The Re-Emergence of Mpox: Old Illness, Modern Challenges
Abstract
:1. Introduction
2. Outbreaks
3. Origin and Emergence
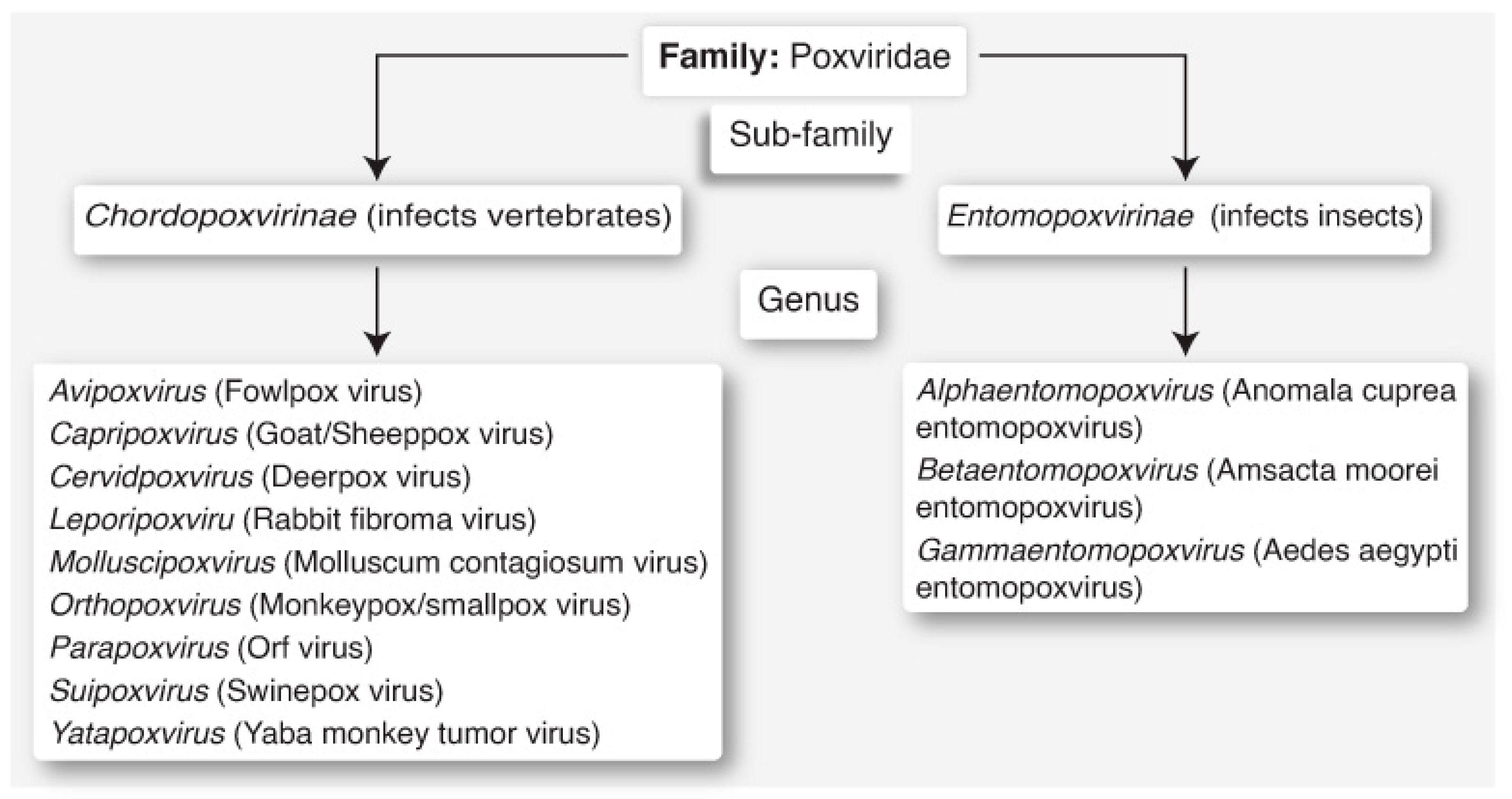
4. Classification and Clades
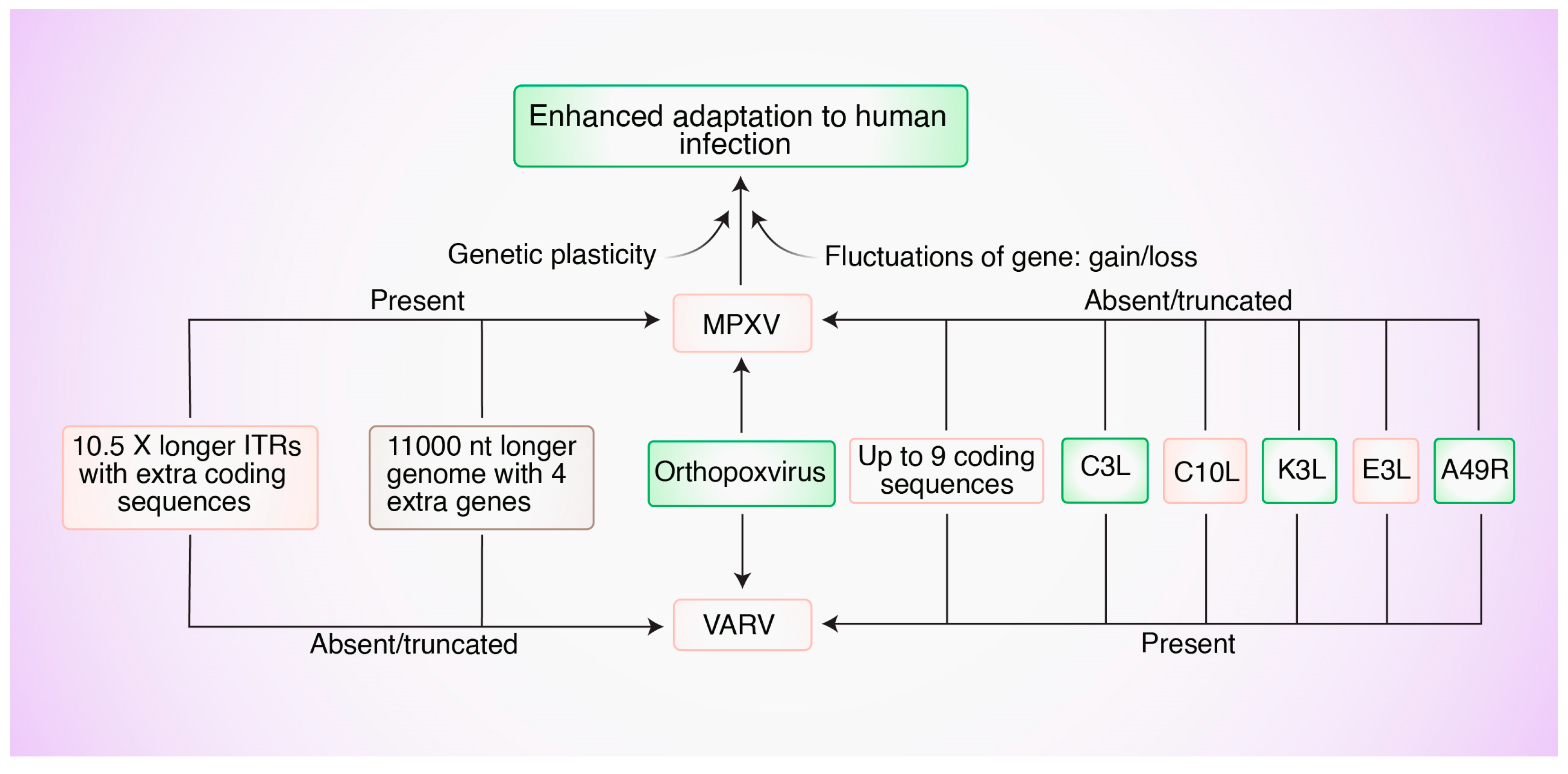
5. Genetic Factors
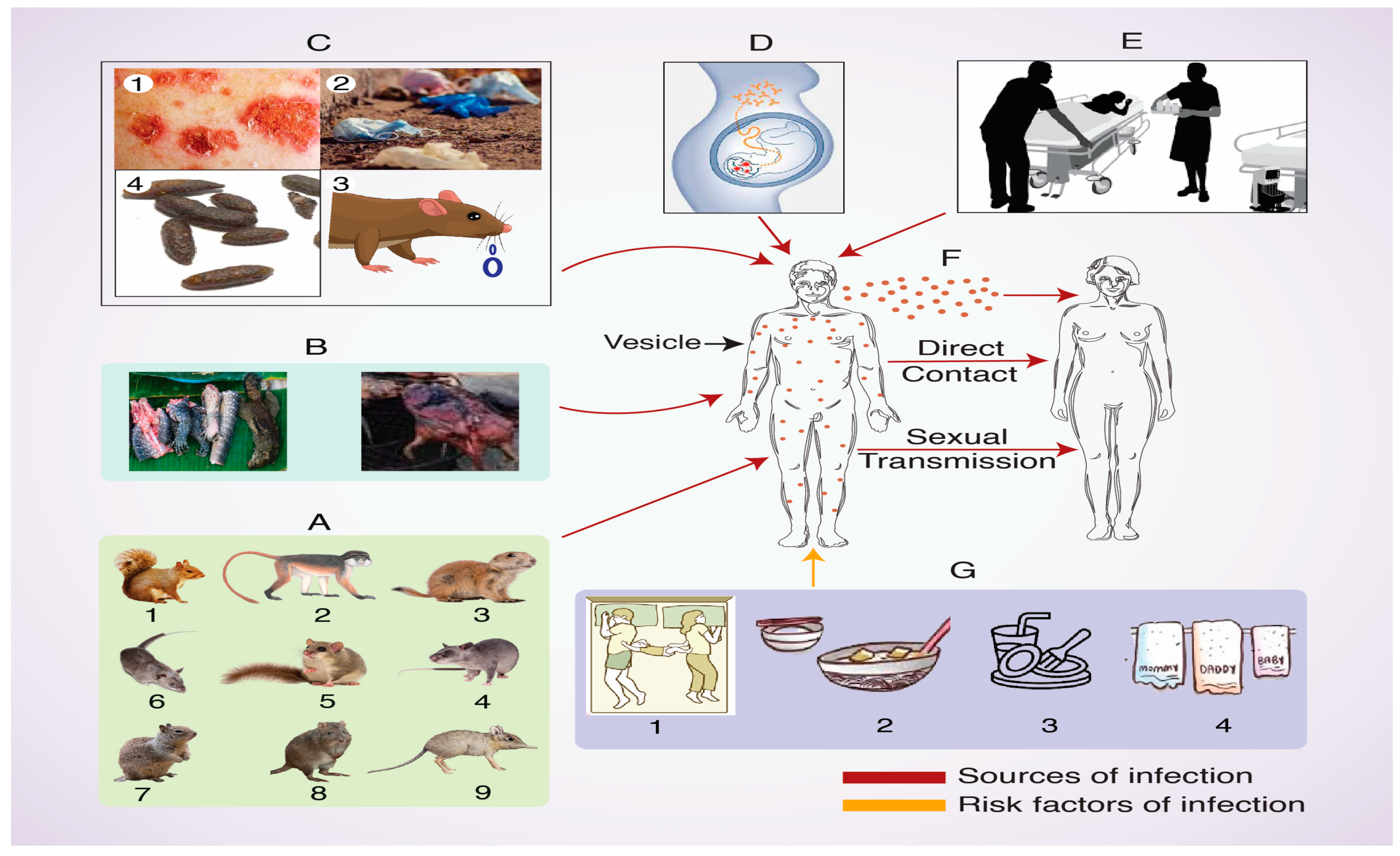
6. Hosts and Transmission
7. Pathogenesis
8. Clinical Hallmarks
9. Pathological Features
10. Host Immune Responses to MPXV Infections
11. Diagnosis
12. Immunoprophylaxis and Therapy
13. Future Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Multistate outbreak of monkeypox-Illinois, Indiana, and Wisconsin, 2003. Morb. Mortal. Wkly. Rep. 2003, 52, 537–540. [Google Scholar] [PubMed]
- Pak, A.; Adegboye, O.A.; Adekunle, A.I.; Rahman, K.M.; McBryde, E.S.; Eisen, D.P. Economic consequences of the COVID-19 outbreak: The need for epidemic preparedness. Front. Pub. Health 2020, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Coinfections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Leon-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldana-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The Never-Ending Global Emergence of Viral Zoonoses After COVID-19? The rising concern of monkeypox in Europe, North America and Beyond. Travel. Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zheng, T. Comparison and analysis of biological agent category lists based on biosafety and biodefense. PLoS ONE 2014, 9, 101163. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.A. Pathogen security-help or hindrance? Front. Bioeng. Biotechnol. 2015, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Sklenovska, N.; Van Ranst, M. Emergence of monkeypox as the most important Orthopoxvirus infection in humans. Front. Pub. Health 2018, 6, 241. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Monkey Pox Cases by Ages and Gender, Race/Ethnicity, and Symptoms. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html (accessed on 31 December 2023).
- World Health Organization. Multi-Country Outbreak of Monkeypox, External Situation Report. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-31---22-december-2023 (accessed on 31 December 2023).
- Sale, T.A.; Melski, J.W.; Stratmen, E.J. Monkeypox: An epidemiologic and clinical comparison of African and US Disease. J. Am. Acad. Dermatol. 2006, 55, 478–481. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical characteristics of human monkeypox and risk factors for severe disease. Clin. Infec. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Update: Multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb. Mortal. Wkly. Rep. 2003, 52, 561–564. [Google Scholar] [PubMed]
- Arita, I.; Jezek, Z.; Khodakevich, L.; Ruti, K. Human monkeypox: A newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am. J. Trop. Med. Hyg. 1985, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human monkeypox: Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in Cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Woolhouse, M.E.; Gowtage-Sequeria, S. Host Range and Emerging and Reemerging Pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Emerson, G.L.; Li, Y.; Frace, M.A.; Olsen-Rasmussen, M.A.; Khristova, M.L.; Govil, D.; Sammons, S.A.; Regnery, R.L.; Karem, K.L.; Damon, I.K.; et al. The phylogenetics and ecology of the orthopoxviruses endemic to North America. PLoS ONE 2009, 4, e7666. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G. Monkeypox: An Emerging Infection for Humans? Scheld, M.W., Craig, W.A., Hughes, J.M., Eds.; Emerging Infections 4; ASM Press: Washington, DC, USA, 2000; pp. 45–76. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Gavrilova, E.V.; Babkin, I.V. Multiplex PCR detection and species differentiation of orthopoxviruses pathogenic to humans. Mol. Cell Probes. 2005, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A New Threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.C.; Davidson, W.B.; Karem, K.L.; et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed]
- McLysaght, A.; Baldi, P.F.; Gaut, B.S. Extensive gene gain associated with adaptive evolution of poxviruses. Proc. Natl. Acad. Sci. USA 2003, 100, 15655–15660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Calabrese, P.; Wang, S.; Qin, C.; Rao, Y.; Feng, P.; Chen, X.S. The Roles of APOBEC-mediated RNA Editing in SARS-CoV-2 Mutations, Replication and Fitness. Sci. Rep. 2022, 12, 14972. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus genome evolution: The role of gene loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Siggs, O.M. Dissecting mammalian immunity through mutation, Immunol. Cell. Biol. 2014, 92, 392–399. [Google Scholar] [CrossRef]
- Martinez, T.; Shapiro, M.; Bhaduri-McIntosh, S.; MacCarthy, T. Evolutionary effects of the AID/APOBEC family of mutagenic enzymes on human gamma-herpesviruses. Virus Evol. 2019, 5, vey040. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, E.L.; Wang, C.; Lefkowitz, E.J. Genome Variability and Gene Content in Chordopoxviruses: Dependence on Microsatellites. Viruses 2015, 7, 2126–2146. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.E.M.; Giombini, E.; Selleri, M.; Tausch, S.H.; Andrusch, A.; Tyshaieva, A.; Cardeti, G.; Lorenzetti, R.; De Marco, L.; Carletti, F.; et al. Whole Genome Characterization of Orthopoxvirus (OPV) Abatino, a Zoonotic Virus Representing a Putative Novel Clade of Old World Orthopoxviruses. Viruses 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, M.; Arenas, M.; Galán, J.C.; Palero, F.; González-Candelas, F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect. Genet. Evol. 2015, 30, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Okeke, M.I.; Hansen, H.; Traavik, T. A naturally occurring cowpox virus with an ectromelia virus A-type inclusion protein gene displays atypical A-type inclusions. Infect. Genet. Evol. 2012, 12, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sasani, T.A.; Cone, K.R.; Quinlan, A.R.; Elde, N.C. Long read sequencing reveals poxvirus evolution through rapid homogenization of gene arrays. eLife 2018, 7, e35453. [Google Scholar] [CrossRef] [PubMed]
- Hoff, N.A.; Morier, D.S.; Kisalu, N.K.; Johnston, S.C.; Doshi, R.H.; Hensley, L.E.; Okitolonda-Wemakoy, E.; Muyembe-Tamfum, J.J.; Lloyd-Smith, J.O.; Rimoin, A.W. Varicella Coinfection in Patients with Active Monkeypox in the Democratic Republic of the Congo. EcoHealth 2017, 14, 564–574. [Google Scholar] [CrossRef]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1986, 8472, 98–99. [Google Scholar] [CrossRef]
- Radonic, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef]
- Parker, S.; Buller, R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef]
- Doty, J.B.; Malekani, J.M.; Kalemba, L.N.; Stanley, W.T.; Monroe, B.P.; Nakazawa, Y.U.; Mauldin, M.R.; Bakambana, T.L.; Liyandja, D.L.T.; Braden, Z.H.; et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Matz-Rensing, K.; Kaup, F.J. Non-human primate models of orthopoxvirus infections. Vet. Sci. 2014, 1, 40–62. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, Y.; Wu, H. Bibliometric analysis of global research trends on monkeypox: Are we ready to face this challenge? J. Med. Virol. 2023, 95, e27892. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Ortiz-Martínez, Y.; Bonilla-Aldana, D.K. What has been researched about Monkeypox? A bibliometric analysis of an old zoonotic virus causing global concern. New Microbes New Infect. 2022, 47, 100993. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Kalemba, L.; Malekani, J.; Kabamba, J.; et al. Introduction of monkeypox into a community and household: Risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Guagliardo, S.A.J.; Doshi, R.H.; Reynolds, M.G.; Dzabatou-Babeaux, A.; Ndakala, N.; Moses, C. Do monkeypox exposures vary by ethnicity? Comparison of Aka and Bantu suspected monkeypox cases. Am. J. Trop. Med. Hyg. 2020, 102, 202–205. [Google Scholar] [CrossRef]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Ogoina, D.; Aworabhi, N.; Eteng, W.; Badaru, S.; Mohammed, A.; Agenyi, J.; Etebu, E.N.; Numbere, T.W.; et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018, 24, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.T. Maternal and Fetal Outcomes Among Pregnant Women with Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Realegeno, S.; Priyamvada, L.; Kumar, A.; Blackburn, J.B.; Hartloge, C.; Puschnik, A.S.; Sambhara, S.; Olson, V.A.; Carette, J.E.; Lupashin, V.; et al. Conserved Oligomeric Golgi (COG) Complex Proteins Facilitate Orthopoxvirus Entry, Fusion and Spread. Viruses 2020, 12, 707. [Google Scholar] [CrossRef]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Brown, K.; Leggat, P.A. Human monkeypox: Current state of knowledge and implications for the future. Trav. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef]
- Guarner, J.; Johnson, B.J.; Paddock, C.D.; Shieh, W.J.; Goldsmith, C.S.; Reynolds, M.G.; Damon, I.K.; Regnery, R.L.; Zaki, S.R. Veterinary Monkeypox Virus Working Group, Monkeypox Transmission and Pathogenesis in Prairie Dogs. Emerg. Infect. Dis. 2004, 10, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cuadrado, F.J.; Nájera, L.; Suárez, D.; Silvestre, G.; García-Fresnadillo, D.; Roustan, G.; Sánchez-Vázquez, L.; Jo, M.; Santonja, C.; Garrido-Ruiz, M.C.; et al. Requena, Clinical, histopathologic, immunohistochemical, and electron microscopic findings in cutaneous monkeypox: A multicenter retrospective case series in Spain. J. Am. Acad. Dermatol. 2023, 88, 856–863. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B. Monkeypox Virus: Histologic, Immunohistochemical and Electron-Microscopic Findings. J. Cutan. Pathol. 2005, 32, 28–34. [Google Scholar] [CrossRef]
- Benedict, C.A.; Ware, C.F. Poxviruses aren’t StuPYD. Immunity 2005, 36, 553–555. [Google Scholar] [CrossRef]
- Townsend, M.B.; Keckler, M.S.; Patel, N.; Davies, D.H.; Felgner, P.; Damon, I.K.; Karem, K.L. Humoral immunity to smallpox vaccines and monkeypox virus challenge: Proteomic assessment and clinical correlations. J. Virol. 2013, 87, 900–911. [Google Scholar] [CrossRef]
- Song, H.; Josleyn, N.; Janosko, K.; Skinner, J.; Reeves, R.K.; Cohen, M.; Jett, C.; Johnson, R.; Blaney, J.E.; Bollinger, L.; et al. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS ONE 2013, 8, e77804. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.W.; McFadden, G. Origin and Evolution of Poxviruses. In Origin and Evolution of Viruses; Elsevier Ltd.: Singapore, 2008; pp. 431–446. ISBN 9780123741530. [Google Scholar]
- Mandl, J.N.; Ahmed, R.; Barreiro, L.B.; Daszak, P.; Epstein, J.H.; Virgin, H.W.; Feinberg, M.B. Reservoir host immune responses to emerging zoonotic viruses. Cell 2015, 160, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.J.; Goldstein, J.; Pohl, J.; Hooper, J.W.; Lee, P.R.; Townsend, M.B.; Bagarozzi, D.; Damon, I.K.; Karem, K.L. A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27. Virology 2014, 464–465, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Davi, S.D.; Kissenkötter, J.; Faye, M.; Böhlken-Fascher, S.; Stahl-Hennig, C.; Faye, O.; Faye, O.; Sall, A.A.; Weidmann, M.; Ademowo, O.G.; et al. Recombinase polymerase amplification assay for rapid detection of Monkeypox virus. Diagn. Microbiol. Infect. Dis. 2019, 95, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.W.; Josleyn, M.; Mucker, E.M.; Hung, C.F.; Loudon, P.T.; Wu, T.C.; Hooper, J.W. Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates. PLoS ONE 2012, 7, e42353. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States. Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Montalti, M.; Di Valerio, Z.; Angelini, R.; Bovolenta, E.; Castellazzi, F.; Cleva, M.; Pandolfi, P.; Reali, C.; Resi, D.; Todeschini, R. Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy. Vaccines 2023, 11, 1163. [Google Scholar] [CrossRef]
- Mucker, E.M.; Wollen-Roberts, S.E.; Kimmel, A.; Shamblin, J.; Sampey, D.; Hooper, J.W. Intranasal monkeypox marmoset model: Prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl. Trop. Dis. 2018, 12, e0006581. [Google Scholar] [CrossRef]
- Parker, S.; D’Angelo, J.; Buller, R.M.; Smee, D.F.; Lantto, J.; Nielsen, H.; Jensen, A.; Prichard, M.; George, S.L. A human recombinant analogue to plasma-derived vaccinia immunoglobulin prophylactically and therapeutically protects against lethal orthopoxvirus challenge. Antiviral Res. 2021, 195, 105179. [Google Scholar] [CrossRef] [PubMed]
- Merchlinsky, M.; Albright, A.; Olson, V.; Schiltz, H.; Merkeley, T.; Hughes, C.; Petersen, B.; Challberg, M. The development and approval of tecoviromat (TPOXX®), the first antiviral against smallpox. Antiviral Res. 2019, 168, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Burgado, J.; Baker-Wagner, M.; Kitaygorodskyy, A.; Olson, V.; Lingappa, V.R.; Satheshkumar, P.S. New methylene blue derivatives suggest novel anti-orthopoxviral strategies. Antivir. Res. 2021, 191, 105086. [Google Scholar] [CrossRef] [PubMed]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Doty, J.B.; McCollum, A.M.; Olson, V.A.; Nakazawa, Y. Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. Expert Rev. Anti-Infect. Ther. 2019, 17, 129–139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinnah, M.A.; Uddin, M.B.; Hasan, T.; Das, S.; Khatun, F.; Hasan, M.H.; Udonsom, R.; Rahman, M.M.; Ashour, H.M. The Re-Emergence of Mpox: Old Illness, Modern Challenges. Biomedicines 2024, 12, 1457. https://doi.org/10.3390/biomedicines12071457
Zinnah MA, Uddin MB, Hasan T, Das S, Khatun F, Hasan MH, Udonsom R, Rahman MM, Ashour HM. The Re-Emergence of Mpox: Old Illness, Modern Challenges. Biomedicines. 2024; 12(7):1457. https://doi.org/10.3390/biomedicines12071457
Chicago/Turabian StyleZinnah, Mohammad Ali, Md Bashir Uddin, Tanjila Hasan, Shobhan Das, Fahima Khatun, Md Hasibul Hasan, Ruenruetai Udonsom, Md Masudur Rahman, and Hossam M. Ashour. 2024. "The Re-Emergence of Mpox: Old Illness, Modern Challenges" Biomedicines 12, no. 7: 1457. https://doi.org/10.3390/biomedicines12071457
APA StyleZinnah, M. A., Uddin, M. B., Hasan, T., Das, S., Khatun, F., Hasan, M. H., Udonsom, R., Rahman, M. M., & Ashour, H. M. (2024). The Re-Emergence of Mpox: Old Illness, Modern Challenges. Biomedicines, 12(7), 1457. https://doi.org/10.3390/biomedicines12071457







