Gender-Related Difference in Skin Oxygenation in Young Patients with Uncomplicated Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. Evaluation of Microcirculation
2.2.1. Capillaroscopy Examination
2.2.2. Transcutaneous Oxygen Pressure Measurement
2.3. Laboratory Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, B.I.; Ambrosio, G.; Pries, A.R.; Struijker-Boudier, H.A.J. Microcirculation in Hypertension: A New Target for Treatment? Circulation 2001, 104, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Sun, D.; Koller, A.; Kaley, G. Gender Difference in Myogenic Tone of Rat Arterioles Is Due to Estrogen-Induced, Enhanced Release of NO. Am. J. Physiol. Circ. Physiol. 1997, 272, H1804–H1809. [Google Scholar] [CrossRef] [PubMed]
- Walli-Attaei, M.; Joseph, P.; Rosengren, A.; Chow, C.K.; Rangarajan, S.; Lear, S.A.; AlHabib, K.F.; Davletov, K.; Dans, A.; Lanas, F.; et al. Variations between Women and Men in Risk Factors, Treatments, Cardiovascular Disease Incidence, and Death in 27 High-Income, Middle-Income, and Low-Income Countries (PURE): A Prospective Cohort Study. Lancet 2020, 396, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular Health after Menopause Transition, Pregnancy Disorders, and Other Gynaecologic Conditions: A Consensus Document from European Cardiologists, Gynaecologists, and Endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M.C. Gender Differences in the Cardiovascular Effect of Sex Hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Santos, R.S.; Criollo, A.; Nelson, M.D.; Palmer, B.F.; Clegg, D.J. The Effects of Oestrogens and Their Receptors on Cardiometabolic Health. Nat. Rev. Endocrinol. 2017, 13, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef]
- Hsu, S.-P.; Lee, W.-S. Effects of Female Sex Hormones on the Development of Atherosclerosis. Chin. J. Physiol. 2020, 63, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sairam, M.R. Sex Hormone Imbalances and Adipose Tissue Dysfunction Impacting on Metabolic Syndrome; a Paradigm for the Discovery of Novel Adipokines. Horm. Mol. Biol. Clin. Investig. 2014, 17, 89–97. [Google Scholar] [CrossRef]
- Cifkova, R.; Pitha, J.; Krajcoviechova, A.; Kralikova, E. Is the Impact of Conventional Risk Factors the Same in Men and Women? Plea for a More Gender-Specific Approach. Int. J. Cardiol. 2019, 286, 214–219. [Google Scholar] [CrossRef]
- Huxley, R.R.; Peters, S.A.E.; Mishra, G.D.; Woodward, M. Risk of All-Cause Mortality and Vascular Events in Women versus Men with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.; Castro, A.; Merino, P.M.; López, P.; Lardone, M.C.; Iñiguez, G.; Cassorla, F.; Codner, E. Oestrogen Activity of the Serum in Adolescents with Type 1 Diabetes. Diabet. Med. 2016, 33, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Smigoc Schweiger, D.; Battelino, T.; Groselj, U. Sex-Related Differences in Cardiovascular Disease Risk Profile in Children and Adolescents with Type 1 Diabetes. Int. J. Mol. Sci. 2021, 22, 10192. [Google Scholar] [CrossRef] [PubMed]
- Szadkowska, A.; Madej, A.; Ziółkowska, K.; Szymańska, M.; Jeziorny, K.; Mianowska, B.; Pietrzak, I. Gender and Age—Dependent Effect of Type 1 Diabetes on Obesity and Altered Body Composition in Young Adults. Ann. Agric. Environ. Med. 2015, 22, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N. Sex-specific Factors Regulating Pressure and Flow. Exp. Physiol. 2017, 102, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R.; Sabharwal, S.C.; Desai, N. Sex Differences in Peripheral Vascular Adrenergic Receptors. Circ. Res. 1987, 61, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.C.; Charkoudian, N.; Wallin, B.G.; Curry, T.B.; Eisenach, J.; Joyner, M.J. Sex and Ageing Differences in Resting Arterial Pressure Regulation: The Role of the Β-adrenergic Receptors. J. Physiol. 2011, 589, 5285–5297. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Moore, S.M.; Lucitti, J.L.; Aghajanian, A.; Zhang, H. Sex Differences in the Cerebral Collateral Circulation. Transl. Stroke Res. 2017, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Rodriguez-Miguelez, P.; Zimmerman, M.A.; Harris, R.A. Differences in Angiotensin (1–7) between Men and Women. Am. J. Physiol. Circ. Physiol. 2015, 308, H1171–H1176. [Google Scholar] [CrossRef]
- Arnetz, L.; Rajamand Ekberg, N.; Alvarsson, M. Sex Differences in Type 2 Diabetes: Focus on Disease Course and Outcomes. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 409. [Google Scholar] [CrossRef]
- Hendriks-Balk, M.; Damianaki, A.; Theiler, K.; Polychronopoulou, E.; Brito, W.; Pruijm, M.; Wuerzner, G. Sex Differences in Renal Microcirculation of Hypertensive Patients. J. Hypertens. 2023, 41, e262. [Google Scholar] [CrossRef]
- Gillis, E.E.; Sasser, J.M.; Sullivan, J.C. Endothelin, Sex, and Pregnancy: Unique Considerations for Blood Pressure Control in Females. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R691–R696. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Rosengren, A.; Ekman, I. Symptoms in Acute Coronary Syndromes: Does Sex Make a Difference? Am. Heart J. 2004, 148, 27–33. [Google Scholar] [CrossRef] [PubMed]
- El-Awaisi, J.; Mitchell, J.L.; Ranasinghe, A.; Kalia, N. Interleukin-36 Is Vasculoprotective in Both Sexes despite Sex-Specific Changes in the Coronary Microcirculation Response to IR Injury. Front. Cardiovasc. Med. 2023, 10, 1227499. [Google Scholar] [CrossRef] [PubMed]
- Neubauer-Geryk, J.; Hoffmann, M.; Wielicka, M.; Piec, K.; Kozera, G.; Brzeziński, M.; Bieniaszewski, L. Current Methods for the Assessment of Skin Microcirculation: Part 1. Adv. Dermatol. Allergol. 2019, 36, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Neubauer-Geryk, J.; Hoffmann, M.; Wielicka, M.; Piec, K.; Kozera, G.; Bieniaszewski, L. Current Methods for the Assessment of Skin Microcirculation: Part 2. Adv. Dermatol. Allergol. 2019, 36, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.; Roustit, M. Current Methods to Assess Human Cutaneous Blood Flow: An Updated Focus on Laser-Based-Techniques. Microcirculation 2016, 23, 337–344. [Google Scholar] [CrossRef]
- Jörneskog, G.; Brismar, K.; Fagrell, B. Skin Capillary Circulation Severely Impaired in Toes of Patients with IDDM, with and without Late Diabetic Complications. Diabetologia 1995, 38, 474–480. [Google Scholar] [CrossRef]
- Tibiriçá, E.; Rodrigues, E.; Cobas, R.A.; Gomes, M.B. Endothelial Function in Patients with Type 1 Diabetes Evaluated by Skin Capillary Recruitment. Microvasc. Res. 2007, 73, 107–112. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Kozera, G.M.; Wolnik, B.; Szczyrba, S.; Nyka, W.M.; Bieniaszewski, L. Decreased Reactivity of Skin Microcirculation in Response to L-Arginine in Later-Onset Type 1 Diabetes. Diabetes Care 2013, 36, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Berger, W. Nailfold Videomicroscopy and Local Cold Test in Type I Diabetics. Angiology 1992, 43, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kuryliszyn-Moskal, A.; Dubicki, A.; Zarzycki, W.; Zonnenberg, A.; Górska, M. Microvascular Abnormalities in Capillaroscopy Correlate with Higher Serum IL-18 and SE-Selectin Levels in Patients with Type 1 Diabetes Complicated by Microangiopathy. Folia Histochem. Cytobiol. 2011, 49, 104–110. [Google Scholar] [CrossRef]
- Hoffmann, M.; Neubauer-Geryk, J.; Wielicka, M.; Kowaleczko, M.; Myśliwiec, M.; Bieniaszewski, L. The Impact of Autoimmune Thyroiditis on Skin Microcirculation in Children with Non-Complicated Type 1 Diabetes Mellitus. Microvasc. Res. 2019, 123, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J. Microvascular Haemodynamics in Diabetes Mellitus. Clin. Sci. 1986, 70, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E.; Lins, P.E.; Ostergren, J.; Fagrell, B. Skin Microvascular Autoregulatory Responses in Type I Diabetes: The Influence of Duration and Control. Int. J. Microcirc. Clin. Exp. 1985, 4, 249–256. [Google Scholar]
- Neubauer-Geryk, J.; Wielicka, M.; Hoffmann, M.; Myśliwiec, M.; Bieniaszewski, L. The Impact of Disease Duration on Microcirculatory Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines 2024, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Anyfanti, P.; Triantafyllou, G.; Zabulis, X.; Aslanidis, S.; Douma, S. Impaired Metabolic Profile Is a Predictor of Capillary Rarefaction in a Population of Hypertensive and Normotensive Individuals. J. Am. Soc. Hypertens. 2016, 10, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Riccieri, V.; Vasile, M.; Stefanantoni, K.; Comberiati, P.; Taverniti, L.; Cavallo, M.G. High Prevalence of Capillary Abnormalities in Patients with Diabetes and Association with Retinopathy. Diabet. Med. 2011, 28, 1039–1044. [Google Scholar] [CrossRef]
- Fahrig, C.; Breitinger, L.; Heidrich, H. Vital Capillary Microscopic Findings in the Nailfold of Patients with Diabetes Mellitus. VASA 2000, 29, 258–263. [Google Scholar] [CrossRef]
- Buss, C.; Maranhão, P.A.; de Souza, M.; Bouskela, E.; Kraemer-Aguiar, L.G. Obesity Blunts Cephalic-Phase Microvascular Responses to Food. Physiol. Behav. 2020, 225, 113087. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M.; Bergman, T.; O’Donnell, G.; Drobny, E.; Borgos, J.; Bonner, R.F. Microvascular Blood Flow, Volume, and Velocity Measured by Laser Doppler Techniques in IDDM. Diabetes 1989, 38, 819–824. [Google Scholar] [CrossRef]
- Hauser, C.J.; Klein, S.R.; Mehringer, C.M.; Appel, P.; Shoemaker, W.C. Assessment of Perfusion in the Diabetic Foot by Regional Transcutaneous Oximetry. Diabetes 1984, 33, 527–531. [Google Scholar] [CrossRef]
- Bauersachs, R.; Shaw, S.; Zeidler, A.; Meiselman, H. Red Blood Cell Aggregation and Blood Viscoelasticity in Poorly Controlled Type 2 Diabetes Mellitus. Clin. Hemorheol. Microcirc. 1989, 9, 935–952. [Google Scholar] [CrossRef]
- Rendell, M.; Fox, M.; Knox, S.; Lastovica, J.; Kirchain, W.; Meiselman, H.J. Effects of Glycemic Control on Red Cell Deformability Determined by Using the Cell Transit Time Analyzer. J. Lab. Clin. Med. 1991, 117, 500–504. [Google Scholar] [PubMed]
- Boulton, A.J.M.; Scarpello, J.H.B.; Ward, J.D. Venous Oxygenation in the Diabetic Neuropathic Foot: Evidence of Arteriovenous Shunting? Diabetologia 1982, 22, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, R.; Küper, M.; Königsrainer, I.; Löb, S.; Wichmann, D.; Königsrainer, A.; Coerper, S.; Beckert, S. Predictive Value of Routine Transcutaneous Tissue Oxygen Tension (TcpO2) Measurement for the Risk of Non-Healing and Amputation in Diabetic Foot Ulcer Patients with Non-Palpable Pedal Pulses. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, CR273–CR277. [Google Scholar]
- Koch, C.; Chauve, E.; Chaudru, S.; Le Faucheur, A.; Jaquinandi, V.; Mahé, G. Exercise Transcutaneous Oxygen Pressure Measurement Has Good Sensitivity and Specificity to Detect Lower Extremity Arterial Stenosis Assessed by Computed Tomography Angiography. Medicine 2016, 95, 4522. [Google Scholar] [CrossRef]
- Nishio, H.; Minakata, K.; Kawaguchi, A.; Kumagai, M.; Ikeda, T.; Shimizu, A.; Yokode, M.; Morita, S.; Sakata, R. Transcutaneous Oxygen Pressure as a Surrogate Index of Lower Limb Amputation. Int. Angiol. 2016, 35, 565–572. [Google Scholar]
- Moosa, H.H.; Peitzman, A.B.; Makaroun, M.S.; Webster, M.W.; Steed, D.L. Transcutaneous Oxygen Measurements in Lower Extremity Ischemia: Effects of Position, Oxygen Inhalation, and Arterial Reconstruction. Surgery 1988, 103, 193–198. [Google Scholar]
- Papa, G.; Spazzapan, L.; Pangos, M.; Delpin, A.; Arnež, Z.M. Compared to Coverage by STSG Grafts Only Reconstruction by the Dermal Substitute Integra® plus STSG Increases TcPO2 Values in Diabetic Feet at 3 and 6 Months after Reconstruction. J. Ital. Assoc. Hosp. Surg. 2014, 35, 141–145. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Wielicka, M.; Kozera, G.M.; Brandt-Varma, A.; Wołoszyn-Durkiewicz, A.; Myśliwiec, M.; Bieniaszewski, L. Skin Oxygenation Impairment Is Associated with Increased Total Cholesterol Level in Children with Short-Lasting Type 1 Diabetes Mellitus. Adv. Dermatol. Allergol. 2021, 38, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Neubauer-Geryk, J.; Wielicka, M.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Relationship between TNF-a, IL-35, VEGF and Cutaneous Microvascular Dysfunction in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines 2023, 11, 2857. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. Clinical Practice Consensus Guidelines 2022: Definition, Epidemiology, and Classification of Diabetes in Children and Adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Dyck, P.J. Detection, Characterization, and Staging of Polyneuropathy: Assessed in Diabetics. Muscle Nerve 1988, 11, 21–32. [Google Scholar] [CrossRef]
- Falkner, B.; Gidding, S.S.; Baker-Smith, C.M.; Brady, T.M.; Flynn, J.T.; Malle, L.M.; South, A.M.; Tran, A.H.; Urbina, E.M. Pediatric Primary Hypertension: An Underrecognized Condition: A Scientific Statement from the American Heart Association. Hypertension 2023, 80, e101–e111. [Google Scholar] [CrossRef]
- Leenstra, B.; Wijnand, J.; Verhoeven, B.; Koning, O.; Teraa, M.; Verhaar, M.C.; de Borst, G.J. Applicability of Transcutaneous Oxygen Tension Measurement in the Assessment of Chronic Limb-Threatening Ischemia. Angiology 2020, 71, 208–216. [Google Scholar] [CrossRef]
- Fife, C.E.; Smart, D.R.; Sheffield, P.J.; Hopf, H.W.; Hawkins, G.; Clarke, D. Transcutaneous Oximetry in Clinical Practice: Consensus Statements from an Expert Panel Based on Evidence. Undersea Hyperb. Med. 2009, 36, 43–53. [Google Scholar] [PubMed]
- Chambliss, K.L.; Shaul, P.W. Estrogen Modulation of Endothelial Nitric Oxide Synthase. Endocr. Rev. 2002, 23, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Slotta, J.E.; Garcia, P.; Seekamp, A.; Menger, M.D.; Pohlemann, T. The Effect of Estrogen on Hepatic Microcirculation after Ischemia/Reperfusion. Int. J. Color. Dis. 2007, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Campisi, R.; Nathan, L.; Pampaloni, M.H.; Schöder, H.; Sayre, J.W.; Chaudhuri, G.; Schelbert, H.R. Noninvasive Assessment of Coronary Microcirculatory Function in Postmenopausal Women and Effects of Short-Term and Long-Term Estrogen Administration. Circulation 2002, 105, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Worboys, S.; Kotsopoulos, D.; Teede, H.; McGrath, B.; Davis, S.R. Evidence That Parenteral Testosterone Therapy May Improve Endothelium-Dependent and -Independent Vasodilation in Postmenopausal Women Already Receiving Estrogen. J. Clin. Endocrinol. Metab. 2001, 86, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.-L.; Sun, C.-W. Gender-Related Effect in Oxygenation Dynamics by Using Far-Infrared Intervention with Near-Infrared Spectroscopy Measurement: A Gender Differences Controlled Trial. PLoS ONE 2015, 10, e0135166. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender Differences in the Regulation of Blood Pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Grau, M.; Cremer, J.M.; Schmeichel, S.; Kunkel, M.; Bloch, W. Comparisons of Blood Parameters, Red Blood Cell Deformability and Circulating Nitric Oxide between Males and Females Considering Hormonal Contraception: A Longitudinal Gender Study. Front. Physiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- White, R.E. Estrogen and Vascular Function. Vascul. Pharmacol. 2002, 38, 73–80. [Google Scholar] [CrossRef]
- Charkoudian, N.; Hart, E.C.J.; Barnes, J.N.; Joyner, M.J. Autonomic Control of Body Temperature and Blood Pressure: Influences of Female Sex Hormones. Clin. Auton. Res. 2017, 27, 149–155. [Google Scholar] [CrossRef]
- Andrade, L.E.C.; Gabriel, A.; Assad, R.L.; Ferrari, A.J.L.; Atra, E. Panoramic Nailfold Capillaroscopy: A New Reading Method and Normal Range. Semin. Arthritis Rheum. 1990, 20, 21–31. [Google Scholar] [CrossRef]
- Terreri, M.T.R.A.; Andrade, L.E.C.; Puccinelli, M.L.; Hilário, M.O.E.; Goldenberg, J. Nail Fold Capillaroscopy: Normal Findings in Childrenand Adolescents. Semin. Arthritis Rheum. 1999, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bogusz-Górna, K.; Polańska, A.; Dańczak-Pazdrowska, A.; Żaba, R.; Sumińska, M.; Fichna, P.; Kędzia, A. Non-Invasive Detection of Early Microvascular Changes in Juveniles with Type 1 Diabetes. Cardiovasc. Diabetol. 2023, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, J.; Hegyi, V.; Messer, G.; Arenberger, P.; Ruzicka, T.; Berking, C. Confocal Laser-scanning Capillaroscopy: A Novel Approach to the Analysis of Skin Capillaries In Vivo. Ski. Res. Technol. 2009, 15, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Heimhalt-El Hamriti, M.; Schreiver, C.; Noerenberg, A.; Scheffler, J.; Jacoby, U.; Haffner, D.; Fischer, D.-C. Impaired Skin Microcirculation in Paediatric Patients with Type 1 Diabetes Mellitus. Cardiovasc. Diabetol. 2013, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Stupin, A.; Stupin, M.; Baric, L.; Matic, A.; Kolar, L.; Drenjancevic, I. Sex-Related Differences in Forearm Skin Microvascular Reactivity of Young Healthy Subjects. Clin. Hemorheol. Microcirc. 2019, 72, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, A.; Mazkereth, R.; Tsur, H. Mapping of the Human Body Skin with the Transcutaneous Oxygen Pressure Method. Ann. Plast. Surg. 1988, 20, 419–425. [Google Scholar] [CrossRef]
- Dooley, J.; King, G.; Slade, B. Establishment of Reference Pressure of Transcutaneous Oxygen for the Comparative Evaluation of Problem Wounds. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. 1997, 24, 235–244. [Google Scholar]
- Rodrigues, L.M.; Contreiras Pinto, P.; Leal, A. Transcutaneous Flow Related Variables Measured In Vivo: The Effects of Gender. BMC Dermatol. 2001, 1, 4. [Google Scholar] [CrossRef]
- Dowd, G.S.E.; Linge, K.; Bentley, G. The Effect of Age and Sex of Normal Volunteers upon the Transcutaneous Oxygen Tension in the Lower Limb. Clin. Phys. Physiol. Meas. 1983, 4, 65–68. [Google Scholar] [CrossRef]
- Rossi, M.; Pistelli, F.; Pesce, M.; Aquilini, F.; Franzoni, F.; Santoro, G.; Carrozzi, L. Impact of Long-Term Exposure to Cigarette Smoking on Skin Microvascular Function. Microvasc. Res. 2014, 93, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lenasi, H.; Štrucl, M. Regular Physical Activity Alters the Postocclusive Reactive Hyperemia of the Cutaneous Microcirculation. Clin. Hemorheol. Microcirc. 2010, 45, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lan, C.; Chen, S.; Wong, M. Tai Chi Chuan Training Is Associated with Enhanced Endothelium-Dependent Dilation in Skin Vasculature of Healthy Older Men. J. Am. Geriatr. Soc. 2002, 50, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
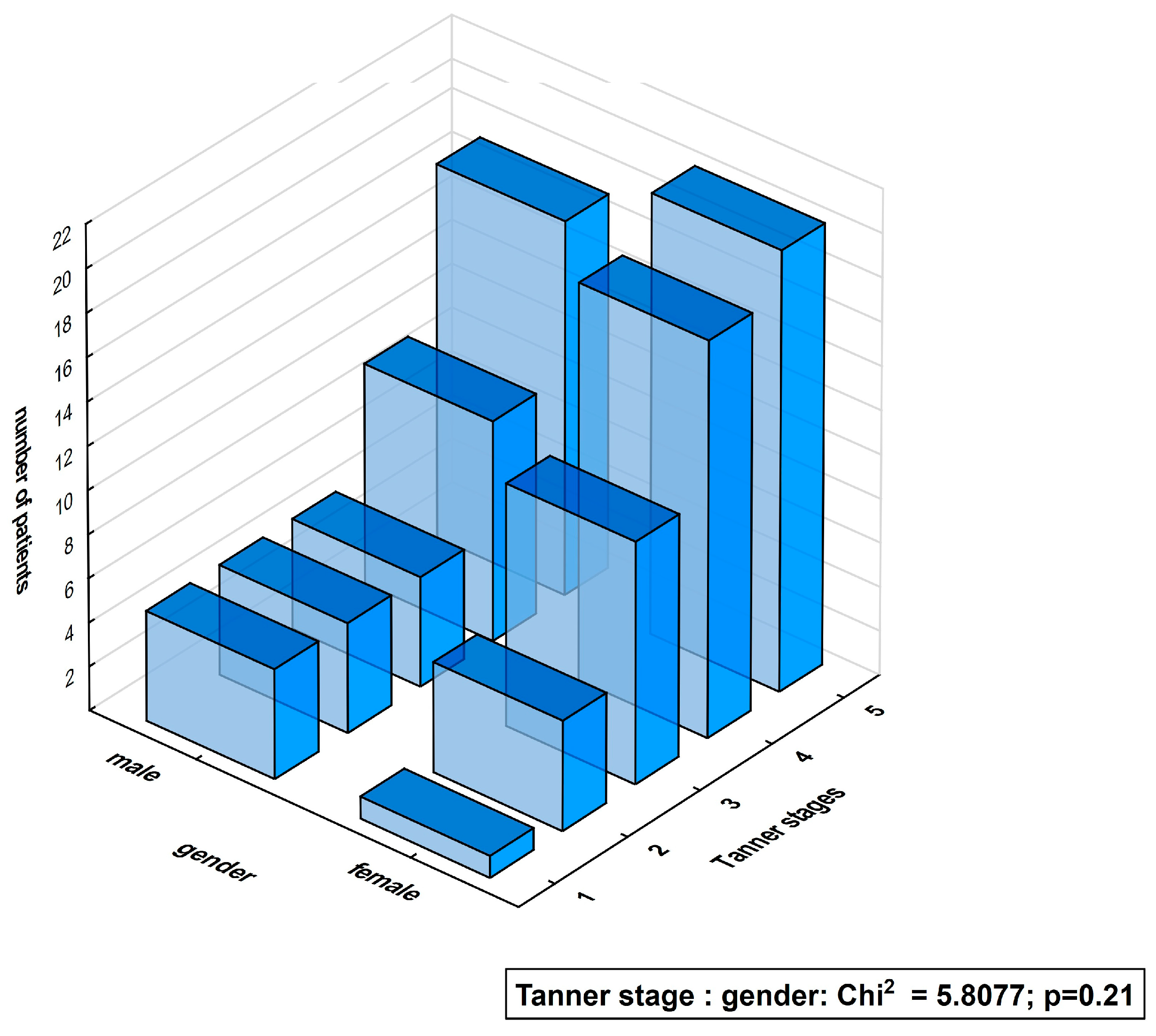
| Characteristics | Diabetic Patients’ Subgroups According to Gender | ||
|---|---|---|---|
| Females (F), n= 55 | p for between-Group Comparison | Males (M), n = 42 | |
| Age [years] | 14.9 (11.5–19.1) 15.2 ± 2.1 | 0.83 | 15.4 (8.4–20.1) 15.2 ± 2.6 |
| Onset of diabetes [age] | 9.4 (2.1–14.9) 9.3 ± 3.4 | 0.92 | 10.2 (1.2–15.0) 9.2 ± 4 |
| T1D duration [years] | 5.5 (0.6–13.1) 5.9 ± 3.4 | 0.96 | 4.8 (0.3–14.5) 6.1 ± 4 |
| Body mass [kg] | 56.8 (37.8–95.7) | 0.13 | 62.2 (28.8–100) |
| BMI [kg/m2] | 20.5 (15.7–29.7) | 0.29 | 20.3 (14.5–28.3) |
| Insulin dose [units/24 h] | 45 (10.3–75) | 0.52 | 45 (1.5–100) |
| Time of pump treatment as ratio to T1D duration [%] | 50 (0–100) | 0.85 | 36 (0–100) |
| HbA1c [%] | 7.8 (5.3–13.6) | 0.93 | 7.6 (5.5–11.3) |
| Episodes of mild hypoglycemia [N/last month] | 6 (0–20) | 0.92 | 5.5 (0–30) |
| Episodes of severe hypoglycemia [N/last year] | 0 (0–2) | 0.57 | 0 (0–3) |
| Systolic blood pressure [mmHg] | 104 (84–125) | 0.01 | 109 (91–138) |
| Diastolic blood pressure [mmHg] | 60 (49–76) | 0.65 | 61 (49–72) |
| Heart rate [beats/min.] | 83 (66–110) | 0.001 | 76 (57–114) |
| Pulse pressure [mmHg] | 44 (27–60) | 0.009 | 49.3 (33.2–69.6) |
| Characteristics | Diabetic Patients’ Subgroups According to Gender | ||
|---|---|---|---|
| Females (F), n = 55 | p for between-Group Comparison | Males (M), n = 42 | |
| Total cholesterol [mg/dL] | 179 (114–288) | 0.35 | 173 (119–246) |
| Cholesterol LDL [mg/dL] | 102 (57–188) | 0.66 | 100 (61–164) |
| Cholesterol HDL [mg/dL] | 57 (33–120) | 0.22 | 54 (35–90) |
| Triglycerides [mg/dL] | 69 (34–294) | 0.76 | 75 (38–154) |
| TSH [mIU/L] | 1.6 (0.6–4.2) | 0.18 | 1.9 (0.6–5.1) |
| fT4 [pmol/L] | 12.4 (9.3–14.5) | 0.28 | 12.8 (9.0–18.1) |
| Serum creatinine [mg/dL] | 0.7 (0.5–0.9) | 0.002 | 0.8 (0.5–1.2) |
| Hemoglobin [g/dL] | 13.4 (10.9–14.5) | <0.001 | 14.5 (12.9–17.2) |
| Characteristics | Diabetic Patients’ Subgroups According to Gender | ||
|---|---|---|---|
| Females (F), n = 55 | p for between-Group Comparison | Males (M), n = 42 | |
| Capillaroscopy | |||
| CoverageBASE [%] | 17.4 (12.7–23.6) | 0.50 | 17.4 (11.5–24.8) |
| CoveragePORH [%] | 16.2 (10.4–24.3) | 0.45 | 15.9 (9.8–24.4) |
| ∆CoveragePB [%] | −0.7 (–8–4.9) | 1 | −1.0 (–5.8–3.3) |
| Capillary reactivity | −4.8 (–41–31) | 0.99 | −6.0 (–35.1–19.5) |
| Coverage_ratio [%] | 95 (59–131) | 0.99 | 94 (65–119) |
| DistanceBASE [µm] | 224.1 (165.8–307.3) | 0.71 | 225.5 (179.4–377.7) |
| DistancePORH [µm] | 225.5 (166.3–334.2) | 0.48 | 234.8 (180.5–345.1) |
| ∆DistancePB [µm] | 6.91 (−63.3–105.8) | 0.97 | 10.4 (−70.4–90.5) |
| Transcutaneous oxygen pressure | |||
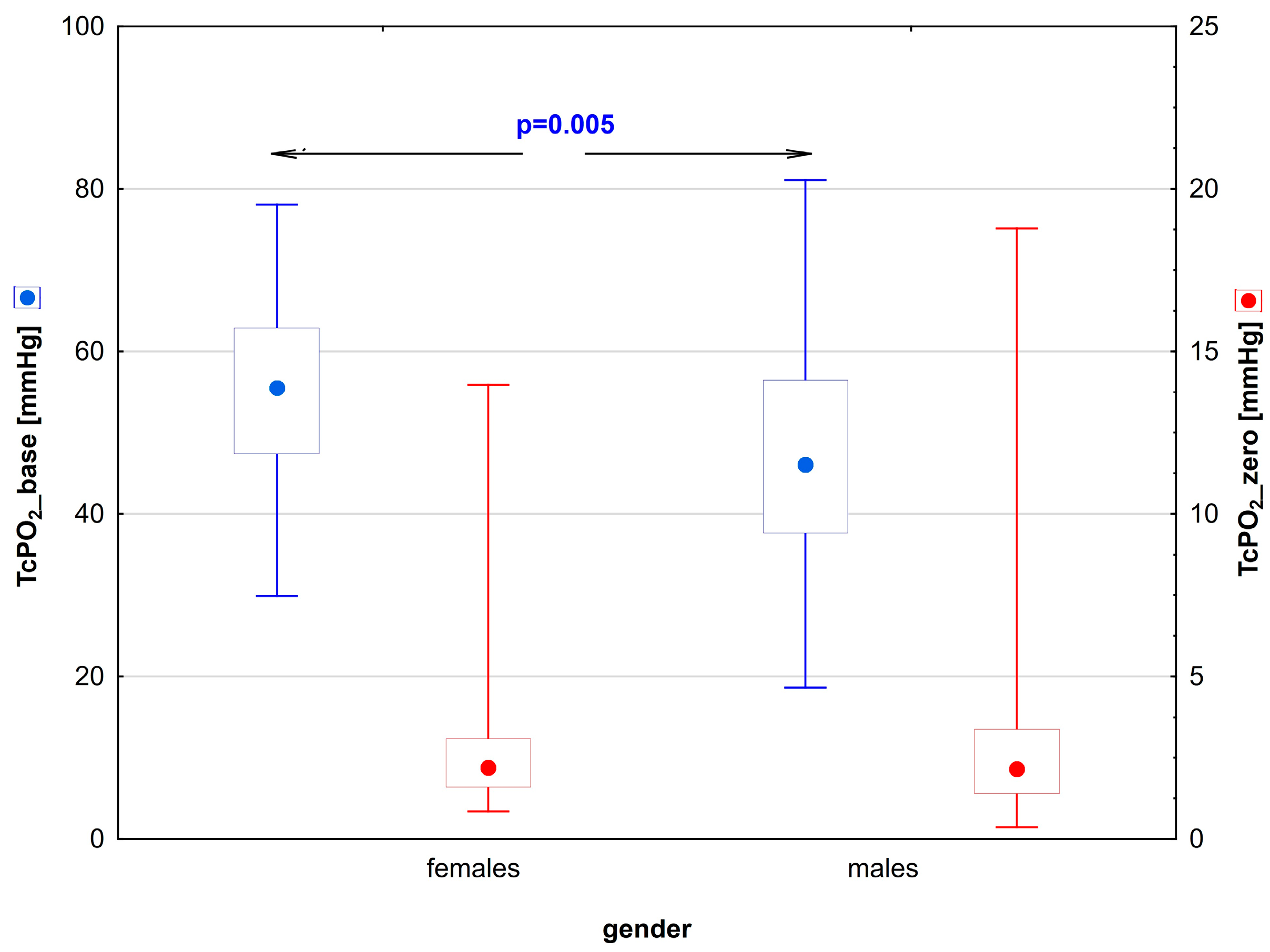
| TcPO2_base [mmHg] | 56 (29.9–78.1) | 0.005 | 46.5 (27.6–80.8) |
| TcPO2_zero [mmHg] | 2.4 (0.9–13.7) | 0.81 | 2.8 (0.7–18.8) |
| TTR [s] | 85 (28–240) | 0.85 | 80.5 (32–240) |
| Parameter | Females | Males | ||
|---|---|---|---|---|
| r | p | r | p | |
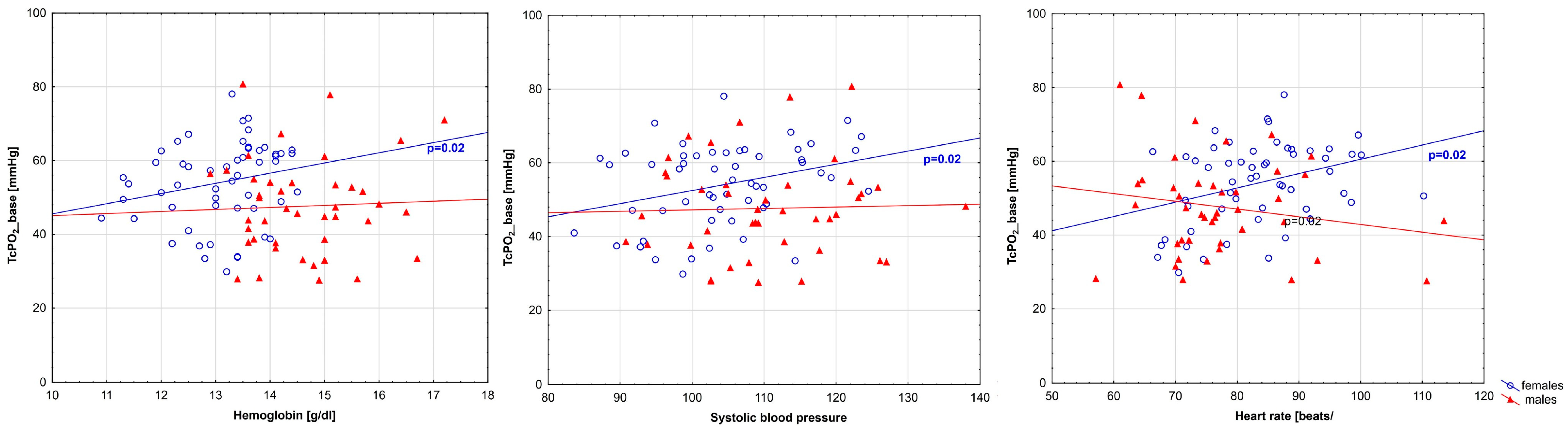
| Hemoglobin | 0.30 | 0.02 | −0.01 | 0.95 |
| Serum creatinine | −0.20 | 0.14 | −0.02 | 0.89 |
| Systolic blood pressure | 0.30 | 0.02 | −0.00 | 1.00 |
| Heart rate | 0.32 | 0.02 | −0.11 | 0.48 |
| Pulse pressure | 0.21 | 0.12 | −0.04 | 0.79 |
| Parameter | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic Blood Pressure | Heart Rate | Pulse Pressure | Systolic Blood Pressure | Heart Rate | Pulse Pressure | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Hemoglobin | 0.07 | 0.59 | 0.26 | 0.06 | −0.20 | 0.15 | 0.37 | 0.02 | −0.30 | 0.06 | 0.25 | 0.1 |
| Systolic blood pressure | 0.41 | 0.002 | 0.77 | <0.001 | −0.20 | 0.21 | 0.84 | <0.001 | ||||
| Heart rate | −0.01 | 0.96 | −0.44 | 0.004 | ||||||||
| Characteristics | Diabetic Patients’ Subgroups According to Gender | p for between-Group Comparison | ||
|---|---|---|---|---|
| Females, n = 55 | Males, n = 42 | |||
| TcPO2_base [mmHg] | 56 (29.9–78.1) | 46.5 (27.6–80.8) | 0.017 | |
| After adjustment for hemoglobin | 0.01 | |||
| Systolic blood pressure | 0.003 | |||
| Heart rate | 0.02 | |||
| Hemoglobin and systolic blood pressure | 0.004 | |||
| Hemoglobin and heart rate | 0.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer-Geryk, J.; Myśliwiec, M.; Bieniaszewski, L. Gender-Related Difference in Skin Oxygenation in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines 2024, 12, 1413. https://doi.org/10.3390/biomedicines12071413
Neubauer-Geryk J, Myśliwiec M, Bieniaszewski L. Gender-Related Difference in Skin Oxygenation in Young Patients with Uncomplicated Type 1 Diabetes. Biomedicines. 2024; 12(7):1413. https://doi.org/10.3390/biomedicines12071413
Chicago/Turabian StyleNeubauer-Geryk, Jolanta, Małgorzata Myśliwiec, and Leszek Bieniaszewski. 2024. "Gender-Related Difference in Skin Oxygenation in Young Patients with Uncomplicated Type 1 Diabetes" Biomedicines 12, no. 7: 1413. https://doi.org/10.3390/biomedicines12071413





