.J.; Bown, S.G. Photodynamic therapy using 5-aminolaevulinic acid for experimental pancreatic cancer–prolonged animal survival. Br. J. Cancer 1994, 70, 248–254. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Li, W.; Wang, Q.; Han, Y.; Liu, H. Differential in vitro sensitivity of oral precancerous and squamous cell carcinoma cell lines to 5-aminolevulinic acid-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101554. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Y.S.; Wright, K.; Melcher, A.; Jayne, D. Anti-cancer effects of oncolytic viral therapy combined with photodynamic therapy in human pancreatic cancer cell lines. Lancet 2015, 385 (Suppl. S1), S56. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Bertagnolo, V.; Pierdomenico, L.; Bascelli, A.; Santavenere, E.; Alinari, L.; Capitani, S.; Miscia, S.; Marchisio, M. Enhancement of TRAIL cytotoxicity by AG-490 in human ALL cells is characterized by downregulation of cIAP-1 and cIAP-2 through inhibition of Jak2/Stat3. Cell Res. 2009, 19, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Bedner, E.; Smolewski, P. Flow cytometry in analysis of cell cycle and apoptosis. Semin. Hematol. 2001, 38, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Telford, W.G.; Komoriya, A.; Packard, B.Z.; Bagwell, C.B. Multiparametric analysis of apoptosis by flow cytometry. Methods Mol. Biol. 2011, 699, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Bologna, G.; Lanuti, P.; D’Ambrosio, P.; Tonucci, L.; Pierdomenico, L.; D’Emilio, C.; Celli, N.; Marchisio, M.; d’Alessandro, N.; Santavenere, E.; et al. Water-soluble platinum phthalocyanines as potential antitumor agents. Biometals 2014, 27, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Bech, O.; Gaullier, J.M.; Stokke, T.; Steen, H.B.; Ma, L.W.; Berg, K. Protoporphyrin IX accumulation in cells treated with 5-aminolevulinic acid: Dependence on cell density, cell size and cell cycle. Int. J. Cancer 1998, 75, 134–139. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Mokoena, D.R.; George, B.P.; Abrahamse, H. Photodynamic Therapy Induced Cell Death Mechanisms in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 10506. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Harada, Y.; Minamikawa, T.; Tanaka, H.; Otsuji, E.; Takamatsu, T. Recent advances in photodynamic diagnosis of gastric cancer using 5-aminolevulinic acid. World J. Gastroenterol. 2016, 22, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Babilas, P.; Karrer, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology—An update 2008. J. Dtsch. Dermatol. Ges. 2008, 6, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Fahey, J.M.; Korbelik, M. Photodynamic Therapy as an Oxidative Anti-Tumor Modality: Negative Effects of Nitric Oxide on Treatment Efficacy. Pharmaceutics 2021, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, P.; Chen, F.; Li, L.; Luo, R. Effect and mechanism of 5-aminolevulinic acid-mediated photodynamic therapy in esophageal cancer. Lasers Med. Sci. 2011, 26, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rosin, F.C.P.; Teixeira, M.G.; Pelissari, C.; Corrêa, L. Photodynamic Therapy Mediated by 5-aminolevulinic Acid Promotes the Upregulation and Modifies the Intracellular Expression of Surveillance Proteins in Oral Squamous Cell Carcinoma. Photochem. Photobiol. 2019, 95, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Shi, S.R.; Wan, G.Y.; Wang, Y.S.; Wang, Y.; Zhang, L.Y.; Zhao, Y.H. Study on the in vivo and in vitro effects of photodynamic therapy mediated by 5-aminolevulinic acid on oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, N.; Meng, J.; Wen, N. The use of topical ALA-photodynamic therapy combined with induction chemotherapy for locally advanced oral squamous cell carcinoma. Am. J. Otolaryngol. 2021, 42, 103112. [Google Scholar] [CrossRef]
- Abd-Elgaliel, W.R.; Cruz-Monserrate, Z.; Wang, H.; Logsdon, C.D.; Tung, C.H. Pancreatic cancer-associated Cathepsin E as a drug activator. J. Control. Release 2013, 167, 221–227. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Antonio, D.L.; Marchetti, S.; Pignatelli, P.; Umme, S.; De Bellis, D.; Lanuti, P.; Piattelli, A.; Curia, M.C. Effect of 5-Aminolevulinic Acid (5-ALA) in “ALADENT” Gel Formulation and Photodynamic Therapy (PDT) against Human Oral and Pancreatic Cancers. Biomedicines 2024, 12, 1316. https://doi.org/10.3390/biomedicines12061316
D’Antonio DL, Marchetti S, Pignatelli P, Umme S, De Bellis D, Lanuti P, Piattelli A, Curia MC. Effect of 5-Aminolevulinic Acid (5-ALA) in “ALADENT” Gel Formulation and Photodynamic Therapy (PDT) against Human Oral and Pancreatic Cancers. Biomedicines. 2024; 12(6):1316. https://doi.org/10.3390/biomedicines12061316
Chicago/Turabian StyleD’Antonio, Domenica Lucia, Simona Marchetti, Pamela Pignatelli, Samia Umme, Domenico De Bellis, Paola Lanuti, Adriano Piattelli, and Maria Cristina Curia. 2024. "Effect of 5-Aminolevulinic Acid (5-ALA) in “ALADENT” Gel Formulation and Photodynamic Therapy (PDT) against Human Oral and Pancreatic Cancers" Biomedicines 12, no. 6: 1316. https://doi.org/10.3390/biomedicines12061316
APA StyleD’Antonio, D. L., Marchetti, S., Pignatelli, P., Umme, S., De Bellis, D., Lanuti, P., Piattelli, A., & Curia, M. C. (2024). Effect of 5-Aminolevulinic Acid (5-ALA) in “ALADENT” Gel Formulation and Photodynamic Therapy (PDT) against Human Oral and Pancreatic Cancers. Biomedicines, 12(6), 1316. https://doi.org/10.3390/biomedicines12061316







