Pharmacological Potential of Three Berberine-Containing Plant Extracts Obtained from Berberis vulgaris L., Mahonia aquifolium (Pursh) Nutt., and Phellodendron amurense Rupr
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
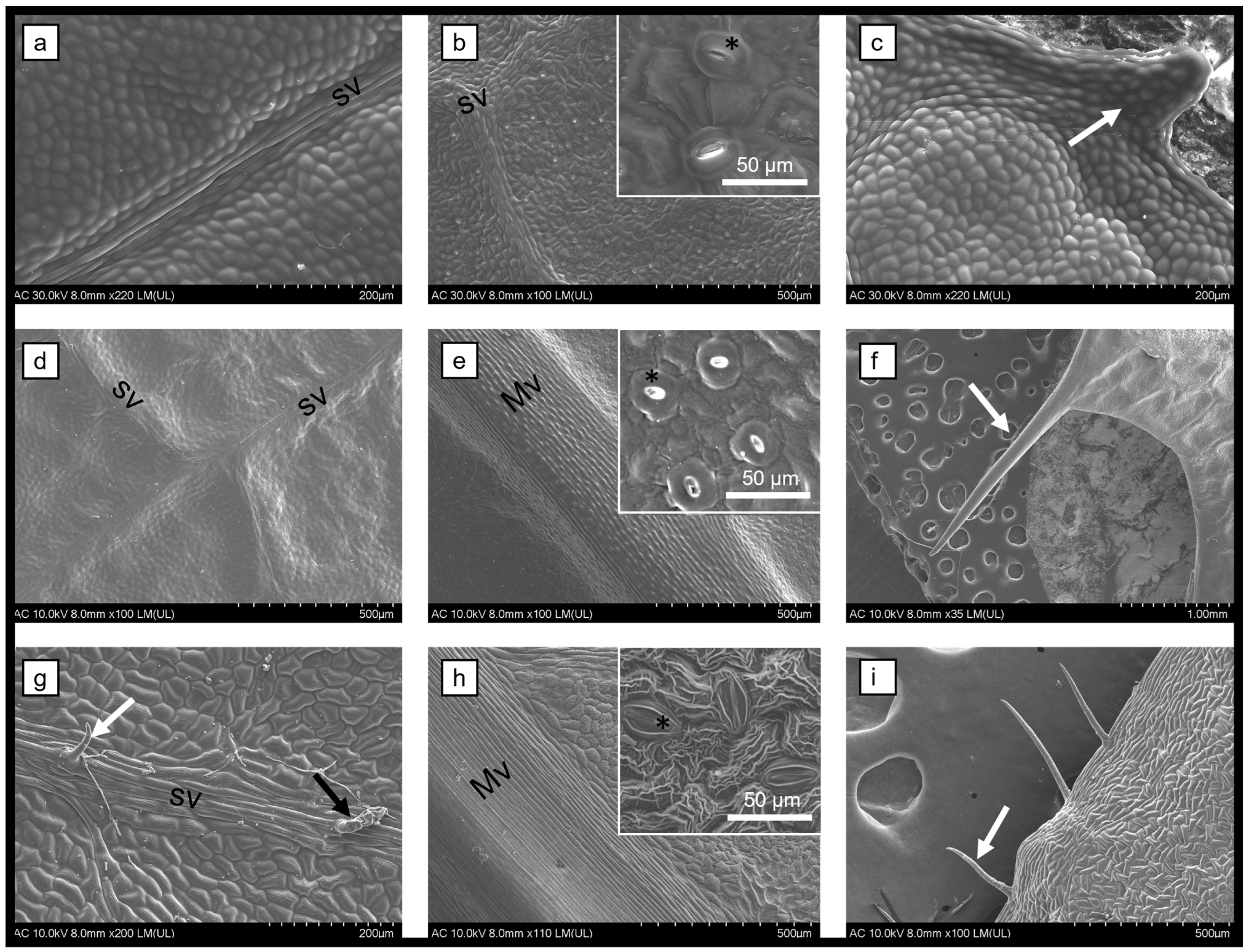
2.2. SEM Examination
2.3. Extract Preparation
2.4. Plant Extract Characterization
2.4.1. Phytochemical Analysis
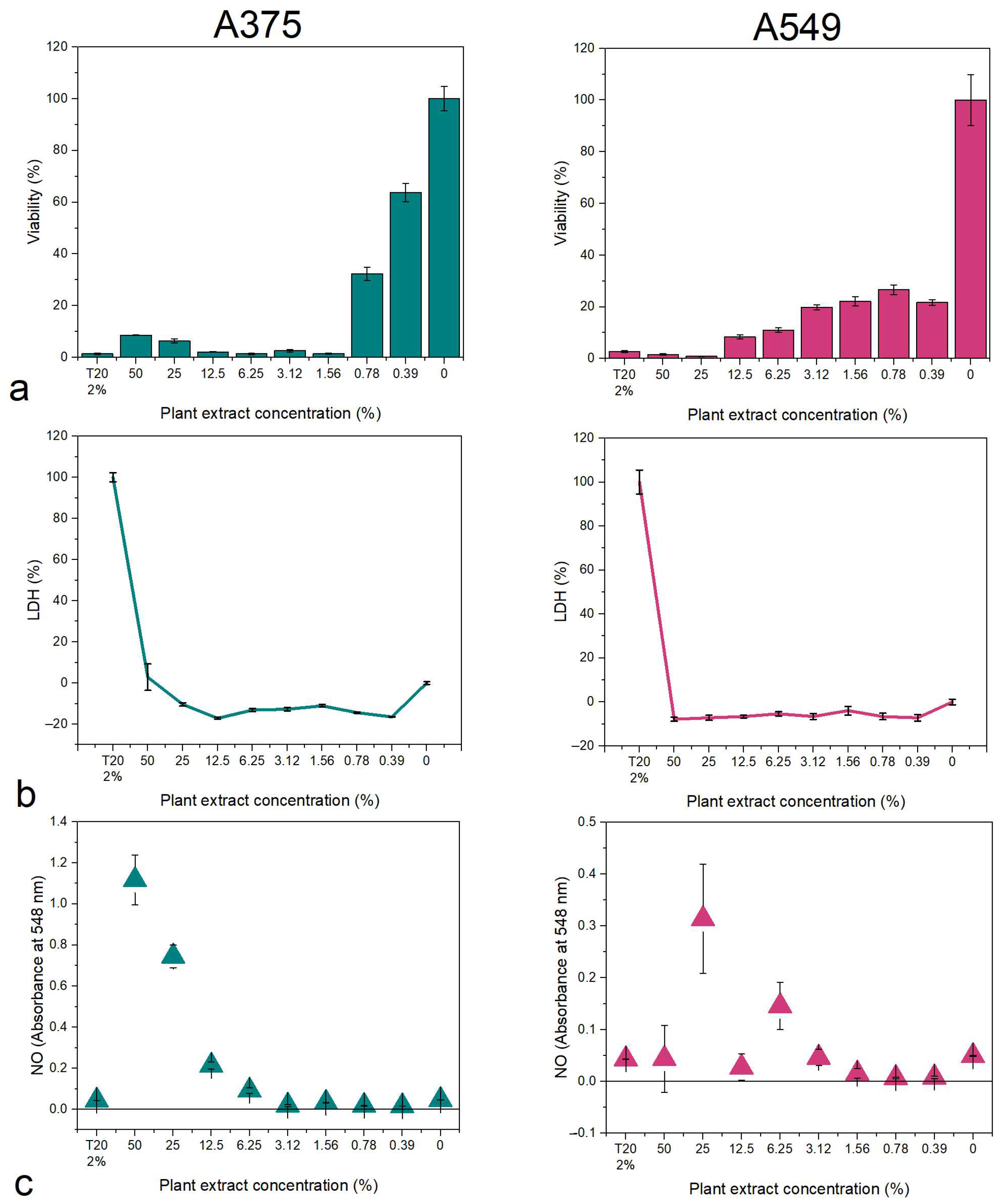
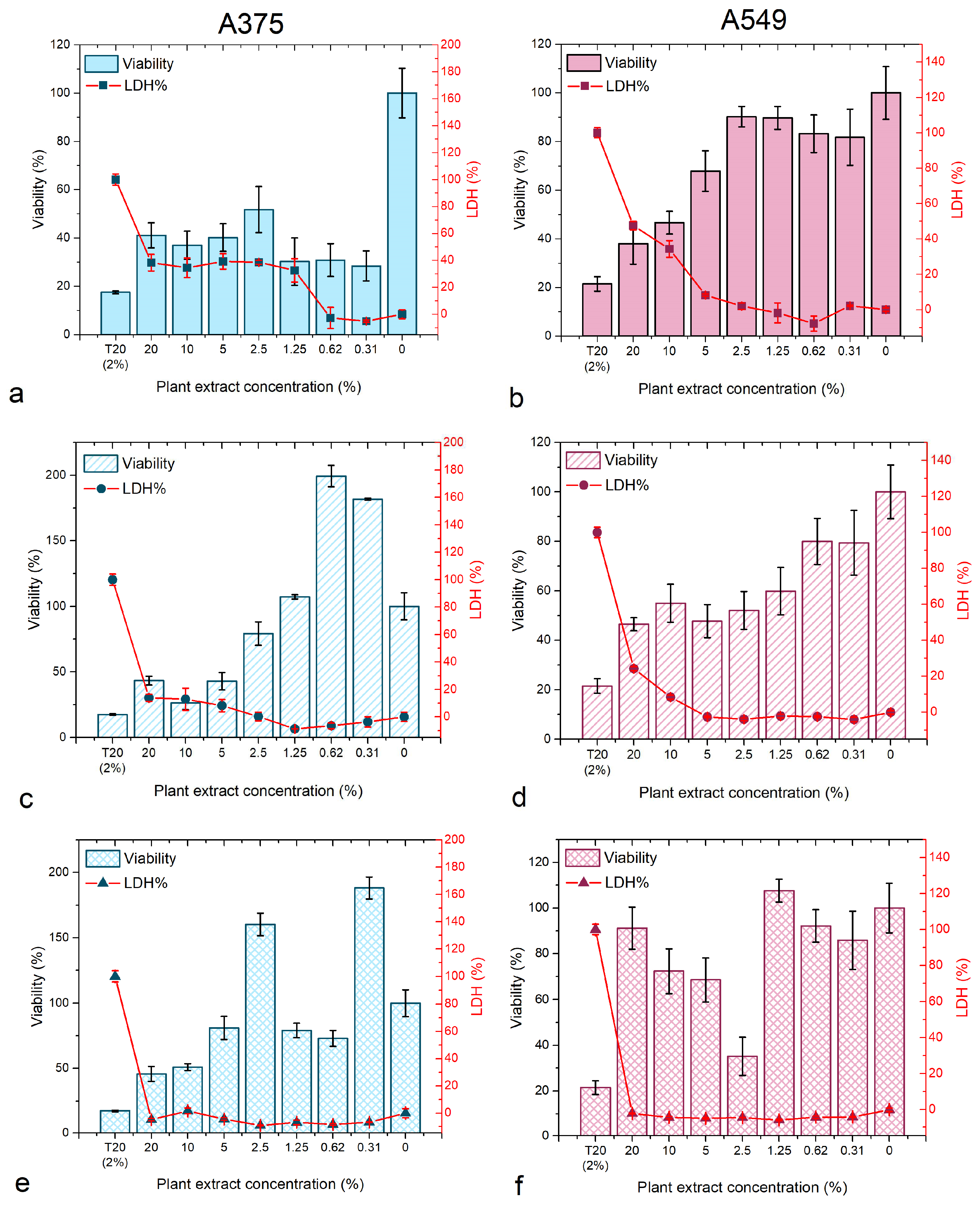
2.4.2. Cytotoxicity Assays
2.4.3. Nanoparticle Synthesis
3. Results
3.1. Plant Characterization
3.1.1. Stems and Leaves of Berberis vulgaris, Mahonia aquifolium, and Phellodendron amurense
3.1.2. Chemical Compounds in Plant Extracts
3.2. Pharmacological Potential
3.2.1. Cytotoxicity Assays
3.2.2. Nanoparticle Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A.-Z. Antioxidant, Antibacterial, and Antifungal Activities of the Ethanolic Extract Obtained from Berberis vulgaris Roots and Leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef] [PubMed]
- Gıdık, B. Antioxidant, Antimicrobial Activities and Fatty Acid Compositions of Wild Berberis spp. by Different Techniques Combined with Chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef] [PubMed]
- Andreicuț, A.D.; Fischer-Fodor, E.; Pârvu, A.E.; Ţigu, A.B.; Cenariu, M.; Pârvu, M.; Cătoi, F.A.; Irimie, A. Antitumoral and immunomodulatory effect of Mahonia aquifolium extracts. Oxidative Med. Cell. Longev. 2019, 2019, 6439021. [Google Scholar] [CrossRef] [PubMed]
- Milata, V.; Svedova, A.; Barbierikova, Z.; Holubkova, E.; Cipakova, I.; Cholujova, D.; Jakubikova, J.; Panik, M.; Jantova, S.; Brezova, V. Synthesis and anticancer activity of novel 9-O-substituted berberine derivatives. Int. J. Mol. Sci. 2019, 20, 2169. [Google Scholar] [CrossRef] [PubMed]
- Andreicut, A.-D.; Pârvu, A.E.; Mot, A.C.; Pârvu, M.; Fischer Fodor, E.; Cătoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical Analysis of Anti-Inflammatory and Antioxidant Effects of Mahonia aquifolium Flower and Fruit Extracts. Oxidative Med. Cell. Longev. 2018, 2018, 2879793. [Google Scholar] [CrossRef] [PubMed]
- Erhan, S.-E.; Pârvu, A.E.; Ciorîță, A.; Putri, A.A.; Molina, A.J.V.; Pârvu, M. Chemical composition and anti-inflammatory effect of Phellodendron amurense Rupr. stem bark extract. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13306. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana, B.; et al. Berberis plants—Drifting from farm to food applications, phytotherapy, and phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Misiurek, J.; Szymczak, G.; Buszewski, B. Determination of Cytotoxic Activity of Selected Isoquinoline Alkaloids and Plant Extracts Obtained from Various Parts of Mahonia aquifolium Collected in Various Vegetation Seasons. Molecules 2021, 26, 816. [Google Scholar] [CrossRef] [PubMed]
- He, J.-M.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef]
- Wang, W.; Zu, Y.; Fu, Y.; Reichling, J.; Suschke, U.; Nokemper, S.; Zhang, Y. In vitro Antioxidant, Antimicrobial and Anti-Herpes Simplex Virus Type 1 Activity of Phellodendron amurense Rupr. from China. Am. J. Chin. Med. 2009, 37, 195–203. [Google Scholar] [CrossRef]
- Do, G.-Y.; Kim, J.-W.; Park, H.-J.; Yoon, S.-B.; Park, J.-Y.; Yang, S.-G.; Jung, B.D.; Kwon, Y.-S.; Kang, M.-J.; Song, B.-S.; et al. Native plants (Phellodendron amurense and Humulus japonicus) extracts act as antioxidants to support developmental competence of bovine blastocysts. Asian-Australas J. Anim. Sci. 2017, 30, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Balážová, Ľ.; Kurhajec, S.; Kello, M.; Bedlovičová, Z.; Zigová, M.; Petrovová, E.; Beňová, K.; Mojžiš, J.; Eftimová, J. Antiproliferative Effect of Phellodendron amurense Rupr. Based on Angiogenesis. Life 2022, 12, 767. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, X.-M.; Liu, Y.-Q.; Du, W.-J.; Ji, J.; He, X.; Zhang, C.-F.; Li, F.; Guo, C.-R. Gastroprotective effect of alkaloids from cortex phellodendri on gastric ulcers in rats through neurohumoral regulation. Planta Medica 2017, 83, 277–284. [Google Scholar] [CrossRef]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Long, Y.; Ni, L.; Yuan, X.; Yu, N.; Wu, R.; Tao, J.; Zhang, Y. Anticancer effect of berberine based on experimental animal models of various cancers: A systematic review and meta-analysis. BMC Cancer 2019, 19, 589. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A natural isoquinoline alkaloid with antitumor activity: Studies of the biological activities of berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Jantova, S.; Cipak, L.; Letasiova, S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol. Vitr. 2007, 21, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Sharvan, R.; Gao, J.; Qu, S. Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed. Pharmacother. 2017, 95, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.-H.; Kim, J.-H.; Kim, S.-J. Induction of G2/M arrest by berberine via activation of PI3K/Akt and p38 in human chondrosarcoma cell line. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2015, 22, 147–157. [Google Scholar] [CrossRef]
- Park, S.; Sung, J.; Kim, E.; Chung, N. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Biol. Res. 2014, 48, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Laux, A.; Hamman, J.; Svitina, H.; Wrzesinski, K.; Gouws, C. In vitro evaluation of the anti-melanoma effects (A375 cell line) of the gel and whole leaf extracts from selected aloe species. J. Herb. Med. 2022, 31, 100539. [Google Scholar] [CrossRef]
- Kamran, M.Z.; Gude, R.P. Preclinical evaluation of the antimetastatic efficacy of Pentoxifylline on A375 human melanoma cell line. Biomed. Pharmacother. 2012, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Jęśkowiak-Kossakowska, I.; Jawień, P.; Krzyżak, E.; Mączyński, M.; Szafran, R.; Szeląg, A.; Janeczek, M.; Wiatrak, B. Search for immunomodulatory compounds with antiproliferative activity against melanoma. Biomed. Pharmacother. 2023, 160, 114374. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- Amann, A.; Zwierzina, M.; Gamerith, G.; Bitsche, M.; Huber, J.M.; Vogel, G.F.; Blumer, M.; Koeck, S.; Pechriggl, E.J.; Kelm, J.M. Development of an innovative 3D cell culture system to study tumour-stroma interactions in non-small cell lung cancer cells. PLoS ONE 2014, 9, e92511. [Google Scholar] [CrossRef] [PubMed]
- Ikari, R.; Mukaisho, K.-i.; Kageyama, S.; Nagasawa, M.; Kubota, S.; Nakayama, T.; Murakami, S.; Taniura, N.; Tanaka, H.; Kushima, R.P.; et al. Differences in the Central Energy Metabolism of Cancer Cells between Conventional 2D and Novel 3D Culture Systems. Int. J. Mol. Sci. 2021, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Hassanpour, S.; Vahid, Z.F.; Hejazi, M.; Hashemi, M.; Ranjbari, J.; Tabarzad, M.; Noorolyai, S.; de la Guardia, M. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J. Control. Release 2017, 266, 166–186. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.-L.; Pârvu, M. Green synthesis of Ag-MnO2 nanoparticles using Chelidonium majus and Vinca minor extracts and their in vitro cytotoxicity. Molecules 2020, 25, 819. [Google Scholar] [CrossRef] [PubMed]
- Hanan, N.A.; Chiu, H.I.; Ramachandran, M.R.; Tung, W.H.; Zain, N.N.M.; Yahaya, N.; Lim, V. Cytotoxicity of plant-mediated synthesis of metallic nanoparticles: A systematic review. Int. J. Mol. Sci. 2018, 19, 1725. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Kaya, G.; Bayram, S.; Kurek-Górecka, A.; Olczyk, P. Green Synthesis, Characterization, Antioxidant, Antibacterial and Enzyme Inhibition Effects of Chestnut (Castanea sativa) Honey-Mediated Silver Nanoparticles. Molecules 2023, 28, 2762. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Almofti, M.R.; Ichikawa, T.; Yamashita, K.; Terada, H.; Shinohara, Y. Silver ion induces a cyclosporine A-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003, 134, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Habas, K.; Shang, L. Silver nanoparticle-mediated cellular responses in human keratinocyte cell line HaCaT in vitro. Nanoscale Rep. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Ciorîță, A.; Tripon, S.C.; Mircea, I.G.; Podar, D.; Barbu-Tudoran, L.; Mircea, C.; Pârvu, M. The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants 2021, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Parvu, M.; Mot, C.A.; Parvu, A.E.; Mircea, C.; Stoeber, L.; Rosca-Casian, O.; Tigu, A.B. Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules 2019, 24, 3958. [Google Scholar] [CrossRef] [PubMed]
- Ciorîță, A.; Zăgrean-Tuza, C.; Moț, A.; Carpa, R.; Pârvu, M. The phytochemical analysis of Vinca L. species leaf extracts is correlated with the antioxidant, antibacterial, and antitumor effects. Molecules 2021, 26, 3040. [Google Scholar] [CrossRef]
- Ciorîță, A.; Gutt, R.; Lung, I.; Soran, M.-L.; Pârvu, M. Green-synthesized Ag-MnO2 nanoparticles as plausible non-invasive antimicrobial treatment of cultural heritage. GeoPatterns 2021, 6, 6–10. [Google Scholar] [CrossRef]
- Csiky, J.; Purger, D. Herbaceous periwinkle, Vinca herbacea Waldst. et Kit. 1799 (Apocynaceae), a new species of the Croatian flora. Acta Bot. Croat. 2013, 72, 399–406. [Google Scholar] [CrossRef][Green Version]
- Ochirova, K.S.; Ovanova, E.A.; Dordzhieva, V.I. Vinca minor L. leaf anatomical structure. J. Pharm. Sci. Res. 2018, 10, 2528–2530. [Google Scholar]
- Petra, S.A.; Georgescu, M.I.; Manescu, C.R.; Toma, F.; Badea, M.L.; Dobrescu, E.; Popa, V.I. Leaves anatomical and physiological adaptations of Vinca major ‘Variegata’ and Hedera helix L. to specific roof garden conditions. Not. Bot. Horti Agrobot. 2020, 47, 318–328. [Google Scholar] [CrossRef]
- Murata, J.; Roepke, J.; Gordon, H.; De Luca, V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 2008, 20, 524–542. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Segev, R.; Nannapaneni, R.; Sindurakar, P.; Kim, H.; Read, H.; Lijek, S. The effect of the stomatal index on the net rate of photosynthesis in the leaves of Spinacia oleracea, Vinca minor, Rhododendron spp., Epipremnum aureum, and Hedera spp. J. Emerg. Investig. 2015, 20, 2018. [Google Scholar] [CrossRef] [PubMed]
- Sood, H.; Kumar, Y.; Gupta, V.K.; Arora, D.S. Scientific validation of the antimicrobial and antiproliferative potential of Berberis aristata DC root bark, its phytoconstituents and their biosafety. AMB Express 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Cai, C.; Wu, X.; Fan, X.; Huang, W.; Zhou, J.; Wu, Q.; Huang, Y.; Zhao, W.; Zhang, F.; et al. An Insight into the Molecular Mechanism of Berberine Towards Multiple Cancer Types through Systems Pharmacology. Front. Pharmacol. 2019, 10, 857. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Zhou, P. In vitro antifungal effects of berberine against Candida spp. in planktonic and biofilm conditions. Drug Des. Dev. Ther. 2020, 14, 87–101. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Huang, S.-Y.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Saimaiti, A.; Zhao, C.-N.; Shang, A.; Zhang, Y.-J.; Gan, R.-Y.; et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules 2022, 27, 4523. [Google Scholar] [CrossRef] [PubMed]
- El khalki, L.; Tilaoui, M.; Jaafari, A.; Ait Mouse, H.; Zyad, A. Studies on the Dual Cytotoxicity and Antioxidant Properties of Berberis vulgaris Extracts and Its Main Constituent Berberine. Adv. Pharmacol. Sci. 2018, 2018, 3018498. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, A.; Zdunić, G.; Savikin, K.; Mandić, B.; Jadranin, M.; Matić, I.Z.; Stanojković, T. Evaluation of the anti-cancer potential of Mahonia aquifolium extracts via apoptosis and anti-angiogenesis. Bangladesh J. Pharmacol. 2016, 11, 741–749. [Google Scholar] [CrossRef]
- Damjanović, A.; Kolundžija, B.; Matić, I.Z.; Krivokuća, A.; Zdunić, G.; Šavikin, K.; Janković, R.; Stanković, J.A.; Stanojković, T.P. Mahonia aquifolium Extracts Promote Doxorubicin Effects against Lung Adenocarcinoma Cells In Vitro. Molecules 2020, 25, 5233. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Mandal, P.; Jagdale, P.R.; Ayanur, A.; Ansari, K.M. Safety studies of Nexrutine, bark extract of Phellodendron amurense through repeated oral exposure to rats for 28 days. Heliyon 2021, 7, e07654. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, T.; Kawada-Matsuo, M.; Migita, H.; Ohta, K.; Oogai, Y.; Yamasaki, Y.; Komatsuzawa, H. Antibacterial activity of phellodendron bark against Streptococcus mutans. Microbiol. Immunol. 2020, 64, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-D.; Chen, L.-J.; Xu, X.-Y.; Liu, Y.-J.; Tao, F.; Zhu, M.-H.; Li, C.-Y.; Zhao, D.; Yang, G.-J.; Chen, J. Berberine as a potential agent for breast cancer therapy. Front. Oncol. 2022, 12, 993775. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, W.; Tong, Y. Berberine inhibits proliferative ability of breast cancer cells by reducing metadherin. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9058. [Google Scholar] [CrossRef]
- El Khalki, L.; Maire, V.; Dubois, T.; Zyad, A. Berberine Impairs the Survival of Triple Negative Breast Cancer Cells: Cellular and Molecular Analyses. Molecules 2020, 25, 506. [Google Scholar] [CrossRef]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef]
- Tong, M.; Liu, H.; Hao, J.; Fan, D. Comparative pharmacoproteomics reveals potential targets for berberine, a promising therapy for colorectal cancer. Biochem. Biophys. Res. Commun. 2020, 525, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Saiman, M.Z.; Abdul Majid, N.; Karsani, S.A.; Yaacob, J.S. Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules 2021, 26, 376. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Peng, W.W.; Wang, Y.; Zhong, L.; Zhang, X.; Zeng, L. β-catenin correlates with the progression of colon cancers and berberine inhibits the proliferation of colon cancer cells by regulating the β-catenin signaling pathway. Gene 2022, 818, 146207. [Google Scholar] [CrossRef]
- Liu, H.; Huang, C.; Wu, L.; Wen, B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. OncoTargets Ther. 2016, 9, 4121–4127. [Google Scholar]
- Chen, Q.; Hou, Y.; Li, D.; Ding, Z.; Xu, X.; Hao, B.; Xia, Q.; Li, M.; Fan, L. Berberine induces non-small cell lung cancer apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann. Transl. Med. 2022, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Li, Z.; Ren, H.; Kong, L.; Chen, X.; Xiong, M.; Zhang, X.; Ning, B.; Li, J. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin. Exp. Pharmacol. Physiol. 2022, 49, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Iqubal, M.K.; Sultana, Y. Tailoring of berberine loaded transniosomes for the management of skin cancer in mice. J. Drug Deliv. Sci. Technol. 2020, 60, 102051. [Google Scholar] [CrossRef]
- Song, Y.C.; Lee, Y.; Kim, H.M.; Hyun, M.Y.; Lim, Y.Y.; Song, K.Y.; Kim, B.J. Berberine regulates melanin synthesis by activating PI3K/AKT, ERK and GSK3β in B16F10 melanoma cells. Int. J. Mol. Med. 2015, 35, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Li, L.; Li, H.; Tan, Y.; Li, B.; Wang, K.; Du, B. Berberine suppressed epithelial mesenchymal transition through cross-talk regulation of PI3K/AKT and RARα/RARβ in melanoma cells. Biochem. Biophys. Res. Commun. 2016, 479, 290–296. [Google Scholar] [CrossRef]
- Parveen, R.; Mohapatra, S.; Ahmad, S.; Husain, S.A. Amalgamation of nanotechnology for delivery of bioactive constituents in solid tumors. Curr. Drug Deliv. 2023, 20, 457–482. [Google Scholar]
- Kabary, D.M.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018, 130, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Anzabi, Y. Biosynthesis of ZnO nanoparticles using barberry (Berberis vulgaris) extract and assessment of their physico-chemical properties and antibacterial activities. Green Process. Synth. 2018, 7, 114–121. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]






| No. | Compounds | Elution Time (min) | B. vulgaris (mg/g) | M. aquifolium (mg/g) | P. amurense (mg/g) |
|---|---|---|---|---|---|
| 1 | Gallic acid | 3.32 | 0.08 ± 0.02 | <LOD | 0.10 ± 0.01 |
| 2 | 4-hydroxybenzoic acid | 9.98 | 0.26 ± 0.03 | <LOD | 0.38 ± 0.03 |
| 3 | Caffeic acid | 12.25 | <LOD | <LOD | <LOD |
| 4 | P-coumaric acid | 15.79 | <LOD | <LOD | <LOD |
| 5 | Ferulic acid | 17.24 | <LOD | <LOD | <LOD |
| 6 | Berbamine | 21.80 | 1.32 ± 0.11 | 1.09 ± 0.12 | <LOD |
| 7 | Jatrorrhizine | 24.70 | 5.27 ± 0.43 | 12.7 ± 1.0 | 0.37 ± 0.03 |
| 8 | Palmatine | 29.70 | 0.15 ± 0.02 | 2.02 ± 0.17 | 0.09 ± 0.01 |
| 9 | Berberine | 31.59 | 10.2 ± 1.1 | 2.84 ± 0.23 | 2.63 ± 0.22 |
| Cell Lines | IC50 Values | ||
|---|---|---|---|
| B. vulgaris | M. aquifolium | P. amurense | |
| A375 | 0.4% | <0.3% | 3.5% |
| A549 | 0.4% | 10.4% | 13.8% |
| Cell Lines | IC50 Values | ||
|---|---|---|---|
| B. vulgaris | M. aquifolium | P. amurense | |
| 3D A375 | 3.79% | 12.9% | 16.14% |
| 3D A549 | 13.41% | 13.68% | 268.58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorîță, A.; Erhan, S.-E.; Soran, M.L.; Lung, I.; Mot, A.C.; Macavei, S.G.; Pârvu, M. Pharmacological Potential of Three Berberine-Containing Plant Extracts Obtained from Berberis vulgaris L., Mahonia aquifolium (Pursh) Nutt., and Phellodendron amurense Rupr. Biomedicines 2024, 12, 1339. https://doi.org/10.3390/biomedicines12061339
Ciorîță A, Erhan S-E, Soran ML, Lung I, Mot AC, Macavei SG, Pârvu M. Pharmacological Potential of Three Berberine-Containing Plant Extracts Obtained from Berberis vulgaris L., Mahonia aquifolium (Pursh) Nutt., and Phellodendron amurense Rupr. Biomedicines. 2024; 12(6):1339. https://doi.org/10.3390/biomedicines12061339
Chicago/Turabian StyleCiorîță, Alexandra, Sabina-Emanuela Erhan, Maria Loredana Soran, Ildiko Lung, Augustin Catalin Mot, Sergiu Gabriel Macavei, and Marcel Pârvu. 2024. "Pharmacological Potential of Three Berberine-Containing Plant Extracts Obtained from Berberis vulgaris L., Mahonia aquifolium (Pursh) Nutt., and Phellodendron amurense Rupr" Biomedicines 12, no. 6: 1339. https://doi.org/10.3390/biomedicines12061339
APA StyleCiorîță, A., Erhan, S.-E., Soran, M. L., Lung, I., Mot, A. C., Macavei, S. G., & Pârvu, M. (2024). Pharmacological Potential of Three Berberine-Containing Plant Extracts Obtained from Berberis vulgaris L., Mahonia aquifolium (Pursh) Nutt., and Phellodendron amurense Rupr. Biomedicines, 12(6), 1339. https://doi.org/10.3390/biomedicines12061339






