Fabrication and Optimization of Poly(ε-caprolactone) Microspheres Loaded with S-Nitroso-N-Acetylpenicillamine for Nitric Oxide Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Fabrication of SNAP-Loaded Microspheres
2.3. Characterization
2.3.1. Size Optimization of SNAP-MSs
2.3.2. Entrapment Efficiency and UV-Vis Spectroscopy
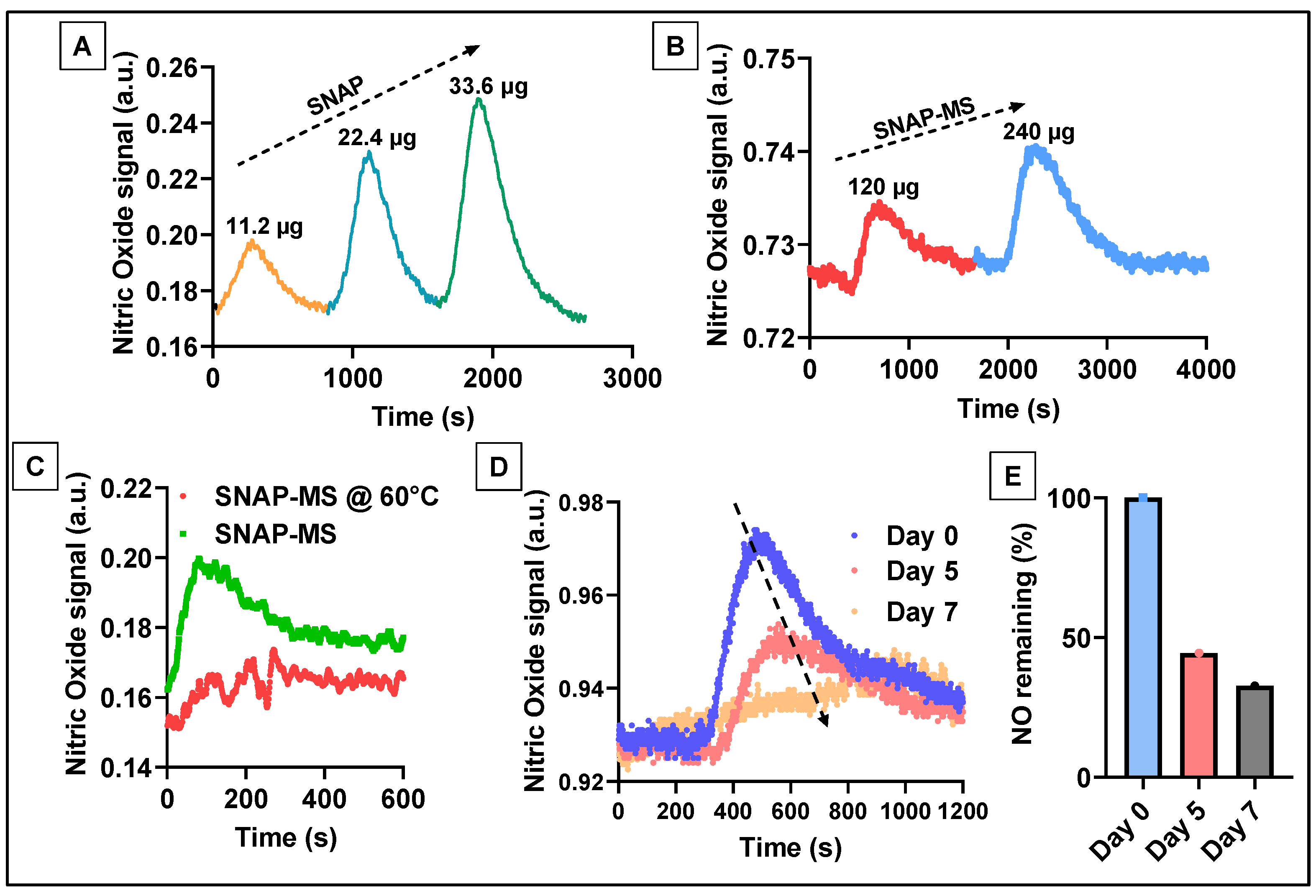
2.3.3. Effect of Homogenization on SNAP Stability
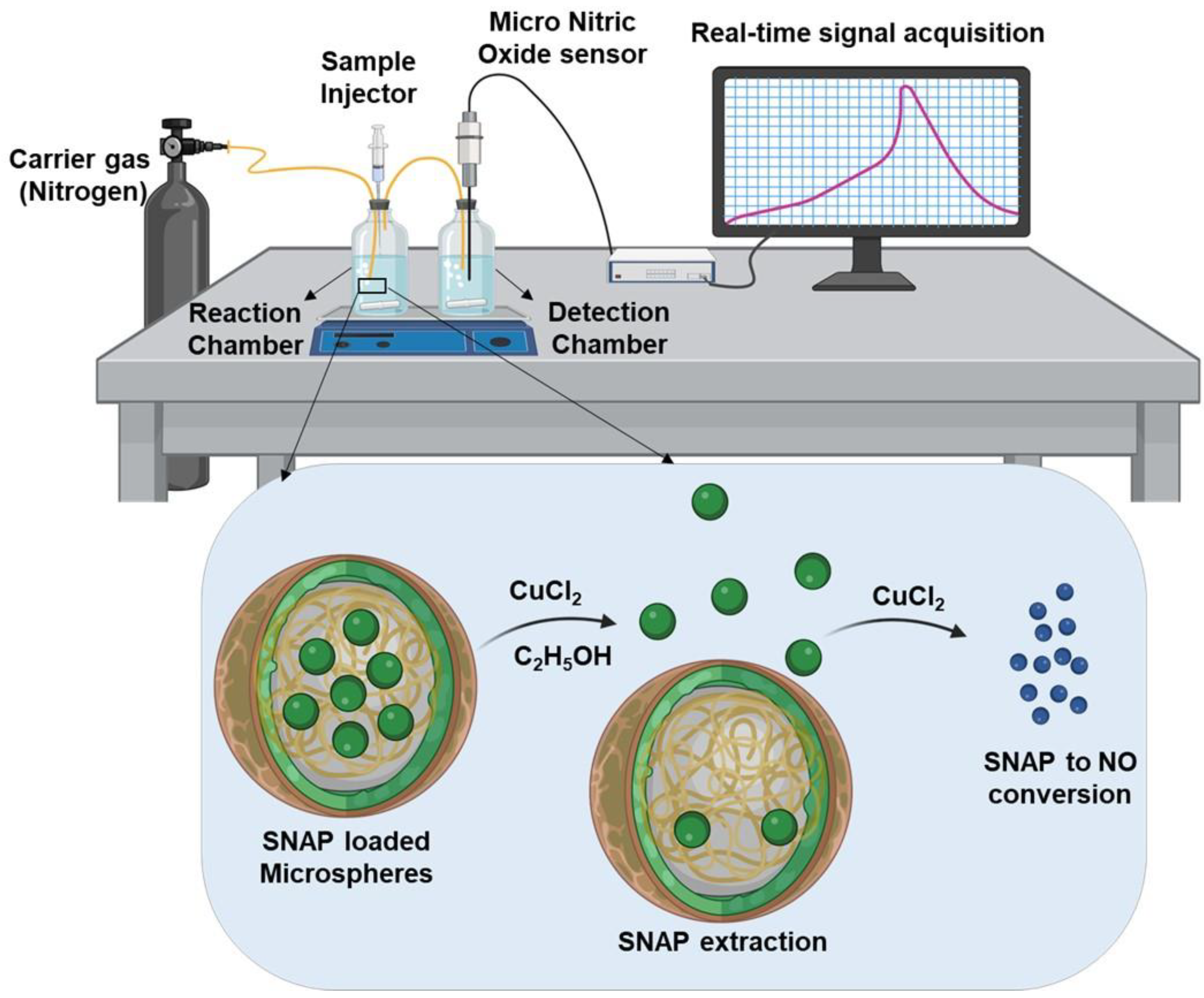
2.3.4. Experimental Setup for Real-Time NO Measurement
2.3.5. Measurement of NO Released from SNAP-MSs
2.3.6. Biocompatibility of SNAP-MS
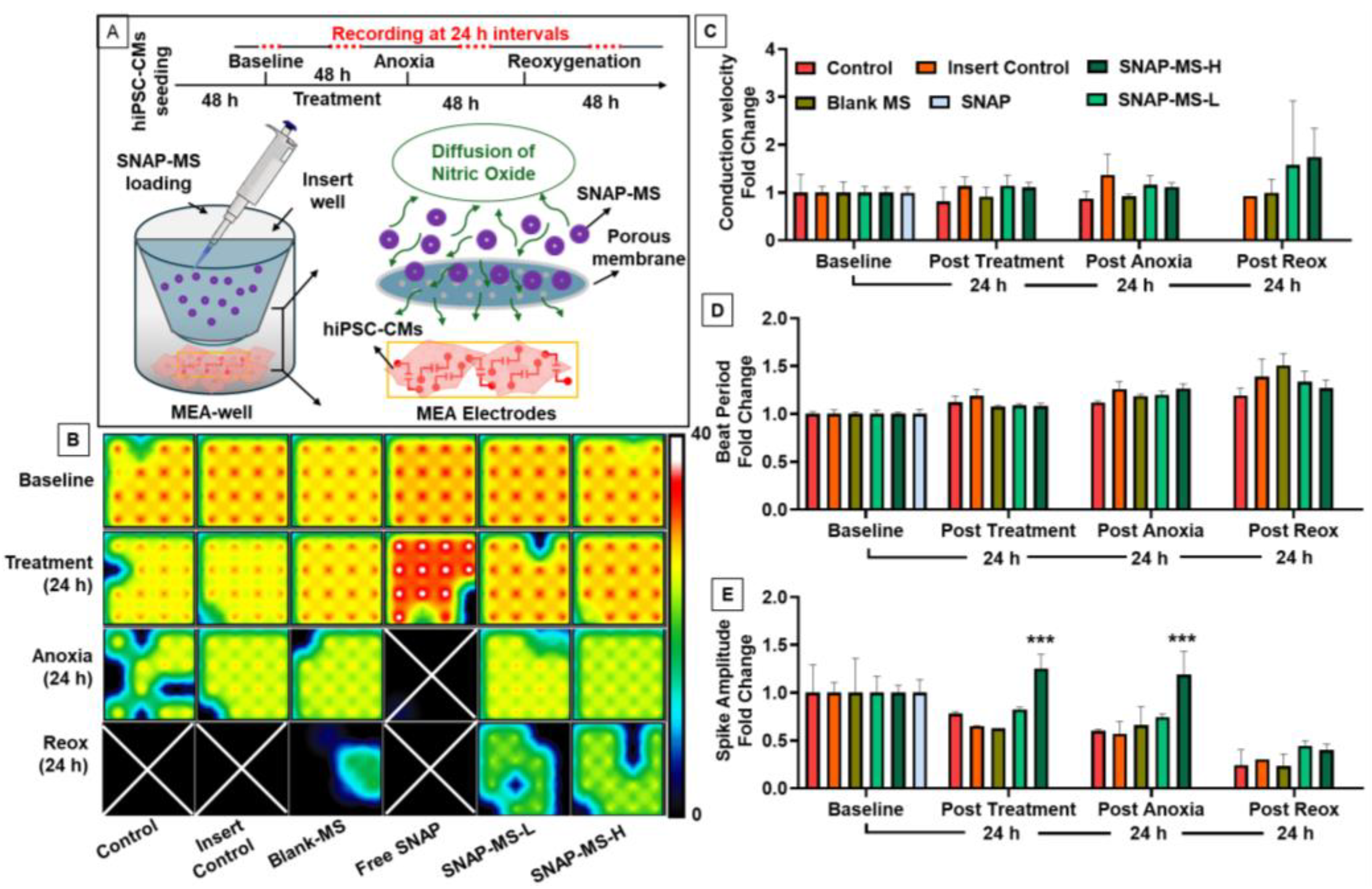
2.3.7. Effect of SNAP-MSs on hiPSC-CMs during Anoxic Stress
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NO | Nitric Oxide |
| MS | Microspheres |
| SNAP | S-Nitroso-N-Acetylpenicillamine |
| PCL | poly(ε-caprolactone) |
| GTN | Glyceryl trinitrate |
| PLGA | poly(lactic-co-glycolic acid) |
| PVA | Polyvinyl alcohol |
| SEM | Scanning Electron Microscopy |
| CuCl2 | Copper (II) chloride |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| DETA NONOate | Diethylenetriamine NONOate |
| DMSO | Dimethylsulfoxide |
| RPM | Rotations Per Minute |
| AUC | Area Under Curve |
| hiPSC-CMs | human induced Pluoripotent Stem Cell derived Cardiomyocytes |
| MEA | Multi-Electrode Array |
References
- Robbins, R.A.; Grisham, M.B. Nitric oxide. Int. J. Biochem. Cell Biol. 1997, 29, 857–860. [Google Scholar] [CrossRef]
- Matsunaga, T.; Weihrauch, D.W.; Moniz, M.C.; Tessmer, J.; Warltier, D.C.; Chilian, W.M. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation 2002, 105, 2185–2191. [Google Scholar] [CrossRef]
- Marin, J.; Rodriguez-Martinez, M.A. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol. Ther. 1997, 75, 111–134. [Google Scholar] [CrossRef]
- Kim, K.H.; Kerndt, C.C.; Adnan, G.; Schaller, D.J. Nitroglycerin; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yu, B.; Ichinose, F.; Bloch, D.B.; Zapol, W.M. Inhaled nitric oxide. Br. J. Pharmacol. 2019, 176, 246–255. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule—Recent developments in cGMP research and development. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 287–302. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Sim, J.Y. Nitric oxide and pulmonary hypertension. Korean J. Anesthesiol. 2010, 58, 4–14. [Google Scholar] [CrossRef]
- Hickok, J.R.; Thomas, D.D. Nitric oxide and cancer therapy: The emperor has NO clothes. Curr. Pharm. Des. 2010, 16, 381–391. [Google Scholar] [CrossRef]
- Lautner, G.; Meyerhoff, M.E.; Schwendeman, S.P. Biodegradable poly(lactic-co-glycolic acid) microspheres loaded with S-nitroso-N-acetyl-D-penicillamine for controlled nitric oxide delivery. J. Control Release 2016, 225, 133–139. [Google Scholar] [CrossRef]
- Korde Choudhari, S.; Sridharan, G.; Gadbail, A.; Poornima, V. Nitric oxide and oral cancer: A review. Oral. Oncol. 2012, 48, 475–483. [Google Scholar] [CrossRef]
- Salvemini, D.; Pistelli, A.; Mollace, V. Release of nitric oxide from glyceryl trinitrate by captopril but not enalaprilat: In vitro and in vivo studies. Br. J. Pharmacol. 1993, 109, 430–436. [Google Scholar] [CrossRef]
- Garatti, L.; Frea, S.; Bocchino, P.P.; Angelini, F.; Cingolani, M.; Sacco, A.; Rondinara, G.M.; Bagnardi, V.; Sala, I.M.; Kapur, N.K.; et al. Sodium nitroprusside in acute heart failure: A multicenter historic cohort study. Int. J. Cardiol. 2022, 369, 37–44. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Napoli, C.; Loscalzo, J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: An overview. Circ. Res. 2002, 90, 21–28. [Google Scholar] [CrossRef]
- Liang, H.; Nacharaju, P.; Friedman, A.; Friedman, J.M. Nitric oxide generating/releasing materials. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Babich, H.; Zuckerbraun, H.L.; Hirsch, S.T.; Blau, L. In vitro cytotoxicity of the nitric oxide donor, S-nitroso-N-acetyl-penicillamine, towards cells from human oral tissue. Pharmacol. Toxicol. 1999, 84, 218–225. [Google Scholar] [CrossRef]
- Christopher, T.A.; Ma, X.L.; Lefer, A.M. Beneficial actions of S-nitroso-N-acetylpenicillamine, a nitric oxide donor, in murine traumatic shock. Shock 1994, 1, 19–24. [Google Scholar] [CrossRef]
- Symington, P.A.; Ma, X.L.; Lefer, A.M. Protective actions of S-nitroso-N-acetylpenicillamine (SNAP) in a rat model of hemorrhagic shock. Methods Find. Exp. Clin. Pharmacol. 1992, 14, 789–797. [Google Scholar]
- Melvin, A.C.; Jones, W.M.; Lutzke, A.; Allison, C.L.; Reynolds, M.M. S-Nitrosoglutathione exhibits greater stability than S-nitroso-N-acetylpenicillamine under common laboratory conditions: A comparative stability study. Nitric Oxide 2019, 92, 18–25. [Google Scholar] [CrossRef]
- Arunkumar, P.; Dougherty, J.A.; Weist, J.; Kumar, N.; Angelos, M.G.; Powell, H.M.; Khan, M. Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials 2019, 9, 1037. [Google Scholar] [CrossRef]
- Vivek, K.; Reddy, L.H.; Murthy, R.S. Comparative study of some biodegradable polymers on the entrapment efficiency and release behavior of etoposide from microspheres. Pharm. Dev. Technol. 2007, 12, 79–88. [Google Scholar] [CrossRef]
- Thackaberry, E.A.; Farman, C.; Zhong, F.; Lorget, F.; Staflin, K.; Cercillieux, A.; Miller, P.E.; Schuetz, C.; Chang, D.; Famili, A.; et al. Evaluation of the Toxicity of Intravitreally Injected PLGA Microspheres and Rods in Monkeys and Rabbits: Effects of Depot Size on Inflammatory Response. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4274–4285. [Google Scholar] [CrossRef]
- Zhou, F.L.; Hubbard Cristinacce, P.L.; Eichhorn, S.J.; Parker, G.J. Preparation and characterization of polycaprolactone microspheres by electrospraying. Aerosol Sci. Technol. 2016, 50, 1201–1215. [Google Scholar] [CrossRef]
- da Silva, T.B.V.; de Oliveira, A.; Moreira, T.F.M.; da Silva, K.C.; Zanin, R.C.; Bona, E.; Goncalves, O.H.; Shirai, M.A.; Peron, A.P.; Leimann, F.V. Analytical validation of an ultraviolet-visible procedure for determining vitamin D(3) in vitamin D(3)-loaded microparticles and toxigenetic studies for incorporation into food. Food Chem. 2021, 360, 129979. [Google Scholar] [CrossRef]
- Anarjan, N.; Jafarizadeh-Malmiri, H.; Nehdi, I.A.; Sbihi, H.M.; Al-Resayes, S.I.; Tan, C.P. Effects of homogenization process parameters on physicochemical properties of astaxanthin nanodispersions prepared using a solvent-diffusion technique. Int. J. Nanomed. 2015, 10, 1109–1118. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical nitric oxide sensors for physiological measurements. Chem. Soc. Rev. 2010, 39, 1925–1935. [Google Scholar] [CrossRef]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas. Res. 2019, 9, 192–207. [Google Scholar] [CrossRef]
- Zhuang, H.; Shao, J.; Wu, P.; Yu, G.; Fu, K.; Sun, Z.; Cao, M.; Liu, Y.; Zhou, Y. Nitric oxide releasing alginate microspheres for antimicrobial application. Int. J. Biol. Macromol. 2023, 224, 1244–1251. [Google Scholar] [CrossRef]
- Lee, P.C.; Kibbe, M.R.; Schuchert, M.J.; Stolz, D.B.; Watkins, S.C.; Griffith, B.P.; Billiar, T.R.; Shears, L.L., 2nd. Nitric oxide induces angiogenesis and upregulates alpha(v)beta(3) integrin expression on endothelial cells. Microvasc. Res. 2000, 60, 269–280. [Google Scholar] [CrossRef]
- Kumar, N.; Dougherty, J.A.; Manring, H.R.; Elmadbouh, I.; Mergaye, M.; Czirok, A.; Greta Isai, D.; Belevych, A.E.; Yu, L.; Janssen, P.M.L.; et al. Assessment of temporal functional changes and miRNA profiling of human iPSC-derived cardiomyocytes. Sci. Rep. 2019, 9, 13188. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Smith, A.F.; Doyeux, V.; Berg, M.; Peyrounette, M.; Haft-Javaherian, M.; Larue, A.E.; Slater, J.H.; Lauwers, F.; Blinder, P.; Tsai, P.; et al. Brain Capillary Networks Across Species: A few Simple Organizational Requirements Are Sufficient to Reproduce Both Structure and Function. Front. Physiol. 2019, 10, 233. [Google Scholar] [CrossRef]
- Zhao, H.; Gagnon, J.; Hafeli, U.O. Process and formulation variables in the preparation of injectable and biodegradable magnetic microspheres. Biomagn. Res. Technol. 2007, 5, 2. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, J.S.; Lee, C.H. Characterization of nitric oxide-releasing microparticles for the mucosal delivery. J. Biomed. Mater. Res. A 2010, 92, 1233–1243. [Google Scholar] [CrossRef]
- Mayer, G.A. Instability of nitroglycerin tablets. Can. Med. Assoc. J. 1974, 110, 788–789, 791. [Google Scholar]
- Xing, L.; Zhang, Z.B.; Liu, C.Y.; Wu, Z.Z.; Lin, C. Amperometric detection of nitric oxide with microsensor in the medium of seawater and its applications. Sensors 2005, 5, 537–545. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, A.; Yang, Z.; Huang, W. Advanced Nitric Oxide Generating Nanomedicine for Therapeutic Applications. ACS Nano 2023, 17, 8935–8965. [Google Scholar] [CrossRef]
- Brisbois, E.J.; Davis, R.P.; Jones, A.M.; Major, T.C.; Bartlett, R.H.; Meyerhoff, M.E.; Handa, H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J. Mater. Chem. B 2015, 3, 1639–1645. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Qin, M.; Landriscina, A.; Rosen, J.M.; Wei, G.; Kao, S.; Olcott, W.; Agak, G.W.; Paz, K.B.; Bonventre, J.; Clendaniel, A.; et al. Nitric Oxide-Releasing Nanoparticles Prevent Propionibacterium acnes-Induced Inflammation by Both Clearing the Organism and Inhibiting Microbial Stimulation of the Innate Immune Response. J. Investig. Dermatol. 2015, 135, 2723–2731. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Paniagua, A.; Diamond, C.; Ferlauto, H.R.; Fernandez-Moure, J.S. Nanomaterial Nitric Oxide Delivery in Traumatic Orthopedic Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 592008. [Google Scholar] [CrossRef]
- Gresele, P.; Momi, S.; Guglielmini, G. Nitric oxide-enhancing or -releasing agents as antithrombotic drugs. Biochem. Pharmacol. 2019, 166, 300–312. [Google Scholar] [CrossRef]
- Soni, S.D.; Song, W.; West, J.L.; Khera, M. Nitric oxide-releasing polymeric microspheres improve diabetes-related erectile dysfunction. J. Sex. Med. 2013, 10, 1915–1925. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Zhou, L.; Liu, J.; Mao, L.; Jia, R.; Zhao, F. Porous gelatin microspheres implanted with adipose mesenchymal stromal cells promote angiogenesis via protein kinase B/endothelial nitric oxide synthase signaling pathway in bladder reconstruction. Cytotherapy 2023, 25, 1317–1330. [Google Scholar] [CrossRef]
- Oh, Y.; Park, K.; Jung, S.; Choi, M.; Kim, T.; Lee, Y.; Choi, J.Y.; Kim, Y.H.; Jung, S.Y.; Hong, J. Inhalable Nitric Oxide Delivery Systems for Pulmonary Arterial Hypertension Treatment. Small 2023, 20, e2308936. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Burgaud, J.L.; Morelli, A. Nitric oxide-releasing NSAIDs: A review of their current status. Drug Saf. 2001, 24, 801–811. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Singh, D.P. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs: Gastrointestinal-sparing potential drugs. J. Med. Food 2009, 12, 208–218. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.S.; Park, S.Y.; Baek, S.W.; Jung, J.W.; Kim, T.H.; Han, D.K. Nitric Oxide-Releasing Bioinspired Scaffold for Exquisite Regeneration of Osteoporotic Bone via Regulation of Homeostasis. Adv. Sci. 2023, 10, e2205336. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvi, S.B.; Pracha, N.; Shalaan, M.; Dholaniya, P.S.; Mergaye, M.; Sridharan, D.; Khan, M. Fabrication and Optimization of Poly(ε-caprolactone) Microspheres Loaded with S-Nitroso-N-Acetylpenicillamine for Nitric Oxide Delivery. Biomedicines 2024, 12, 1363. https://doi.org/10.3390/biomedicines12061363
Alvi SB, Pracha N, Shalaan M, Dholaniya PS, Mergaye M, Sridharan D, Khan M. Fabrication and Optimization of Poly(ε-caprolactone) Microspheres Loaded with S-Nitroso-N-Acetylpenicillamine for Nitric Oxide Delivery. Biomedicines. 2024; 12(6):1363. https://doi.org/10.3390/biomedicines12061363
Chicago/Turabian StyleAlvi, Syed Baseeruddin, Nooruddin Pracha, Mahmoud Shalaan, Pankaj Singh Dholaniya, Muhamad Mergaye, Divya Sridharan, and Mahmood Khan. 2024. "Fabrication and Optimization of Poly(ε-caprolactone) Microspheres Loaded with S-Nitroso-N-Acetylpenicillamine for Nitric Oxide Delivery" Biomedicines 12, no. 6: 1363. https://doi.org/10.3390/biomedicines12061363





