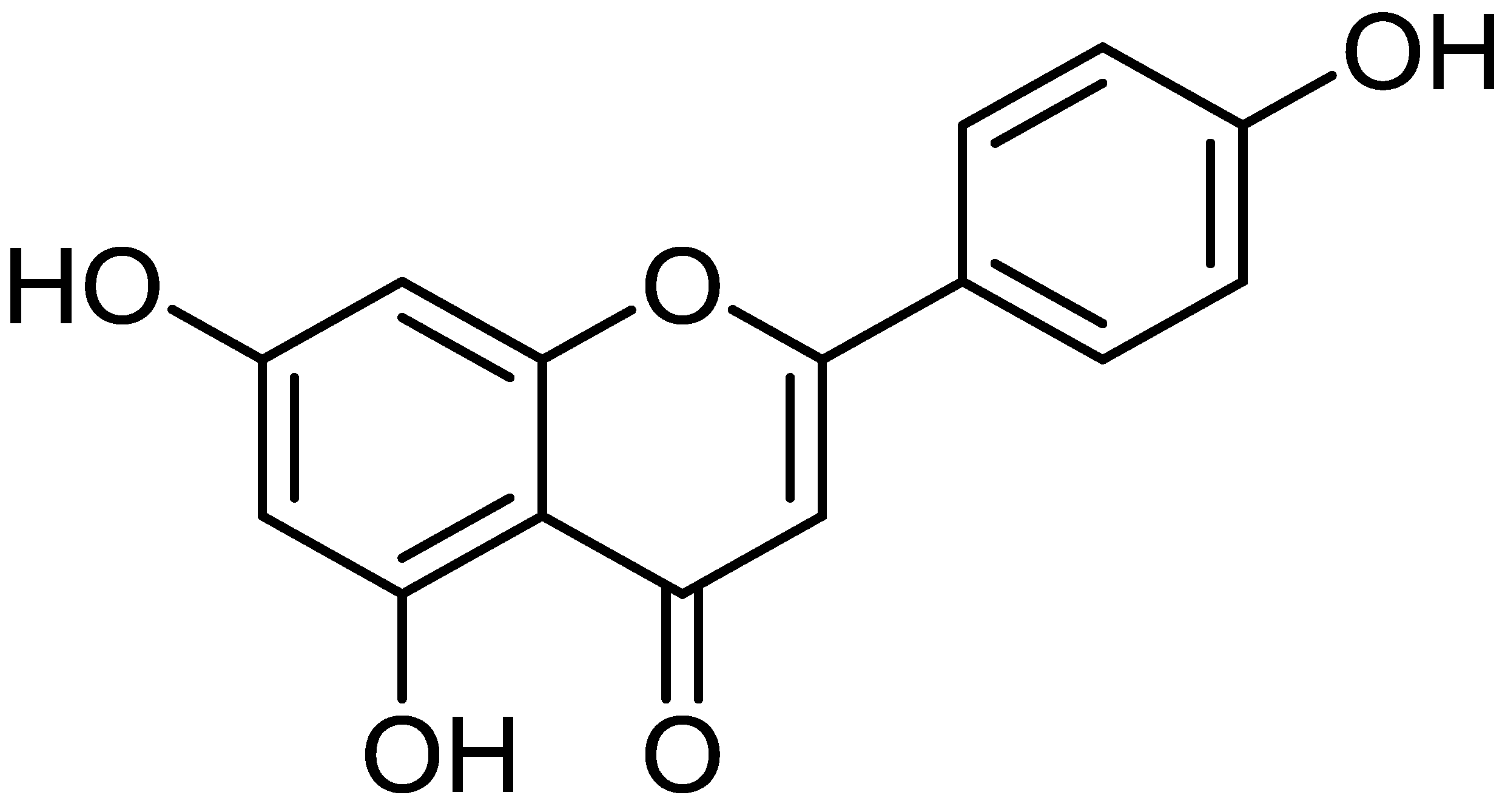
Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment
Abstract
1. Introduction
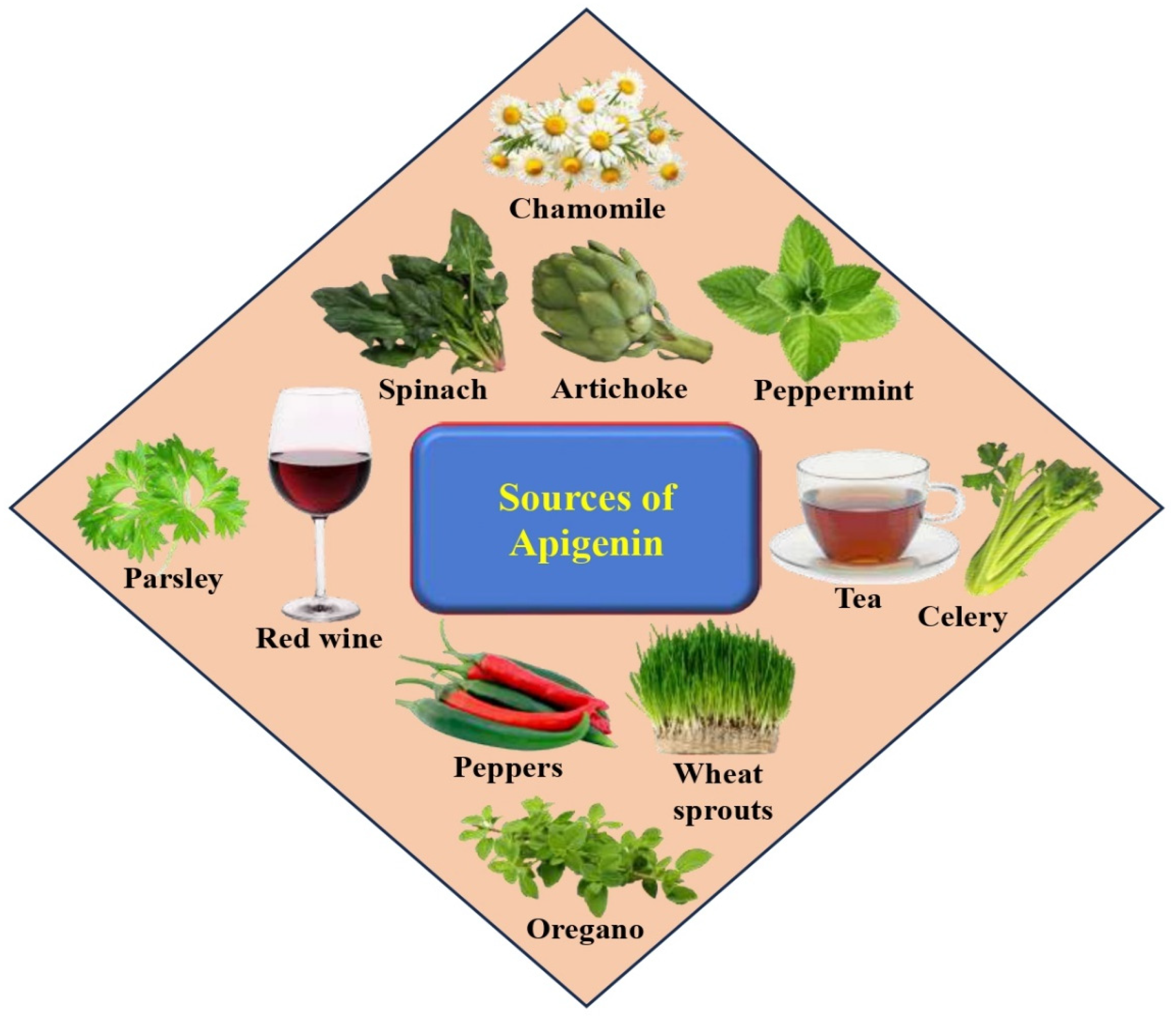
2. Sources, Intake, and Safety of Apigenin
3. Exploring the Pharmacological Potential of Apigenin through the Modulation of Biological Activities
3.1. Oxidative Stress
3.2. Anti-Inflammatory Potential
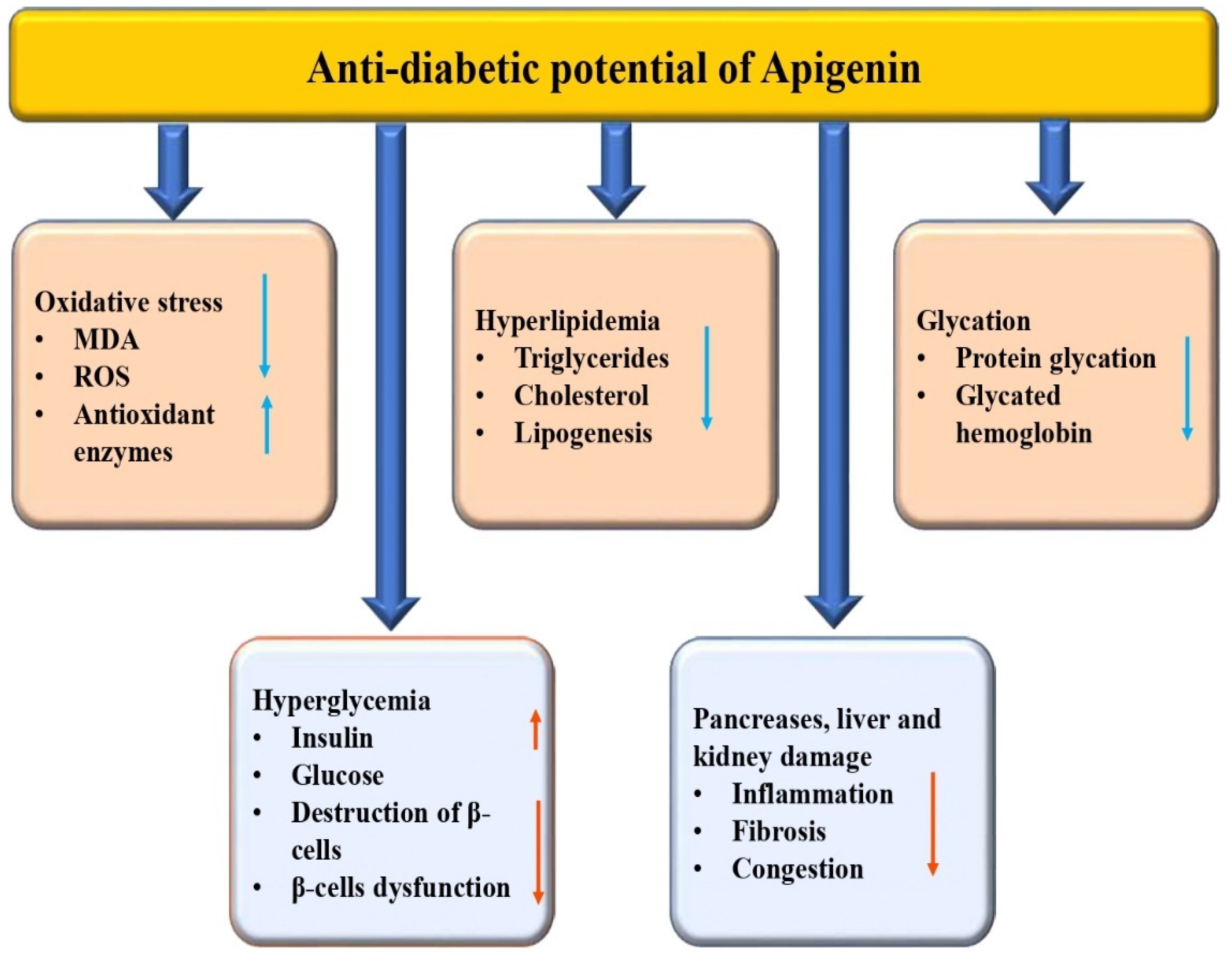
3.3. Anti-Diabetic Potential
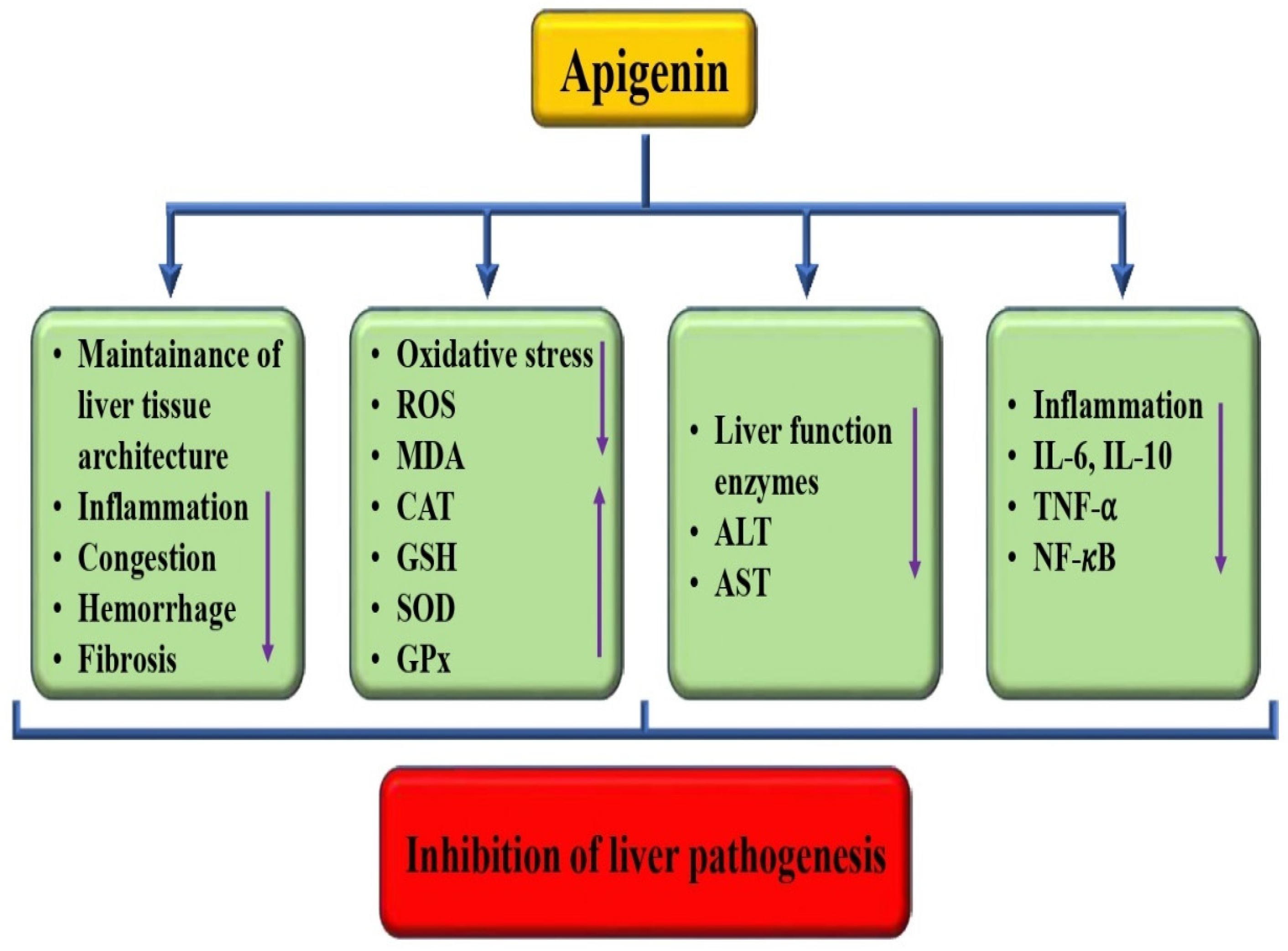
3.4. Hepatoprotective Effects
3.5. Renoprotective Effects
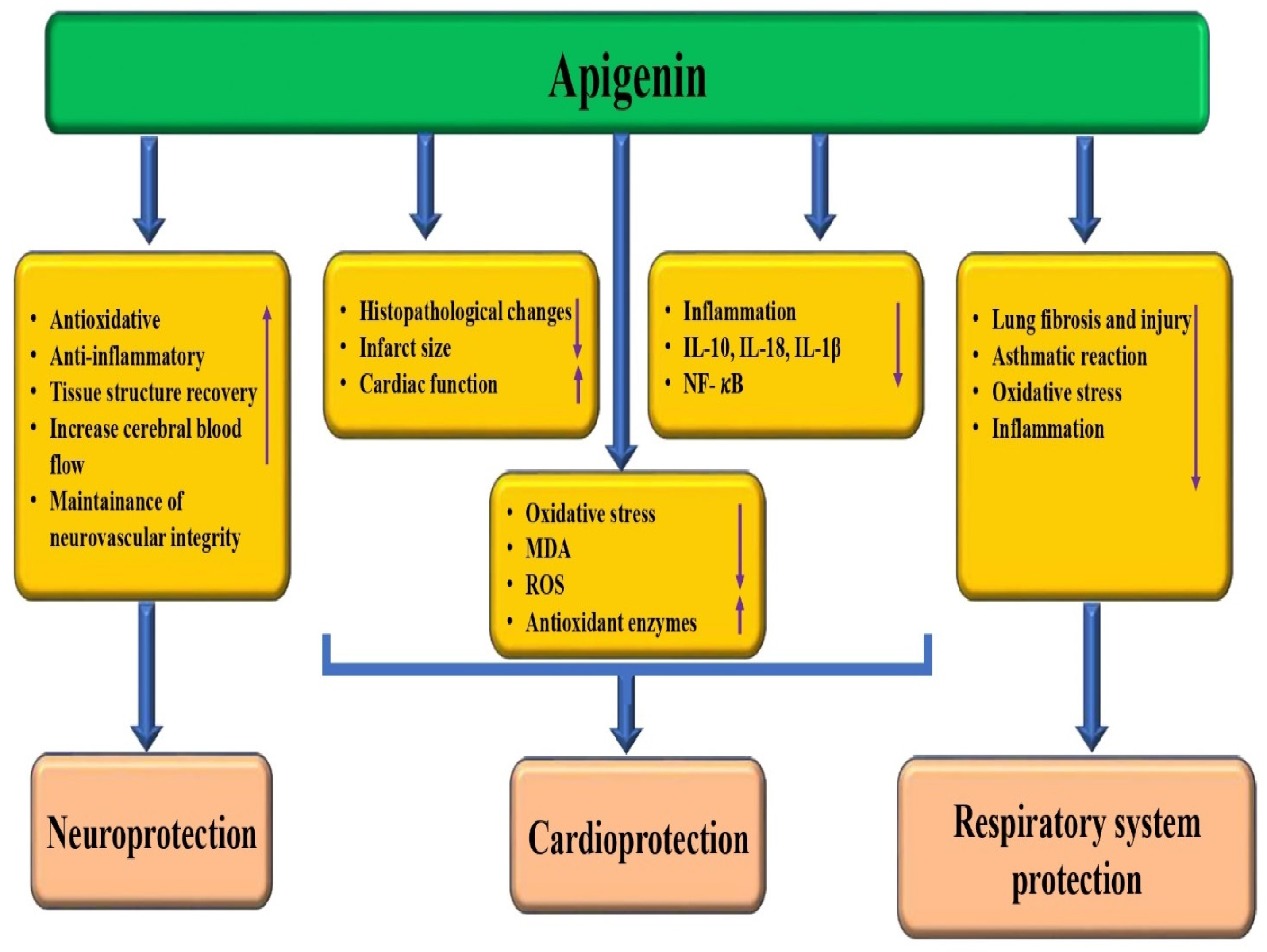
3.6. Cardioprotective Effects
3.7. Neuroprotective Potential
3.8. Role in the Respiratory System
3.9. Role in the Reproductive System
3.10. Role in Skin Disease
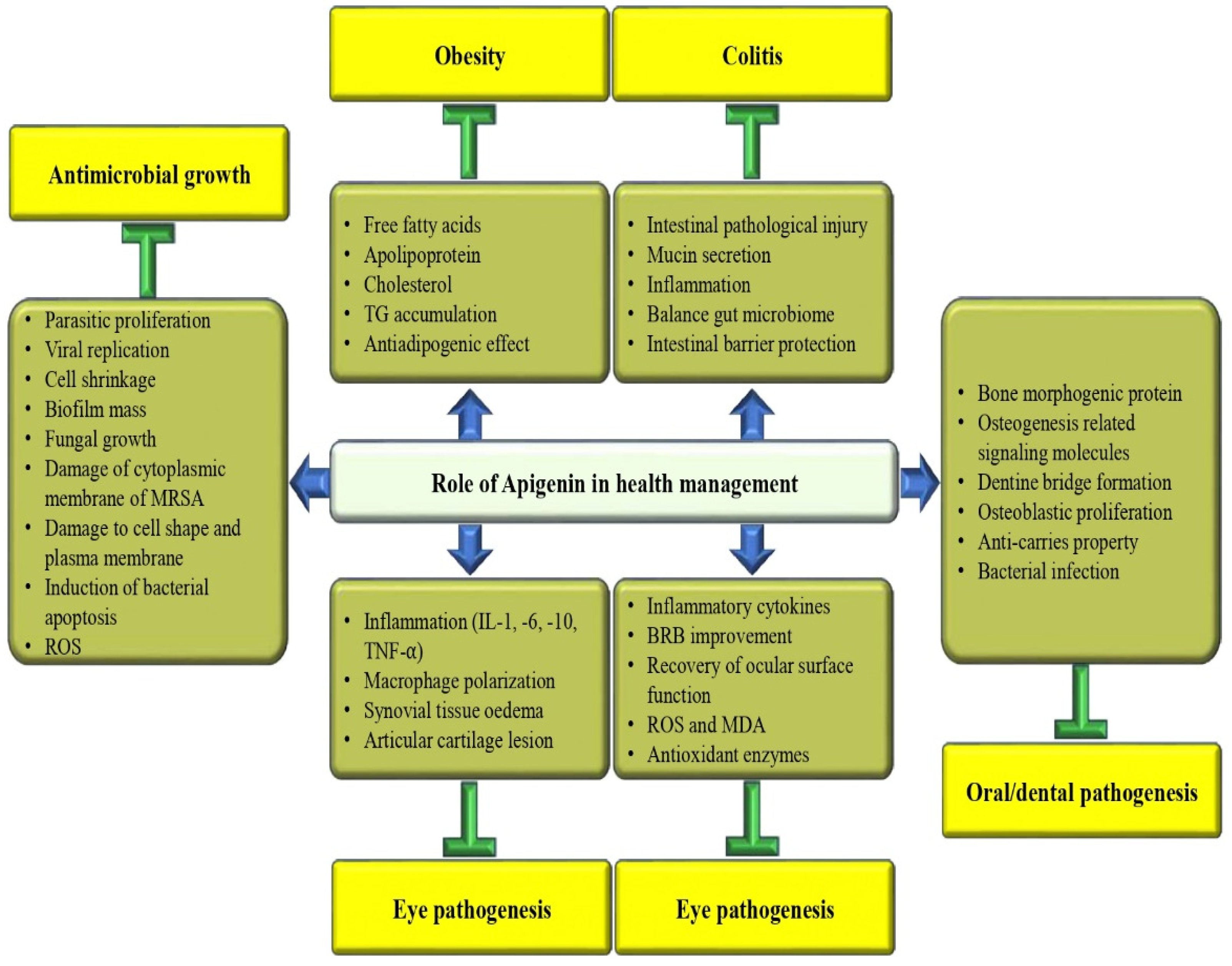
3.11. Anti-Obesity Potential
3.12. Anti-Depressive Effects
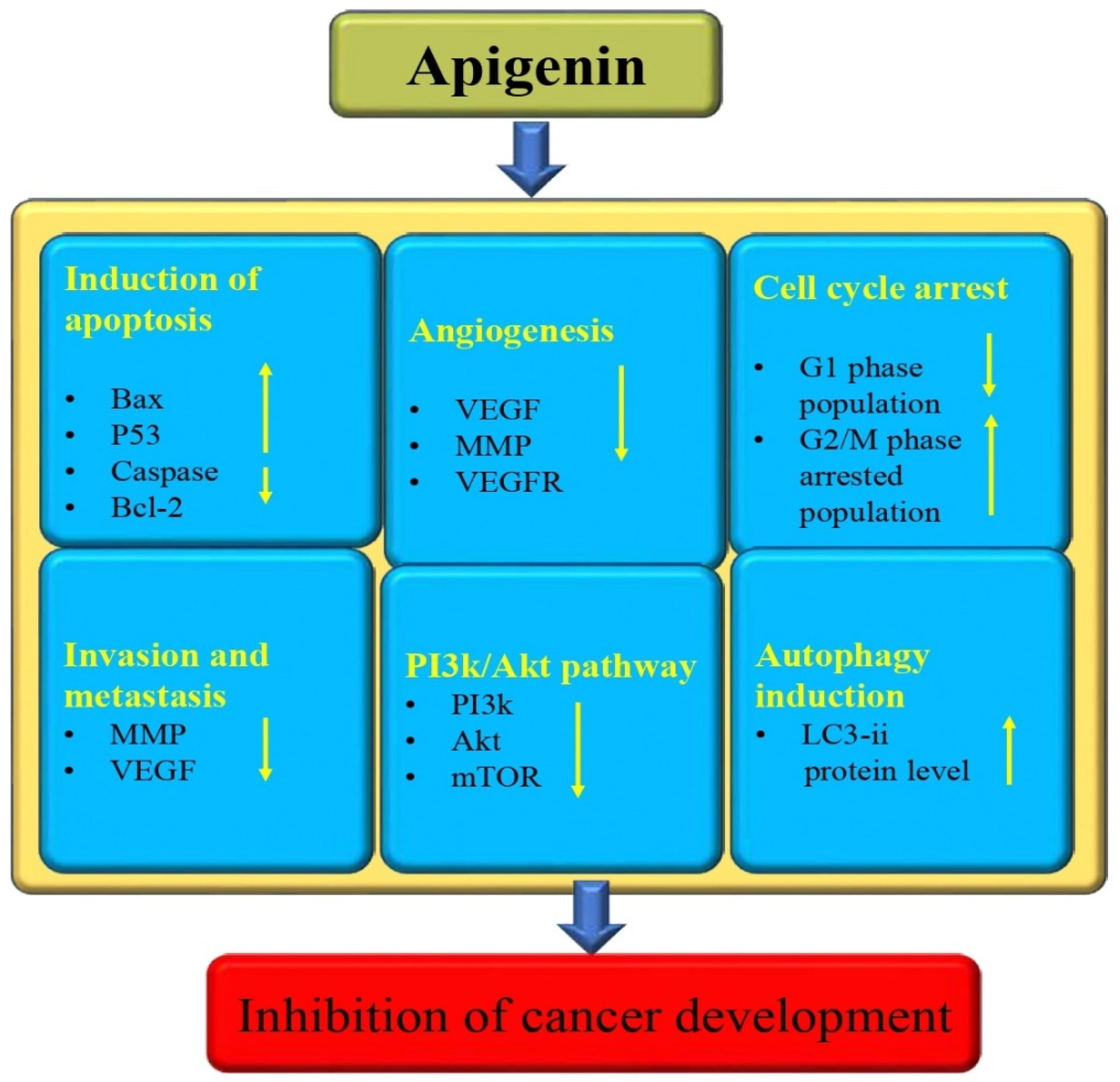
3.13. Anti-Cancer Potential
3.14. Anti-Arthritis Activity
3.15. Role in Eye Health
3.16. Role in Oral Health
3.17. Radioprotective Effects
3.18. Anti-Colitis Effects
3.19. Wound-Healing Activity
3.20. Immunomodulatory Effects
| Activity | Study Type | Dose | Mechanism | Outcome | Refs. |
|---|---|---|---|---|---|
| Anti-inflammatory | In vitro | 10, 20, 40 Μm | TNF-α ↓, IL-1ß ↓, and IL-6 ↓ |
| [52] |
| In vivo | 20 and 40 mg/kg | TNF α ↓, IL-1-ß ↓, and IL-6 ↓ |
| [55] | |
| In vitro | 30 μM | IL-10 ↓ and TNF-α ↓ |
| [56] | |
| In vitro | 10, 50 μM | ICAM-1 ↓, COX-2 ↓, VCAM-1 ↓ NO ↓ |
| [61] | |
| Anti-diabetic | In vivo | 20 mg/kg | Fibrosis ↓, oxidative stress ↓ |
| [69] |
| In vivo | 0.78 mg/kg | Insulin ↑, glucose ↓ |
| [71] | |
| Hepatoprotective | In vivo | 50, 100, 200 mg/kg | Liver function enzyme ↓, oxidative stress ↓, inflammation ↓ |
| [75] |
| In vitro | 10, 20, and 40 μM | OxidativesStress ↓, inflammation ↓ |
| [75] | |
| In vivo | 25 mg/kg | Oxidative stress ↓, fibrosis ↓, inflammation ↓ |
| [76] | |
| In vivo | 150–300 mg/kg | Lipid peroxidation ↓ and oxidative stress ↓ |
| [78] | |
| Reno protective | In vivo | 10, 15, and 20 mg/kg | Lipid hydroperoxides ↓, antioxidant level ↑ |
| [82] |
| In vivo | 20, 40 mg/kg | Infarct size ↓, LDH ↓, CPK ↓ |
| [83] | |
| Cardioprotective | In vivo | 50 and 100 mg/kg | Inflammation ↓, nuclear translocation of NF-κB ↓ |
| [86] |
| In vitro | 1, 6, and 25 μM | Pyroptosis and apoptosis ↓ |
| [87] | |
| In vivo | 5 mg/kg | LDH activity, intracellular reactive oxygen species generation ↓, improved the loss of mitochondrial membrane potential, antioxidant enzymes ↑ |
| [88] | |
| In vitro | 20 μM | LDH activity, intracellular reactive oxygen species generation ↓, Hes1 expression ↑ |
| [88] | |
| Neuroprotective | In vivo | 20 and 40 mg/kg | Lucigenin and luminol ↓ and IL-10 levels ↑ |
| [94] |
| In vivo | 10 and 20 mg/kg | Inflammation and pxidative stress ↓, apoptosis ↓ |
| [95] | |
| Role in the respiratory system | In vivo | 10, 15, and 20 mg/kg | Fibrosis ↓, antioxidant enzymes ↑, tumor necrosis factor (TNF)-α, and transforming growth factor ↓ |
| [101] |
| In vivo | 20 mg/kg | Expression of NF-κB ↓ |
| [102] | |
| Role in the reproductive system | In vivo | 25 mg/kg | Sperm density ↓, seminiferous epithelium cells ↑ |
| [107] |
| In vivo | 20 or 40 mg/kg | Testosterone levels and sperm ↑, antioxidant enzymes ↑, and inflammation ↓ |
| [110] | |
| Role in skin health | In vitro | 5, 10, and 20 µM | Cellular senescence ↓ MMP-1 ↓ |
| [111] |
| In vivo | Cream containing 1% apigenin | Dermal thickness and skin texture ↑ |
| [111] | |
| In vivo | 0.1% apigenin | Transepidermal water loss ↓, skin surface pH ↓, and hydration increased ↑ |
| [114] | |
| Anti-obesity | In vivo | 0.005% (w/w) apigenin | Free fatty acid ↓, apolipoprotein B ↓, total cholesterol C ↓ |
| [117] |
| Anti-depression | In vivo | 12.5–25 mg/kg | Antioxidant enzymes ↑, corticosterone and lipid peroxidation ↑ |
| [121] |
| In vivo | 20 and 40 mg/kg | Hippocampal brain-derived neurotrophic factor ↑ and corticosterone levels ↓ |
| [124] | |
| Anti-cancer | In vitro | 0, 50, and 100 µM | Bax, p53 ↑ Bcl2 ↓ |
| [134] |
| In vitro | 0, 10, 20, and 40 μM | Apoptosis and autophagy ↑, PI3K/Akt/mTOR ↓ |
| [136] | |
| 10, 20, and 40 μM | G2/M phase cell-cycle arrest, Bax, caspases 9 and 3 ↑ |
| [137] | ||
| In vitro | 0, 10, 20, and 30 μg/mL | BCL2 and NF-κB/MMP-9 ↓ miR-16 ↑ |
| [140] | |
| Anti-arthritis | In vivo | 20 mg/kg | Pro-inflammatory cytokines ↓, Langerhans cells and Maturation and migration of dendritic cell ↓ |
| [142] |
| In vivo | 20 and 40 mg/kg | Inflammation ↓ P2X7/NF-κB signal-related proteins ↓ |
| [143] | |
| Role in eye-associated pathogenesis | In vivo and in vitro | 20 mg/kg and 20 and 40 μM | Inflammatory cytokines ↓, BRB disruption ↓, microglial M1 polarization inhibited |
| [144] |
| In vivo | 10, 20, and 50 mg/kg | Inflammation ↓ |
| [145] | |
| Role in dental-associated pathogenesis | In vitro and in vivo | 5 μM and 50 μM | Bone morphogenetic protein and osteogenesis-related signaling molecules ↑, TNF-α ↓ |
| [148] |
| Radioprotective | In vitro | 2.5, 5, 10, and 25 µg/mL | Cytokinesis-block proliferation index ↓, cell proliferation ↓ |
| [152] |
| Anti-colitis | In vivo | 3 mg/kg/day | MDA and NOx contents ↓, MPO activity ↓, mucosal addressin cell adhesion molecule-1 ↓, IL-1β content ↓ |
| [159] |
| In vivo | 10, 20, and 40 mg/kg | MPO activity ↓ inflammation ↓ |
| [161] | |
| Wound healing | In vivo | Apigenin cream 2% | wound size ↓ and wound contraction ↑ |
| [164] |
| Immunomodulatory | In vitro | 1–20 μM | mRNA and protein levels of RelB ↓, pro-inflammatory cytokine production ↓ |
| [166] |
3.21. Anti-Microbial Activity
- i.
- Antibacterial activity
| Activity | Species | Mechanism | Outcome | Refs. |
|---|---|---|---|---|
| Antibacterial | Enterococcus caccae | Alters the gene expression; membrane synthesis and protein misfolding are affected |
| [167] |
| Streptococcus mutans | Polysaccharide content affected |
| [168] | |
| Enterobacter cloacae | Leakage of intracellular constituents and damage of the CREC cytoplasmic membrane |
| [169] | |
| Staphylococcus aureus | Leakage of intracellular constituents and damaged MRSA cytoplasmic membrane |
| [170] | |
| Escherichia coli | Increase in intracellular calcium and induces bacterial apoptosis |
| [171] | |
| Antifungal | Candida albicans | Morphological changes, cell shrinkage membrane disturbances |
| [172] |
| Candida albicans | Induced uptake of calcium and induced apoptosis activation |
| [173] | |
| Antiviral | EV71 | Suppressing viral IRES, modulating cellular JNK pathway |
| [174] |
| Influenza A virus | Inhibiting upregulation of retinoic acid-inducible gene-I expression and interferons and pro-inflammatory cytokines |
| [175] | |
| EV71 | Disrupting the viral RNA’s suppressed the expression of GFP |
| [176] | |
| Anti-parasitic | Leishmania amazonensis | Inhibition of cellular proliferation increased reactive oxygen species generation |
| [177] |
- ii.
- Antifungal activity
- iii.
- Antiviral activity
- iv
- Anti-parasitic activity
4. Synergistic Effects of Apigenin with Drugs/Natural Compounds
| Apigenin | Compound/Drugs | Study Type | Outcome | Refs. |
|---|---|---|---|---|
| Apigenin | Ampicillin and ceftriaxone | In vitro |
| [170] |
| Naringenin | In vitro |
| [184] | |
| Curcumin | In vitro |
| [185] | |
| Tyroservatide | In vitro and in vivo |
| [186] | |
| Myricetin | In vivo |
| [187] | |
| Curcumin | In vitro |
| [188] | |
| Abiraterone acetate | In vitro |
| [189] | |
| Curcumin | In vitro |
| [190] | |
| Sorafenib | In vitro |
| [191] | |
| Paclitaxel | In vitro |
| [192] | |
| Etoposide or cyclophosphamide | In vitro |
| [193] | |
| Cisplatin | In vitro |
| [194] | |
| In vitro |
| [195] |
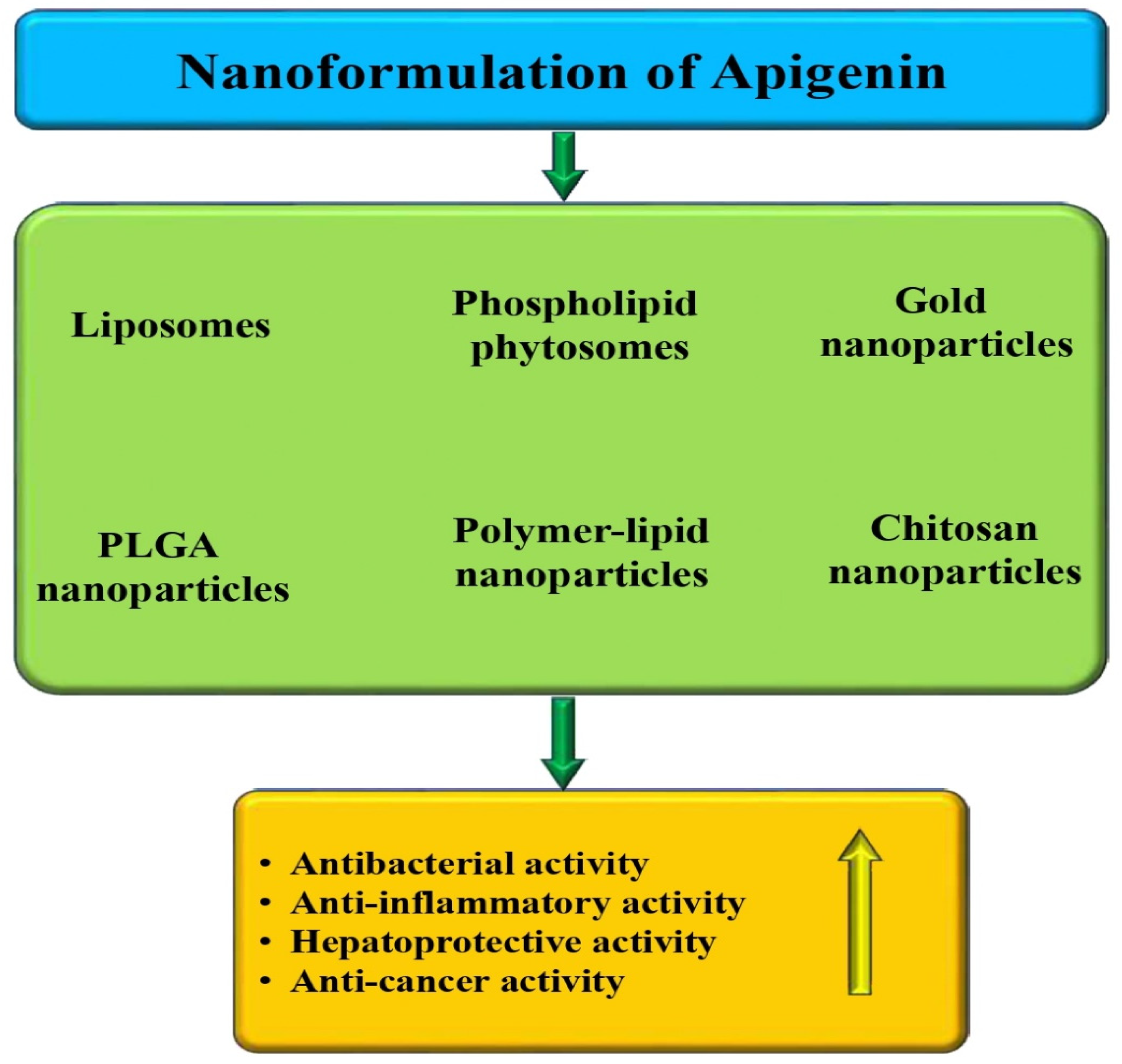
5. Nanoformulation of Apigenin and Pharmacological Action
| Nanoformulation Types | Study Types | Outcomes of the Study | Refs. |
|---|---|---|---|
| Liposomal formulation | In vitro |
| [206] |
| Liposomal formulation | In vitro and in vivo |
| [207] |
| Apigenin–phospholipid phytosome | In vitro and in vivo |
| [208] |
| Apigenin-coated gold nanoparticles | In vitro and in vivo |
| [209] |
| Apigenin-coated gold nanoparticles | In vitro |
| [210] |
| Apigenin-loaded PLGA-nanoparticles | In vitro and in vivo |
| [211] |
| Lipid–polymer hybrid nanoparticles | In vitro |
| [212] |
| Apigenin–gold nanoparticles | In vitro |
| [213] |
| Apigenin-loaded polymer–lipid hybrid nanoparticles | In vitro |
| [214] |
| Apigenin-Mn (II) loaded sodium hyaluronate nanoparticles | In vivo |
| [215] |
| Apigenin-loaded nanostructured lipid carriers | In vitro |
| [216] |
| Apigenin-loaded chitosan/gelatin membranes | In vitro |
| [217] |
6. Conclusions and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ROS | Reactive oxygen species |
| API | Apigenin |
| AGEs | Advanced glycation end products |
| DOX | Doxorubicin |
| MDA | Malondialdehyde |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| COX-2 | Cyclooxygenase-2 |
| MAPK | Mitogen-activated protein kinase |
| SOD | Superoxide dismutase |
| CCl4 | Carbon tetrachloride |
| CAT | Catalase |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| ALT | Alanine transaminase |
| TBI | Traumatic brain injury |
| OVA | Ovalbumin |
| HFD | High-fat diet |
| VEGFs | Vascular endothelial growth factors |
| MMPs | Matrix metalloproteinases |
| BW | Body weight |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MPO | Myeloperoxidase |
| AuNPs | Gold nanoparticles |
| ApNp | Apigenin-loaded nanoparticles |
| VCAM-1 | Vascular cell adhesion protein 1 |
| NPs | Nanoparticles |
References
- Fang, Y.; Yang, C.; Yu, Z.; Li, X.; Mu, Q.; Liao, G.; Yu, B. Natural products as LSD1 inhibitors for cancer therapy. Acta Pharm. Sin. B 2021, 11, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Flavonoids Living Syst. 1998, 439, 175–182. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280443 (accessed on 8 May 2024).
- Ali, F.; Rahul Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Fossatelli, L.; Maroccia, Z.; Fiorentini, C.; Bonucci, M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. Int. J. Mol. Sci. 2023, 25, 251. [Google Scholar] [CrossRef] [PubMed]
- Grignon-Dubois, M.; Rezzonico, B. First Phytochemical Evidence of Chemotypes for the Seagrass Zostera noltii. Plants 2012, 1, 27–38. [Google Scholar] [CrossRef]
- Zhao, J.; Dasmahapatra, A.K.; Khan, S.I.; Khan, I.A. Anti-Aromatase Activity of the Constituents from Damiana (Turnera diffusa). J. Ethnopharmacol. 2008, 120, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Chen, K.; Shi, Q.; Kilkuskie, R.E.; Cheng, Y.C.; Lee, K.H. Anti-AIDS Agents, 10. Acacetin-7-O-β-D-Galactopyranoside, an Anti-HIV Principle from Chrysanthemum Morifolium and a Structure-Activity Correlation with Some Related Flavonoids. J. Nat. Prod. 1994, 57, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Zahmatkeshan, M.; Akbarzadeh, A.; Rabiee, N.; Ahmadi, S.; Keyhanvar, P.; Rezayat, S.M.; Seifalian, A.M. Chemotherapeutic effects of Apigenin in breast cancer: Preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol. Rep. 2022, 34, e00730. [Google Scholar] [CrossRef]
- Xiao, M.; Shao, Y.; Yan, W.; Zhang, Z. Measurement and correlation of solubilities of apigenin and apigenin 7-O-rhamnosylglucoside in seven solvents at different temperatures. J. Chem. Thermodyn. 2011, 43, 240–243. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Robinson, D.H.; Birt, D.F. Evaluation of properties of apigenin and [G-3H] apigenin and analytic method development. J. Pharm. Sci. 1997, 86, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Sikder, M.A.; Lee, H.J.; Ryu, J.; Lee, C.J. Apigenin Inhibits Tumor Necrosis Factor-α-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells. Biomol. Ther. 2014, 22, 525–531. [Google Scholar] [CrossRef]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise. Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; United States Department of Agriculture: Washington, DC, USA, 2011; pp. 1–156. [Google Scholar]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, L.; Buiarelli, F.; Coccioli, F.; Jasionowska, R. Identification of compounds in wine by HPLC-tandem mass spectrometry. Ann Chim. 2004, 94, 679–689. [Google Scholar] [CrossRef]
- Gerhauser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B. Content of Potentially Anti-Carcinogenic Flavonoids of 28 Vegetables and 9 Fruits Commonly Consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xu, S.; Gu, W.; Zhu, W. Apigenin Accumulation and Expression Analysis of Apigenin Biosynthesis Relative Genes in Celery. Sci. Hortic. 2014, 165, 218–224. [Google Scholar] [CrossRef]
- Avallone, R.; Zanoli, P.; Puia, G. Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological Profile of Apigenin, a Flavonoid Isolated from Matricaria Chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Lugast, A.; Hovari, J. Flavonoid aglycons in foods of plant origin I. Vegetables. Acta Aliment. 2000, 29, 345–352. [Google Scholar] [CrossRef]
- Leth, T.; Justesen, U. Analysis of flavonoids in fruits, vegetables and beverages by HPLC-UV and LC-MS and estimation the total daily flavonoid intake in Denmark. In Polyphenols in Food; Amado, R., Andersson, H., Bardócz, S., Serra, F., Eds.; Office for Official Publications of the European Communities: Luxembourgh, 1998; p. 3940. [Google Scholar]
- Aravanis, C.; Corcondilas, A.; Dontas, A.S.; Lekos, D.; Keys, A. Coronary heart disease in seven countries. IX. The Greek islands of Crete and Corfu. Circulation 1970, 41, 186–195. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C. DASH and Mediterranean-type Dietary Patterns to Maintain Cognitive Health. Curr. Nutr. Rep. 2014, 3, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kühnau, J. The Flavonoids. A Class of Semi-Essential Food Components: Their Role in Human Nutrition. In World Review of Nutrition and Dietetics; Karger Publishers: Basel, Switzerland, 1976. [Google Scholar]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of Apigenin from Apiin-Rich Parsley in Humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary Flavonoid Sources in Australian Adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Chen, W.; Zhao, X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010, 103, 249–255. [Google Scholar] [CrossRef]
- Shoubaky, G.A.E.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Isolation and identification of a flavone apigenin from marine red alga Acanthophora spicifera with antinociceptive and anti-Inflammatory activities. J. Exp. Neurosci. 2016, 10, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Czeczot, H.; Tudek, B.; Kusztelak, J.; Szymczyk, T.; Dobrowolska, B.; Glinkowska, G.; Malinowski, J.; Strzelecka, H. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat. Res. Genet. Toxicol. 1990, 240, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Fujii, H.; Nakai, K.; Fukagawa, M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther. Apher. Dial. 2011, 15, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; King, B.L.; Rieger, S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing. Sci. Rep. 2016, 6, 20328. [Google Scholar] [CrossRef]
- Barygina, V.; Becatti, M.; Prignano, F.; Lotti, T.; Taddei, N.; Fiorillo, C. Fibroblasts to keratinocytes redox signaling: The possible role of ROS in psoriatic plaque formation. Antioxidants 2019, 8, 566. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant apigenin relieves age-related muscle atrophy by inhibiting oxidative stress and hyperactive mitophagy and apoptosis in skeletal muscle of mice. J. Gerontol. Ser. A 2020, 75, 2081–2088. [Google Scholar] [CrossRef]
- Jung, W.W. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int. J. Mol. Med. 2014, 33, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cheng, K.W.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Zhao, G.; Lin, M.; Lang, Y.; Zhang, D.; Feng, D.; Tu, C. Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones 2020, 25, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, T.; Su, J.; Zhao, Y.; Li, X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 2017, 40, 157–162. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, 140–146. [Google Scholar] [CrossRef]
- Mehta, K.; Aggarwal, B.B. Recombinant Organisms As Source of Cancer biotherapeutics. In Principles of Cancer Biotherapy; Springer: Dordrecht, The Netherlands, 1998; pp. 51–77. [Google Scholar]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zhao, H. Apigenin attenuates inflammatory response in allergic rhinitis mice by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Environ. Toxicol. 2023, 38, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Sahoo, D.; Agrahari, K.; Khan, A.; Mukhopadhyay, P.; Chanda, D.; Yadav, N.P. Anti-inflammatory, anti-proliferative and anti-psoriatic potential of apigenin in RAW 264.7 cells, HaCaT cells and psoriasis like dermatitis in BALB/c mice. Life Sci. 2023, 328, 121909. [Google Scholar] [CrossRef]
- Karamese, M.; Erol, H.S.; Albayrak, M.; Findik Guvendi, G.; Aydin, E.; Aksak Karamese, S. Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: An immunological, biochemical, and histopathological study. Immunopharmacol. Immunotoxicol. 2016, 38, 228–237. [Google Scholar] [CrossRef]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Dross, R.T.V.; Abu-Yousif, A.; Morrison, A.R.; Pelling, J.C. Apigenin Prevents UVB-Induced Cyclooxygenase 2 Expression: Coupled mRNA Stabilization and Translational Inhibition. Mol. Cell. Biol. 2007, 27, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Umeda, D.; Yamashita, S.; Yamada, K.; Tachibana, H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Biochem. 2009, 20, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Charalabopoulos, A.; Davakis, S.; Lambropoulou, M.; Papalois, A.; Simopoulos, C.; Tsaroucha, A. Apigenin exerts anti-inflammatory effects in an experimental model of acute pancreatitis by down-regulating TNF-α. Vivo 2019, 33, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Husain Rahmani, A. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, the most prominent constituent of Nigella sativa, attenuates liver damage in streptozotocin-induced diabetic rats via regulation of oxidative stress, inflammation and cyclooxygenase-2 protein expression. Appl. Sci. 2021, 11, 3223. [Google Scholar] [CrossRef]
- Aldebasi, Y.H.; Aly, S.M.; Rahmani, A.H. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 194–202. [Google Scholar]
- Aggarwal, N.; Aggarwal, S. A Review of Recent Investigations on Medicinal Herbs Possessing AntiDiabetic Properties. J. Nutr. Disord. Ther. 2011, 1, 2. [Google Scholar] [CrossRef]
- Kayarohanam, S.; Kavimani, S. Current Trends of Plants Having Antidiabetic Activity: A Review. J. Bioanal. Biomed. 2015, 7, 55–65. [Google Scholar] [CrossRef]
- Sidhu, M.C.; Sharma, T. Medicinal Plants From Twelve Families Having Antidiabetic Activity: A Review. Am. J. PharmTech Res. 2013, 3, 36–52. [Google Scholar]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Fan, Y.L.; Liao, H.H.; Liu, Y.; Chen, S.; Ma, Z.G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Apigenin (4’,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Alharbi, H.M.; Khan, A.A.; Husain Rahmani, A. Epigallocatechin-3-gallate (EGCG), an active constituent of green tea: Implications in the prevention of liver injury induced by diethylnitrosamine (DEN) in rats. Appl. Sci. 2019, 9, 4821. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective effects of garlic extract against carbon tetrachloride (CCl4)-induced liver injury via modulation of antioxidant, anti-inflammatory activities and hepatocyte architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-canonical NF-κB Pathway In Vivo and In Vitro. Inflammation 2020, 43, 1634–1648. [Google Scholar] [CrossRef]
- Ali, A.A.; Mansour, A.B.; Attia, S.A. The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ. Sci. Pollut. Res. 2021, 28, 27577–27592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, N.; Yang, D.; Yang, M.; Guo, X.; He, J.; Wu, W.; Ji, B.; Cheng, Q.; Zhou, F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients 2018, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.C.; Zhou, R.J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.L. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem. Biol. Interact. 2017, 275, 171–177. [Google Scholar] [CrossRef]
- Berköz, M.; Ünal, S.; Karayakar, F.; Yunusoğlu, O.; Özkan-Yılmaz, F.; Özlüer-Hunt, A.; Aslan, A. Prophylactic effect of myricetin and apigenin against lipopolysaccharide-induced acute liver injury. Mol. Biol. Rep. 2021, 48, 6363–6373. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Z.; Cheng, B.; Liu, M.; Tao, S.; Guan, S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp. Ther. Med. 2019, 18, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Shrivastava, N. Apigenin and kaempferol as novel renoprotective agent against cisplatin-induced toxicity: An in vitro study. Nat. Prod. Res. 2022, 36, 6085–6090. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Chakravarthi, S.; Kulur, A.B.; Yee, T.M. Plant flavone apigenin protects against cyclosporine-induced histological and biochemical changes in the kidney in rats. Biomed. Prev. Nutr. 2014, 4, 589–593. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, C.; Wang, X.; Li, X.; Han, J.; Xue, W.; Wu, P.; Peng, X.; Xia, X. Protective effects of apigenin against 3-MCPD-induced renal injury in rat. Chem. Biol. Interact. 2018, 296, 9–17. [Google Scholar] [CrossRef]
- Ju, S.M.; Kang, J.G.; Bae, J.S.; Pae, H.O.; Lyu, Y.S.; Jeon, B.H. The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/Akt pathway in human renal proximal tubular epithelial cells. Evid. Based Complement. Altern. Med. 2015, 2015, 186436. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Du, Y.; Chen, Z.; Guo, J.; Weng, X.; Wang, X.; Wang, M.; Chen, D.; Liu, X. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomed. Pharmacother. 2017, 95, 1799–1808. [Google Scholar] [CrossRef]
- Quan, W.; Ma, S.; Zhu, Y.; Shao, Q.; Hou, J.; Li, X. Apigenin-7-O-β-d-(6″-p-Coumaroyl)-Glucopyranoside Reduces Myocardial Ischaemia/Reperfusion Injury in an Experimental Model via Regulating the Inflammation Response. Pharm. Biol. 2020, 58, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, L.; Xiao, Y. Apigenin protects against ischemia-/hypoxia-induced myocardial injury by mediating pyroptosis and apoptosis. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lai, S.; Luo, Y.; Wan, Q.; Wu, Q.; Wan, L.; Qi, W.; Liu, J. Nutritional Preconditioning of Apigenin Alleviates Myocardial Ischemia/Reperfusion Injury via the Mitochondrial Pathway Mediated by Notch1/Hes1. Oxidative Med. Cell. Longev. 2019, 2019, 7973098. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]
- Thangaiyan, R.; Robert, B.M.; Arjunan, S.; Govindasamy, K.; Nagarajan, R.P. Preventive effect of apigenin against isoproterenol-induced apoptosis in cardiomyoblasts. J. Biochem. Mol. Toxicol. 2018, 32, e22213. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.F.R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin Attenuates Doxorubicin Induced Cardiotoxicity via Reducing Oxidative Stress and Apoptosis in Male Rats. Life Sci. 2019, 232, 116623. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; de Almeida, M.M.A.; Bispo da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zheng, Y.; Lin, K.; Wang, H.; Chen, T.; Li, L.; Huang, J.; Lin, W.; Zhu, J.; Li, P.; et al. Neuroprotective effect of apigenin against hypoxic-ischemic brain injury in neonatal rats via activation of the PI3K/Akt/Nrf2 signaling pathway. Food Funct. 2021, 12, 2270–2281. [Google Scholar] [CrossRef]
- Kuru Bektaşoğlu, P.; Demir, D.; Koyuncuoğlu, T.; Yüksel, M.; Peker Eyüboğlu, İ.; Karagöz Köroğlu, A.; Akakın, D.; Yıldırım, A.; Çelikoğlu, E.; Gürer, B. Possible anti-inflammatory, antioxidant, and neuroprotective effects of apigenin in the setting of mild traumatic brain injury: An investigation. Immunopharmacol. Immunotoxicol. 2023, 45, 185–196. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β 25-35-induced toxicity in mice. J. Alzheimer’s Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alrumaihi, F.; Alsahli, M.A.; Alhommrani, M.F.; Khan, A.; Rahmani, A.H. Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BaP) in Rats. Molecules 2020, 25, 724. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective Effects of Thymoquinone, an Active Compound of Nigella sativa, on Rats with Benzo(a)pyrene-Induced Lung Injury through Regulation of Oxidative Stress and Inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Syed, M.A.; Rahmani, A.H. Therapeutic potential of Tamarix aphylla in the prevention of lung injury through the regulation of inflammation, oxidative stress and cell-signaling molecules. Appl. Sci. 2022, 12, 9925. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Ali Syed, M.; Rahmani, A.H. Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo (a) pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules. Appl. Sci. 2022, 12, 6784. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W. Apigenin protects against bleomycin-induced lung fibrosis in rats. Exp. Ther. Med. 2016, 11, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin attenuates PM2.5-induced airway hyperresponsiveness and inflammation by down-regulating NF-κB in murine model of asthma. Int. J. Clin. Exp. Pathol. 2019, 12, 3700–3709. [Google Scholar] [PubMed]
- Choi, J.-R.; Lee, C.-M.; Jung, I.D.; Lee, J.S.; Jeong, Y.-I.; Chang, J.H.; Park, H.-J.; Choi, I.-W.; Kim, J.-S.; Shin, Y.K.; et al. Apigenin Protects Ovalbumin-Induced Asthma Through the Regulation of GATA-3 Gene. Int. Immunopharmacol. 2009, 9, 918–924. [Google Scholar] [PubMed]
- Li, K.C.; Ho, Y.L.; Hsieh, W.T.; Huang, S.S.; Chang, Y.S.; Huang, G.J. Apigenin-7-glycoside prevents LPS-induced acute lung injury via downregulation of oxidative enzyme expression and protein activation through inhibition of MAPK phosphorylation. Int. J. Mol. Sci. 2015, 16, 1736–1754. [Google Scholar] [CrossRef]
- Li, R.R.; Pang, L.L.; Du, Q.; Shi, Y.; Dai, W.J.; Yin, K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2010, 32, 364–370. [Google Scholar] [CrossRef]
- Dang, Y.; Li, Z.; Luo, B.; Pan, L.; Wei, Q.; Zhang, Y. Protective effects of apigenin against acrylonitrile-induced subchronic sperm injury in rats. Food Chem. Toxicol. 2017, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.B.; Zhang, M.; Yan, F.; Zhang, Z.X.; Li, Z.L. Effect of apigenin on the reproductive system in male mice. Health 2010, 2, 435. [Google Scholar] [CrossRef]
- Shi, X.R.; Liu, S.Y.; Chen, Y.; Li, F.L.; Xue, H.L.; Dang, Y.H.; Li, Z.L. Apigenin affects semen parameters in male mice. Zhonghua Nan Ke Xue 2010, 16, 778–782. [Google Scholar] [PubMed]
- Yao, L.; Fan, Z.; Han, S.; Sun, N.; Che, H. Apigenin acts as a partial agonist action at estrogen receptors in vivo. Eur. J. Pharmacol. 2021, 906, 174175. [Google Scholar] [CrossRef]
- Alfwuaires, M.A. Protective Effect of Apigenin Against Cyclophosphamide-Induced Testicular Damage in Mice by Modulating Inflammation, Oxidative Stress, and Apoptosis and Upregulating Nrf2/HO-1 Pathway. J. Biol. Regul. Homeost. Agents 2024, 38, 1079–1091. [Google Scholar]
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct. Meridian Stud. 2013, 6, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Park, J.; Lee, E.; Lim, S.; Yu, J.G.; Lee, S.J.; Chen, H.; Dong, Z.; Lee, K.W.; Lee, H.J. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis 2013, 34, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical apigenin alleviates cutaneous inflammation in murine models. Evid. Based Complement. Altern. Med. 2012, 2012, 912028. [Google Scholar] [CrossRef]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef]
- Su, T.; Huang, C.; Yang, C.; Jiang, T.; Su, J.; Chen, M.; Fatima, S.; Gong, R.; Hu, X.; Bian, Z.; et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol. Res. 2020, 152, 104586. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Yu, S.; Wang, K.; Xiong, X.; Xia, M.; Zeng, G.; Huang, Q. Dietary Apigenin Relieves Body Weight and Glycolipid Metabolic Disturbance via Pro-Browning of White Adipose Mediated by Autophagy Inhibition. Mol. Nutr. Food Res. 2023, 67, 2200763. [Google Scholar] [CrossRef]
- Okla, M.; Al Madani, J.O.; Chung, S.; Alfayez, M. Apigenin Reverses Interleukin-1β-Induced Suppression of Adipocyte Browning via COX2/PGE2 Signaling Pathway in Human Adipocytes. Mol. Nutr. Food Res. 2020, 64, e1900925. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernández-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M.P.; Arbonés-Mainar, J.M.; Portillo, M.P. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, J.N.; Akawa, O.B.; Ogbu, E.K.; Eduviere, A.T.; Ozolua, R.I.; Soliman, M. Apigenin attenuates depressive-like behavior via modulating monoamine oxidase A enzyme activity in chronically stressed mice. Curr. Res. Pharmacol. Drug Discov. 2023, 5, 100161. [Google Scholar] [CrossRef]
- Bijani, S.; Dizaji, R.; Sharafi, A.; Hosseini, M.J. Neuroprotective Effect of Apigenin on Depressive-Like Behavior: Mechanistic Approach. Neurochem. Res. 2022, 47, 644–655. [Google Scholar] [CrossRef]
- Zhang, X.; Bu, H.; Jiang, Y.; Sun, G.; Jiang, R.; Huang, X.; Duan, H.; Huang, Z.; Wu, Q. The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol. Med. Rep. 2019, 20, 2867–2874. [Google Scholar] [CrossRef]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016, 774, 50–54. [Google Scholar] [CrossRef]
- Chen, L.; He, M.; Zhang, M.; Sun, Q.; Zeng, S.; Zhao, H.; Yang, H.; Liu, M.; Ren, S.; Meng, X.; et al. The Role of non-coding RNAs in colorectal cancer, with a focus on its autophagy. Pharmacol. Ther. 2021, 226, 107868. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; De Felice, F.; Romito, A.; Iacobelli, V.; Sassu, C.M.; Corrado, G.; Ricci, C.; Scambia, G.; Fagotti, A. Chemotherapy resistance in epithelial ovarian cancer: Mechanisms and emerging treatments. Semin. Cancer Biol. 2021, 77, 144–166. [Google Scholar] [CrossRef]
- Madhuri, S.; Pandey, G. Some anticancer medicinal plants of foreign origin. Curr. Sci. 2009, 96, 779–783. [Google Scholar]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, an Active Compound of Nigella sativa: Role in Prevention and Treatment of Cancer. Curr. Pharm. Biotechnol. 2020, 21, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and mechanisms of kaempferol in the management of cancers through modulation of inflammation and signal transduction pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Formononetin, a Type of Methoxylated Isoflavone, and Its Role in Cancer Therapy through the Modulation of Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9719. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A. Myricetin: A Significant Emphasis on Its Anticancer Potential via the Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9665. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Choo, G.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Kim, H.J.; Jung, S.H.; Park, Y.S.; Kim, B.S.; et al. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol. Med. Rep. 2020, 22, 4877–4889. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, W.; Chen, M.; Huang, L.; Li, S.; Li, Z.; Pan, Q.; Lv, S.; Yang, X.; Wang, Y.; et al. Apigenin suppresses tumor angiogenesis and growth via inhibiting HIF-1α expression in non-small cell lung carcinoma. Chem.-Biol. Interact. 2022, 361, 109966. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhu, Y.; Li, J.F.; Wang, X.; Liang, Z.; Li, S.Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.Y.; et al. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 2017, 8, 19834. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yuan, M.; Li, S.; Thuan, U.T.; Nguyen, T.T.; Kang, T.W.; Liao, W.; Lian, S.; Jung, Y.D. Apigenin Suppresses the IL-1β-Induced Expression of the Urokinase-Type Plasminogen Activator Receptor by Inhibiting MAPK-Mediated AP-1 and NF-κB Signaling in Human Bladder Cancer T24 Cells. J. Agric. Food Chem. 2018, 66, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.; Li, D.; You, J. Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol. Med. Rep. 2016, 14, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, W.; Che, W.; Wang, L.; Zhao, L. Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules 2023, 28, 2915. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Zhou, Q.; Jie, H.; He, Y.; Han, J.; He, J.; Jiang, Y.; Sun, E. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 2016, 20, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; He, H.; Zhu, L.; Gao, J.; Wei, T.; Ma, Z.; Yan, T. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB pathway. Chem. Biol. Interact. 2015, 236, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Shu, N.; Zhang, Z.; Wang, X.; Li, R.; Li, W.; Liu, X.; Zhang, Q.; Jiang, Z.; Tao, L.; Zhang, L.; et al. Apigenin Alleviates Autoimmune Uveitis by Inhibiting Microglia M1 Pro-Inflammatory Polarization. Investig. Ophthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef]
- Liu, L.; Wei, D.; Xu, H.; Liu, C. Apigenin ameliorates ocular surface lesions in a rat model of dry eye disease. Eur. J. Inflamm. 2019, 17, 2058739218818681. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Tai, Z.; Li, T.; Luo, L.; Tong, Z.; Zhu, W. Apigenin and Ethaverine Hydrochloride Enhance Retinal Vascular Barrier In Vitro and In Vivo. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef] [PubMed]
- Aryal, Y.P.; Yeon, C.Y.; Kim, T.Y.; Lee, E.S.; Sung, S.; Pokharel, E.; Kim, J.Y.; Choi, S.Y.; Yamamoto, H.; Sohn, W.J.; et al. Facilitating Reparative Dentin Formation Using Apigenin Local Delivery in the Exposed Pulp Cavity. Front. Physiol. 2021, 12, 773878. [Google Scholar] [CrossRef]
- D’Amico, E.; Pierfelice, T.V.; Iezzi, G.; Di Pietro, N.; Lepore, S.; Lorusso, F.; Scarano, A.; Pandolfi, A.; Piattelli, A.; Petrini, M. Apigenin Promotes Proliferation and Mineralization of Human Osteoblasts and Up-Regulates Osteogenic Markers. Appl. Sci. 2022, 12, 8510. [Google Scholar] [CrossRef]
- André, C.B.; Rosalen, P.L.; Giannini, M.; Bueno-Silva, B.; Pfeifer, C.S.; Ferracane, J.L. Incorporation of Apigenin and tt-Farnesol into dental composites to modulate the Streptococcus mutans virulence. Dent. Mater. 2021, 37, e201–e212. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- Rithidech, K.N.; Tungjai, M.; Whorton, E.B. Protective effect of apigenin on radiation-induced chromosomal damage in human lymphocytes. Mutat. Res. 2005, 585, 96–104. [Google Scholar] [CrossRef]
- Liu, D.; Peng, R.; Chen, Z.; Yu, H.; Wang, S.; Dong, S.; Li, W.; Shao, W.; Dai, J.; Li, F.; et al. The Protective Effects of Apigenin Against Radiation-Induced Intestinal Injury. Dose Response 2022, 20, 15593258221113791. [Google Scholar] [CrossRef]
- Begum, N.; Prasad, N.R.; Thayalan, K. Apigenin protects gamma-radiation induced oxidative stress, hematological changes and animal survival in whole body irradiated Swiss albino mice. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 45–52. [Google Scholar]
- Begum, N.; Prasad, N.R. Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damages in human peripheral blood lymphocytes. Biomed. Prev. Nutr. 2012, 2, 16–24. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, L.; Meng, Y.; Xue, W.; Liang, J.; Peng, Z.; Meng, J.; Zhang, M. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front. Nutr. 2022, 9, 1062961. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, X.; He, Z.; Xie, Y.; Niu, Z.; Zhang, W.; Wang, A.; Zhang, J.; Si, C.; Li, F.; et al. Apigenin-7-O-glucoside alleviates DSS-induced colitis by improving intestinal barrier function and modulating gut microbiota. J. Funct. Foods 2023, 104, 105499. [Google Scholar] [CrossRef]
- Shibrya, E.E.; Rashed, R.R.; Abd El Fattah, M.A.; El-Ghazaly, M.A.; Kenawy, S.A. Apigenin and Exposure to Low Dose Gamma Radiation Ameliorate Acetic Acid-Induced Ulcerative Colitis in Rats. Dose Response 2023, 21, 15593258231155787. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; González, R.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Br. J. Nutr. 2015, 113, 618–626. [Google Scholar] [CrossRef]
- Sadraei, H.; Asghari, G.; Khanabadi, M.; Minaiyan, M. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res. Pharm. Sci. 2017, 12, 322. [Google Scholar]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, L.; Jiang, J. Apigenin accelerates wound healing in diabetic mice by promoting macrophage M2-type polarization via increasing miR-21 expression. Mol. Cell. Biochem. 2024; ahead of print. [Google Scholar]
- Rajab, A.A.; Al-Wattar, W.T.; Taqa, G.A. The roles of apigenin cream on wound healing in rabbits model. J. Appl. Vet. Sci. 2022, 7, 1–5. [Google Scholar] [CrossRef]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491s–499s. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Moore, P.; Bernui, M.; Singh, N.; Bearoff, F.; Nagarkatti, M.; Khan, Z.K.; Jain, P. Apigenin Modulates Dendritic Cell Activities and Curbs Inflammation Via RelB Inhibition in the Context of Neuroinflammatory Diseases. J. Neuroimmune Pharmacol. 2021, 16, 403–424. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin Impacts the Growth of the Gut Microbiota and Alters the Gene Expression of Enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Chukrathok, S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine 2013, 20, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Akilandeswari, K.; Ruckmani, K. Synergistic antibacterial effect of apigenin with β-lactam antibiotics and modulation of bacterial resistance by a possible membrane effect against methicillin resistant Staphylococcus aureus. Cell. Mol. Biol. 2016, 62, 74–82. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.R.; Lee, D.G. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J. Basic Microbiol. 2020, 60, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, E.R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, E.R.; Lee, D.G. Effect of apigenin isolated from Aster yomena against Candida albicans: Apigenin-triggered apoptotic pathway regulated by mitochondrial calcium signaling. J. Ethnopharmacol. 2019, 231, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Qiu, M.; Chen, D.; Zheng, N.; Jin, Y.; Wu, Z. Apigenin inhibits enterovirus 71 replication through suppressing viral IRES activity and modulating cellular JNK pathway. Antivir. Res. 2014, 109, 30–41. [Google Scholar] [CrossRef]
- Xu, X.; Miao, J.; Shao, Q.; Gao, Y.; Hong, L. Apigenin suppresses influenza A virus-induced RIG-I activation and viral replication. J. Med. Virol. 2020, 92, 3057–3066. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, H.; Lv, Y.; Wang, J.; Chen, X.; Hou, Y.; Tan, R.; Li, E. Apigenin Inhibits Enterovirus-71 Infection by Disrupting Viral RNA Association with trans-Acting Factors. PLoS ONE 2014, 9, e110429. [Google Scholar] [CrossRef]
- Fonseca-Silva, F.; Canto-Cavalheiro, M.M.; Menna-Barreto, R.F.; Almeida-Amaral, E.E. Effect of Apigenin on Leishmania amazonensis Is Associated with Reactive Oxygen Species Production Followed by Mitochondrial Dysfunction. J. Nat. Prod. 2015, 78, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, P.; Joshi, S.C. Treatment of dermatophytosis by a new antifungal agent ‘apigenin’. Mycoses 2014, 57, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Jaber, S. Apigenin Inhibits Infectious Bronchitis Virus Replication Inovo. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5367–5371. [Google Scholar] [CrossRef] [PubMed]
- Emiliano, Y.S.; Almeida-Amaral, E.E. Apigenin is a promising molecule for treatment of visceral leishmaniasis. Front. Cell. Infect. Microbiol. 2023, 13, 1066407. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, T.; Shi, Z.; Hu, C.; Li, Q.; Sun, C. Synergism Antiproliferative Effects of Apigenin and Naringenin in NSCLC Cells. Molecules 2023, 28, 4947. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dia, V.P.; Zhong, Q. Synergistic anti-inflammatory activity of apigenin and curcumin co-encapsulated in caseins assessed with lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 193 Pt A, 702–712. [Google Scholar] [CrossRef]
- Jin, X.; Yang, Q.; Zhang, Y. Synergistic apoptotic effects of apigenin TPGS liposomes and tyroservatide: Implications for effective treatment of lung cancer. Int. J. Nanomed. 2017, 12, 5109–5118. [Google Scholar] [CrossRef]
- Hassan, S.M.; Khalaf, M.M.; Sadek, S.A.; Abo-Youssef, A.M. Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm. Biol. 2017, 55, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Kayacan, S.; Yilancioglu, K.; Akdemir, A.S.; Kaya-Dagistanli, F.; Melikoglu, G.; Ozturk, M. Synergistic effect of apigenin and curcumin on apoptosis, paraptosis and autophagy-related cell death in HeLa cells. Anticancer. Res. 2021, 41, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Genc, F.; Atabey, U.S.; Serttas, R.; Erdogan, S. Abiraterone Acetate, in Combination with Apigenin, Attenuates the Survival of Human Castration-Sensitive Prostate Cancer Cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2022, 22, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Ganguli, A.; Dastidar, D.G.; Acharya, B.R.; Das, A.; Chakrabarti, G. Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin. Biochimie 2013, 95, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Şirin, N.; Elmas, L.; Seçme, M.; Dodurga, Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene 2020, 737, 144428. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.A.; Le Maitre, C.L.; Cross, N.A.; Jordan-Mahy, N. The effect of apigenin and chemotherapy combination treatments on apoptosis-related genes and proteins in acute leukaemia cell lines. Sci. Rep. 2022, 12, 8858. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; He, W.; Xia, S.; Jiang, X.; Li, X.; Bai, J.; Li, N.; Chen, L.; Yang, B. Apigenin Enhanced Antitumor Effect of Cisplatin in Lung Cancer via Inhibition of Cancer Stem Cells. Nutr. Cancer 2021, 73, 1489–1497. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Wang, W.; Gu, C.B.; Zhao, C.J.; Jiao, J.; Efferth, T. Enzyme pretreatment and negative pressure cavitation extraction of genistein and apigenin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.] and the evaluation of antioxidant activity. Ind. Crops Prod. 2012, 37, 311–320. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, L.P.; Lu, X.Y.; Jiang, H.D.; Zeng, S. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J. Agric. Food Chem. 2007, 55, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Fong, R.Y.; Ensunsa, J.L.; Kimball, J.; Medici, V.; Ottaviani, J.I.; Crozier, A. Absorption, distribution, metabolism and excretion of apigenin and its glycosides in healthy male adults. Free. Radic. Biol. Med. 2022, 185, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Verwilst, P.; Won, M.; Lee, J.; Sessler, J.L.; Han, J.; Kim, J.S. A small molecule strategy for targeting cancer stem cells in hypoxic microenvironments and preventing tumorigenesis. J. Am. Chem. Soc. 2021, 143, 14115–14124. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; Rodriguez-Torres, M.D.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449. [Google Scholar] [CrossRef] [PubMed]
- Medina, O.P.; Zhu, Y.; Kairemo, K. Targeted liposomal drug delivery in cancer. Curr. Pharm. Des. 2004, 10, 2981–2989. [Google Scholar] [CrossRef]
- Banerjee, K.; Banerjee, S.; Das, S.; Mandal, M. Probing the potential of apigenin liposomes in enhancing bacterial membrane perturbation and integrity loss. J. Colloid Interface Sci. 2015, 453, 48–59. [Google Scholar] [CrossRef]
- Arsić, I.; Tadić, V.; Vlaović, D.; Homšek, I.; Vesić, S.; Isailović, G.; Vuleta, G. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother. Res. 2011, 25, 228–233. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdam, Z.; Amini, S.M.; Dalouchi, F.; Behrooz, A.B.; Azizi, Y. Apigenin-coated gold nanoparticles as a cardioprotective strategy against doxorubicin-induced cardiotoxicity in male rats via reducing apoptosis. Heliyon 2023, 9, e14024. [Google Scholar] [CrossRef] [PubMed]
- Ngernyuang, N.; Wongwattanakul, M.; Charusirisawad, W.; Shao, R.; Limpaiboon, T. Green synthesized apigenin conjugated gold nanoparticles inhibit cholangiocarcinoma cell activity and endothelial cell angiogenesis in vitro. Heliyon 2022, 8, e12028. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mondal, L.; Mukherjee, B.; Dutta, L.; Ehsan, I.; Debnath, M.C.; Gaonkar, R.H.; Pal, M.M.; Majumdar, S. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1905–1917. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Hashem, A.M.; Abujamel, T.S.; Alhakamy, N.A.; Kalam, M.A.; Riadi, Y.; Md, S. Apigenin Loaded Lipoid–PLGA–TPGS Nanoparticles for Colon Cancer Therapy: Characterization, Sustained Release, Cytotoxicity, and Apoptosis Pathways. Polymers 2022, 14, 3577. [Google Scholar] [CrossRef]
- Jiang, J.; Mao, Q.; Li, H.; Lou, J. Apigenin stabilized gold nanoparticles increased radiation therapy efficiency in lung cancer cells. Int. J. Clin. Exp. Med. 2017, 10, 13298–13305. [Google Scholar]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation and Evaluation of Apigenin-Loaded Hybrid Nanoparticles. Pharmaceutics 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Zhang, Y.; Peng, Q.; Zhao, X.; Hu, D.; Wen, J.; Liu, K.; Li, R.; Wang, K.; Sun, J. Apigenin-Mn (II) loaded hyaluronic acid nanoparticles for ulcerative colitis therapy in mice. Front. Chem. 2022, 10, 969962. [Google Scholar] [CrossRef]
- Bonilla-Vidal, L.; Świtalska, M.; Espina, M.; Wietrzyk, J.; García, M.L.; Souto, E.B.; Gliszczyńska, A.; Sánchez López, E. Dually Active Apigenin-Loaded Nanostructured Lipid Carriers for Cancer Treatment. Int. J. Nanomed. 2023, 31, 6979–6997. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Bozorgi, M.; Jamalpoor, Z. Fabrication and characterization of apigenin-loaded chitosan/gelatin membranes for bone tissue engineering applications. J. Bioact. Compat. Polym. 2023, 38, 142–157. [Google Scholar] [CrossRef]
- Mabrouk Zayed, M.M.; Sahyon, H.A.; Hanafy, N.A.; El-Kemary, M.A. The effect of encapsulated apigenin nanoparticles on HePG-2 cells through regulation of P53. Pharmaceutics 2022, 14, 1160. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment. Biomedicines 2024, 12, 1353. https://doi.org/10.3390/biomedicines12061353
Allemailem KS, Almatroudi A, Alharbi HOA, AlSuhaymi N, Alsugoor MH, Aldakheel FM, Khan AA, Rahmani AH. Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment. Biomedicines. 2024; 12(6):1353. https://doi.org/10.3390/biomedicines12061353
Chicago/Turabian StyleAllemailem, Khaled S., Ahmad Almatroudi, Hajed Obaid A. Alharbi, Naif AlSuhaymi, Mahdi H. Alsugoor, Fahad M. Aldakheel, Amjad Ali Khan, and Arshad Husain Rahmani. 2024. "Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment" Biomedicines 12, no. 6: 1353. https://doi.org/10.3390/biomedicines12061353
APA StyleAllemailem, K. S., Almatroudi, A., Alharbi, H. O. A., AlSuhaymi, N., Alsugoor, M. H., Aldakheel, F. M., Khan, A. A., & Rahmani, A. H. (2024). Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment. Biomedicines, 12(6), 1353. https://doi.org/10.3390/biomedicines12061353







