Analgesic Effect of SKI306X on Chronic Postischemic Pain and Spinal Nerve Ligation-Induced Neuropathic Pain in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Animal Models
2.3. Drug Administration
2.4. Behavioral Testing
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
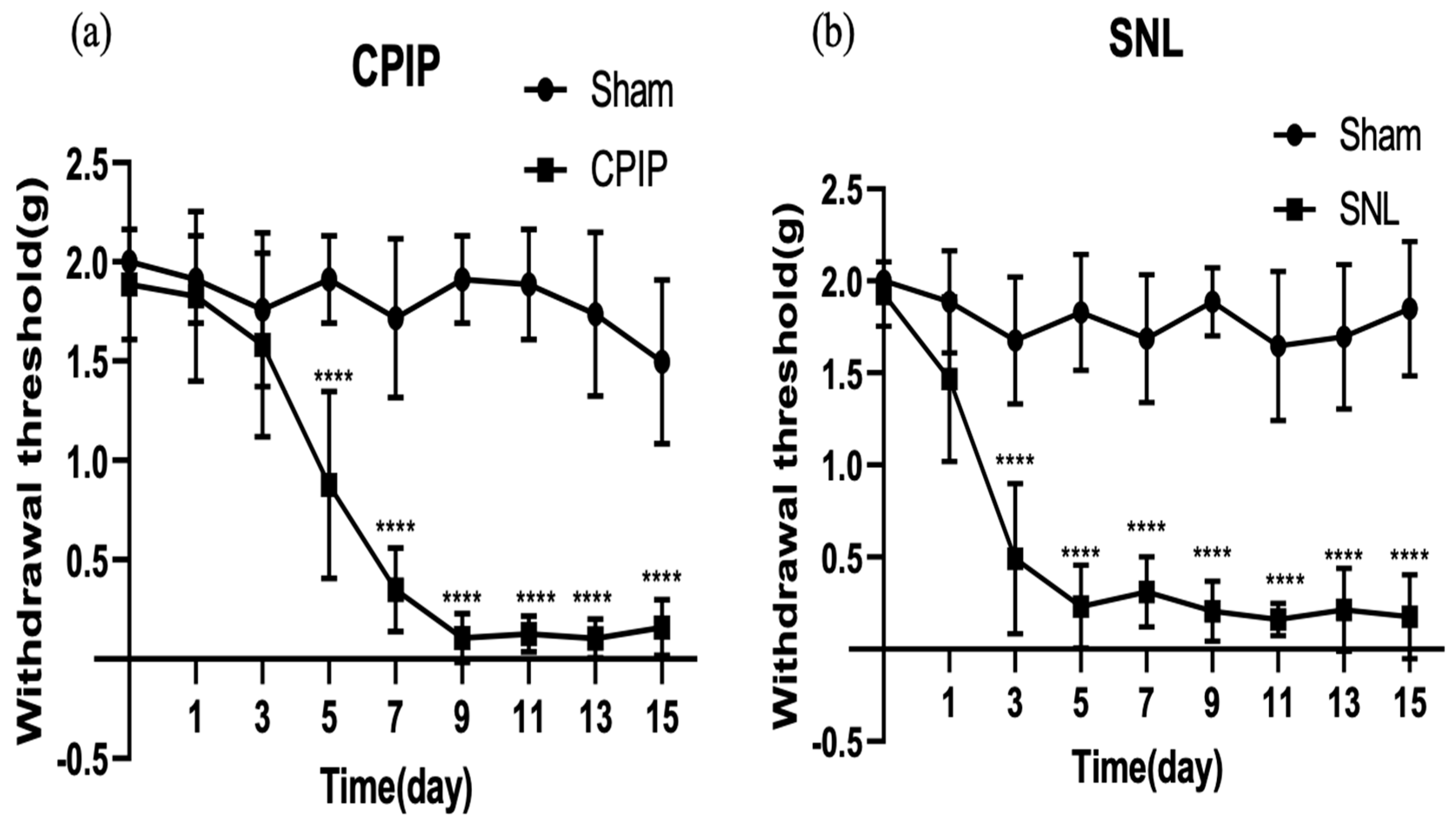
3.1. Mechanical Allodynia in the CPIP and SNL Models
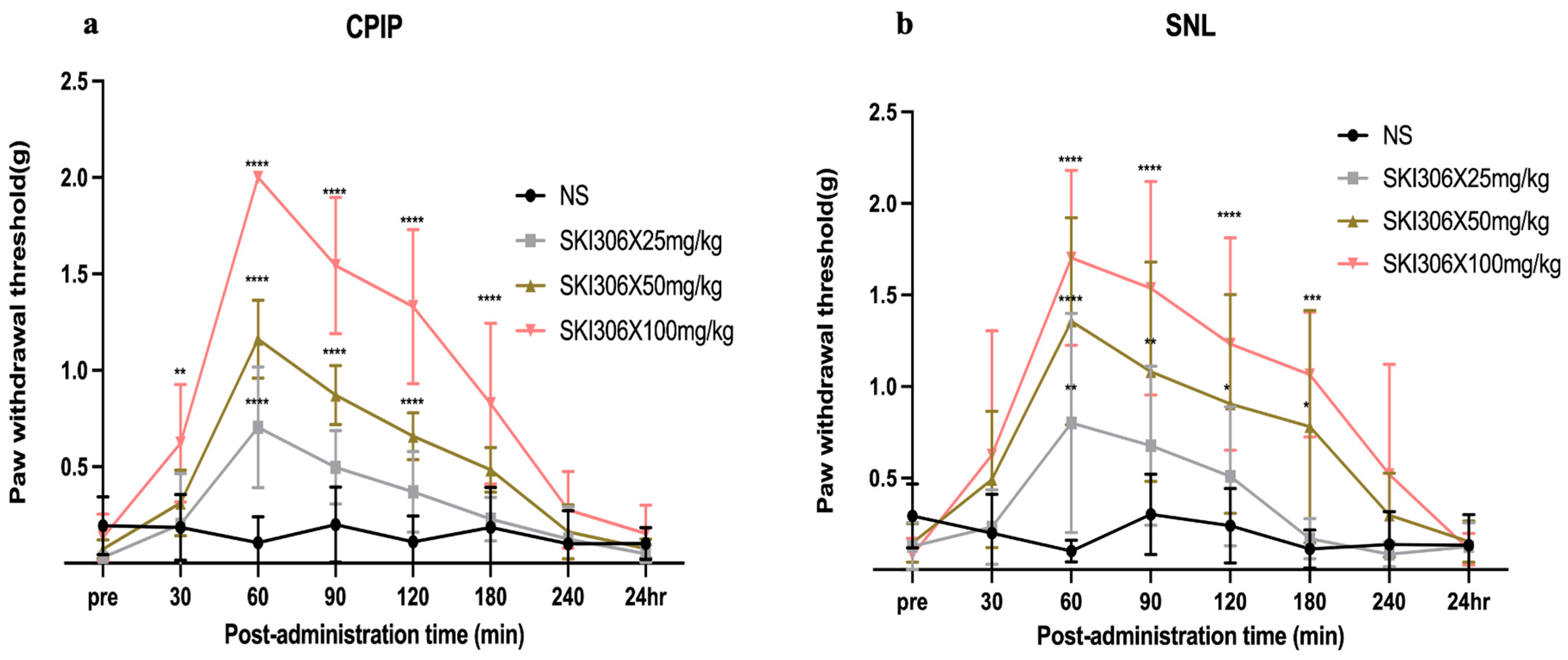
3.2. Ameliorative Effect of SKI306X on Mechanical Allodynia in CPIP and SNL Model Mice
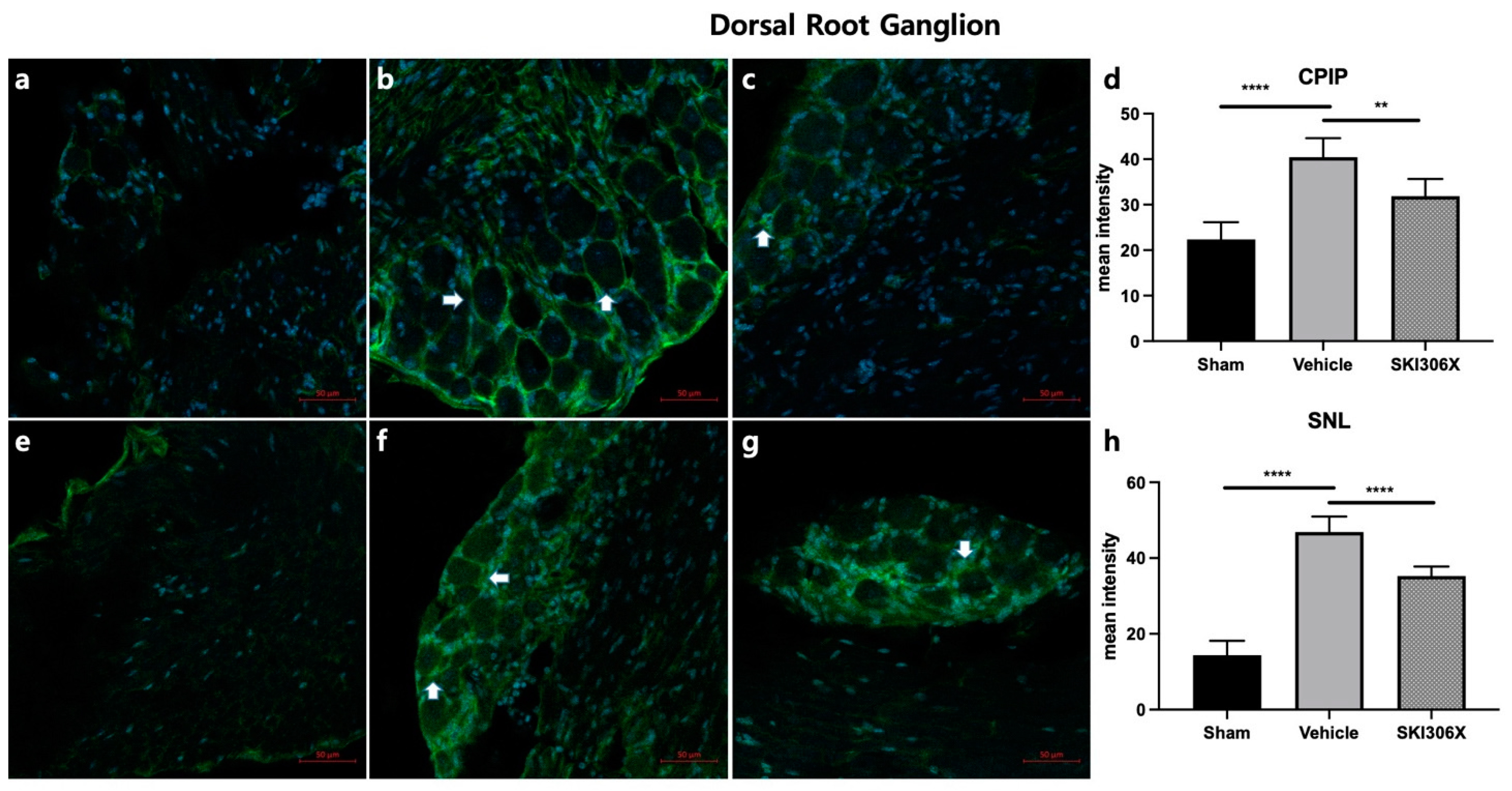
3.3. GFAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, S.H.; Lee, S.H.; Lee, S.; Park, J.H.; Lee, S.; Jin, H.; Park, H.J. The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J. Pain 2020, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Surolia, A. Lysozyme elicits pain during nerve injury by neuronal Toll-like receptor 4 activation and has therapeutic potential in neuropathic Pain. Sci. Transl. Med. 2019, 11, aav4176. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Busse, J.W.; Wang, L.; Kamaleldin, M.; Craigie, S.; Riva, J.J.; Montoya, L.; Mulla, S.M.; Lopes, L.C.; Vogel, N.; Chen, E.; et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 2018, 320, 2448–2460. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, Y.O.; Choi, J.I.; Han, X.H.; Shin, Y.U.; Yoon, M.H. Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic Pain. Korean J. Pain 2022, 35, 59–65. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, J.H.; Kim, D.Y.; Yoon, J.H.; Youn, H.Y.; Yi, J.B.; Rhee, H.I.; Ryu, K.H.; Jung, K.; Han, C.K.; et al. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthr. Cartil. 2002, 10, 471–478. [Google Scholar] [CrossRef]
- Chawla, R.; Kumar, S.; Sharma, A. The genus Clematis (Ranunculaceae): Chemical and pharmacological perspectives. J. Ethnopharmacol. 2012, 143, 116–150. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Al Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K.; Al-Khulaidi, A. Ethnopharmacological Survey of Medicinal Plants in Albaha Region, Saudi Arabia. Pharmacogn. Res. 2017, 9, 401–407. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, K.W.; Kim, B.M.; Won, J.Y.; Min, H.K.; Lee, K.A.; Kim, T.Y.; Lee, S.H. Regulation of Th17 Cytokine-Induced Osteoclastogenesis via SKI306X in Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 1012. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, K.H.; Jung, K.W.; Han, C.K.; Kwak, W.J.; Cho, Y.B. Effects of SKI306X on arachidonate metabolism and other inflammatory mediators. Biol. Pharm. Bull. 2005, 28, 1615–1620. [Google Scholar] [CrossRef][Green Version]
- Fu, Q.; Zan, K.; Zhao, M.; Zhou, S.; Shi, S.; Jiang, Y.; Tu, P. Triterpene saponins from Clematis chinensis and their potential anti-inflammatory activity. J. Nat. Prod. 2010, 73, 1234–1239. [Google Scholar] [CrossRef]
- Guo, L.X.; Wang, H.Y.; Liu, X.D.; Zheng, J.Y.; Tang, Q.; Wang, X.N.; Liu, J.Q.; Yin, H.Q.; Miao, B.; Liang, Y.L.; et al. Saponins from Clematis mandshurica Rupr. regulates gut microbiota and its metabolites during alleviation of collagen-induced arthritis in rats. Pharmacol. Res. 2019, 149, 104459. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J.; Xanthos, D.N.; Francis, L.; Bennett, G.J. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 2004, 112, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.D.; Jung, H.C.; Cheong, Y.K. The effect of intrathecal curcumin on mechanical allodynia in rats after L5 spinal nerve ligation. Korean J. Anesthesiol. 2014, 67, S122–S123. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Nazarinia, M.; Raieskarimian, F. The prevalence and predictors of herbal medicines usage among adult rheumatoid arthritis patients: A case-control study. Complement. Ther. Med. 2018, 41, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Sung, Y.K.; Cho, S.K.; Kim, D.; Won, S.; Choi, C.B.; Bang, S.Y.; Cha, H.S.; Choe, J.Y.; Chung, W.T.; et al. Factors Associated with the Use of Complementary and Alternative Medicine for Korean Patients with Rheumatoid Arthritis. J. Rheumatol. 2015, 42, 2075–2081. [Google Scholar] [CrossRef]
- Taylor, S.S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef]
- Seo, Y.H.; Gil, Y.W.; Choe, G.; Lee, S.H.; Yoo, S.H.; Park, H.J. Comparative Study of Chronic Postischemic Pain Models in Mice: O-Ring Versus Tie Method. Pain Physician 2020, 23, E51–E60. [Google Scholar]
- Hu, Q.; Zheng, X.; Chen, R.; Liu, B.; Tai, Y.; Shao, X.; Fang, J.; Liu, B. Chronic Post-Ischemia Pain Model for Complex Regional Pain Syndrome Type-I in Rats. J. Vis. Exp. 2020, 155, e60562. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, D.N.; Coderre, T.J. Sympathetic vasoconstrictor antagonism and vasodilatation relieve mechanical allodynia in rats with chronic postischemia Pain. J. Pain 2008, 9, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Lee, J.Y.; Choi, H.; Kim, Y.C.; Yang, S.; Jeong, J.; Park, H.J. Effect of Pregabalin Combined with Duloxetine and Tramadol on Allodynia in Chronic Postischemic Pain and Spinal Nerve Ligation Mouse Models. Pharmaceutics 2022, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- LaBuda, C.J.; Little, P.J. Pharmacological evaluation of the selective spinal nerve ligation model of neuropathic pain in the rat. J. Neurosci. Methods 2005, 144, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, H.; Singh, A. Neuropathic Pain models caused by damage to central or peripheral nervous system. Pharmacol. Rep. 2018, 70, 206–216. [Google Scholar] [CrossRef]
- Yang, P.P.; Yeh, G.C.; Huang, E.Y.; Law, P.Y.; Loh, H.H.; Tao, P.L. Effects of dextromethorphan and oxycodone on treatment of neuropathic pain in mice. J. Biomed. Sci. 2015, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Kiso, T.; Watabiki, T.; Tsukamoto, M.; Okabe, M.; Kagami, M.; Nishimura, K.; Aoki, T.; Matsuoka, N. Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience 2008, 153, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, J.D.; Kim, K.H.; Jeong, N.Y. G protein-coupled receptor, family C, group 5 (GPRC5B) downregulation in spinal cord neurons is involved in neuropathic Pain. Korean J. Anesthesiol. 2014, 66, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic Pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- Takeda, K.; Sawamura, S.; Tamai, H.; Sekiyama, H.; Hanaoka, K. Role for cyclooxygenase 2 in the development and maintenance of neuropathic pain and spinal glial activation. Anesthesiology 2005, 103, 837–844. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, T.H.; Sung, Y.K.; Choi, C.B.; Na, Y.I.; Yoo, H.; Jun, J.B. SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes. Korean J. Intern. Med. 2014, 29, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, N.; Zhao, S.; Jiao, T.; Fu, W.; Yang, L.; Zhang, N. miR-410-3p Suppresses Cytokine Release from Fibroblast-Like Synoviocytes by Regulating NF-κB Signaling in Rheumatoid Arthritis. Inflammation 2019, 42, 331–341. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, T.; Fu, W.; Zhao, S.; Yang, L.; Xu, N.; Zhang, N. miR-410-3p regulates proliferation and apoptosis of fibroblast-like synoviocytes by targeting YY1 in rheumatoid arthritis. Biomed. Pharmacother. 2019, 119, 109426. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Sun, Y.; Jiang, H.; Chen, Y.; Jiang, Y.; Han, Y.; Wang, Y. Total Saponins of Radix Clematis Regulate Fibroblast-Like Synoviocyte Proliferation in Rheumatoid Arthritis via the LncRNA OIP5-AS1/MiR-410-3p/Wnt7b Signaling Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 8393949. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, S.H.; Lee, H.J.; Han, D.; Lee, J.; Jeon, S.H.; Cho, E.A.; Park, H.J. Anti-Allodynic Effects of Polydeoxyribonucleotide in an Animal Model of Neuropathic Pain and Complex Regional Pain Syndrome. J. Korean Med. Sci. 2020, 35, e225. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, W. The Role of Satellite Glial Cells, Astrocytes, and Microglia in Oxaliplatin-Induced Neuropathic Pain. Biomedicines 2020, 8, 324. [Google Scholar] [CrossRef]
- Dang, S.J.; Wei, W.B.; Li, R.L.; Song, C.X.; Xu, J. Z-Guggulsterone Relieves Neuropathic Pain by Inhibiting the Expression of Astrocytes and Proinflammatory Cytokines in the Spinal Dorsal Horn. J. Pain Res. 2022, 15, 1315–1324. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, J.; He, C.J.; Kim, J.Y.; Lee, J.Y.; Kim, C.J.; Jeon, Y.J.; Im, C.W.; Lee, D.K.; Kim, J.E.; Park, H.J. Analgesic Effect of SKI306X on Chronic Postischemic Pain and Spinal Nerve Ligation-Induced Neuropathic Pain in Mice. Biomedicines 2024, 12, 1379. https://doi.org/10.3390/biomedicines12071379
Quan J, He CJ, Kim JY, Lee JY, Kim CJ, Jeon YJ, Im CW, Lee DK, Kim JE, Park HJ. Analgesic Effect of SKI306X on Chronic Postischemic Pain and Spinal Nerve Ligation-Induced Neuropathic Pain in Mice. Biomedicines. 2024; 12(7):1379. https://doi.org/10.3390/biomedicines12071379
Chicago/Turabian StyleQuan, Jie, Chun Jing He, Ji Yeon Kim, Jin Young Lee, Chang Jae Kim, Young Jae Jeon, Chang Woo Im, Do Kyung Lee, Ji Eun Kim, and Hue Jung Park. 2024. "Analgesic Effect of SKI306X on Chronic Postischemic Pain and Spinal Nerve Ligation-Induced Neuropathic Pain in Mice" Biomedicines 12, no. 7: 1379. https://doi.org/10.3390/biomedicines12071379
APA StyleQuan, J., He, C. J., Kim, J. Y., Lee, J. Y., Kim, C. J., Jeon, Y. J., Im, C. W., Lee, D. K., Kim, J. E., & Park, H. J. (2024). Analgesic Effect of SKI306X on Chronic Postischemic Pain and Spinal Nerve Ligation-Induced Neuropathic Pain in Mice. Biomedicines, 12(7), 1379. https://doi.org/10.3390/biomedicines12071379




