GNPNAT1 Serves as a Prognostic Biomarker Correlated with Immune Infiltration and Promotes Cancer Cell Metastasis through Stabilization of Snai2 in Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Immune Infiltration of GNPNAT1 in LUAD
2.3. Cell Lines and Cell Culture
2.4. Cell Transfection
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot Assay
2.7. Co-Immunoprecipitation Assay
2.8. Wound-Healing Assay
2.9. Transwell Assay
2.10. Cell Proliferation Assay
2.11. Immunohistochemistry (IHC) Staining Assay
2.12. Ubiquitination Assay
2.13. Cycloheximide (CHX) Pulse-Chase Assay
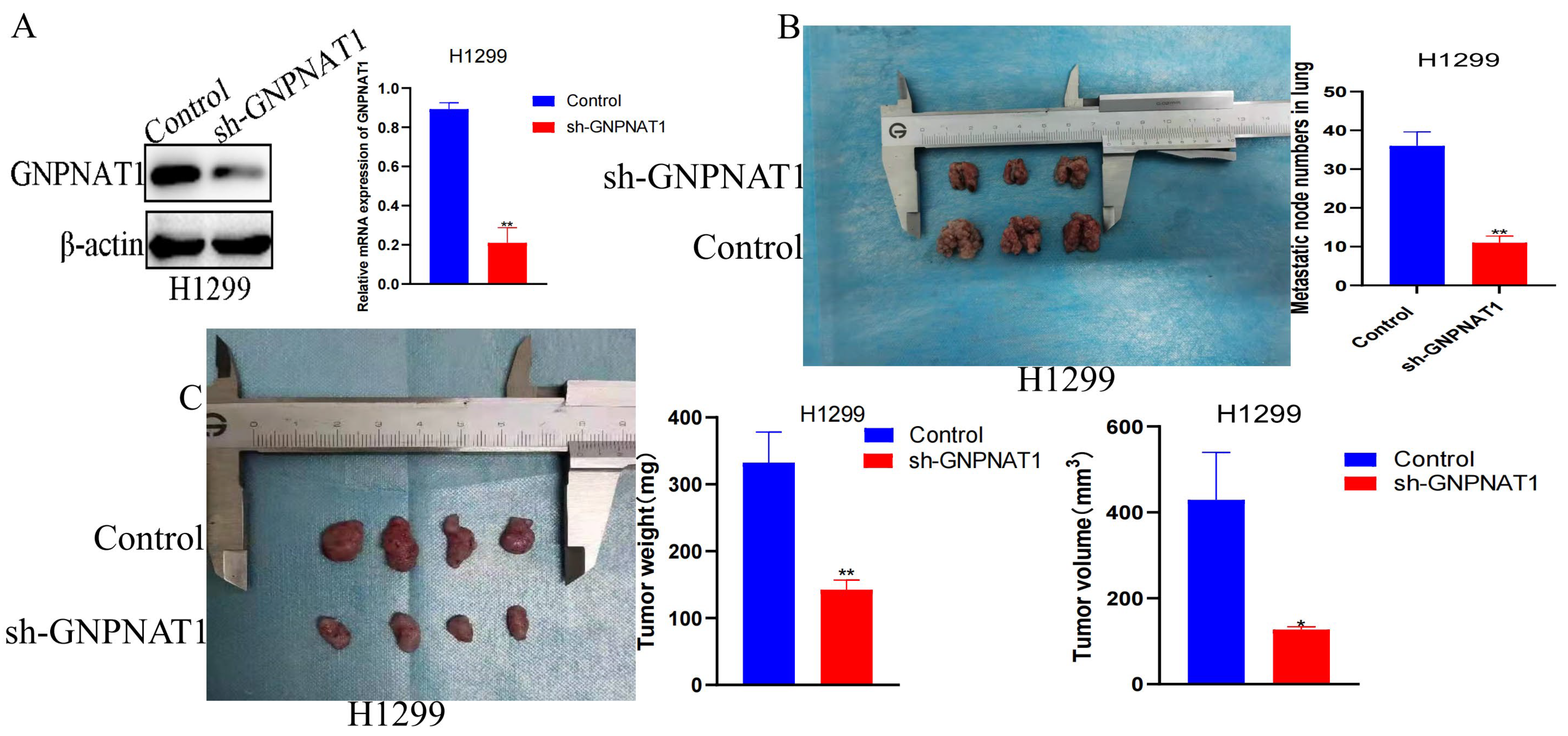
2.14. In Vivo Assay
2.15. Statistical Analysis
3. Results
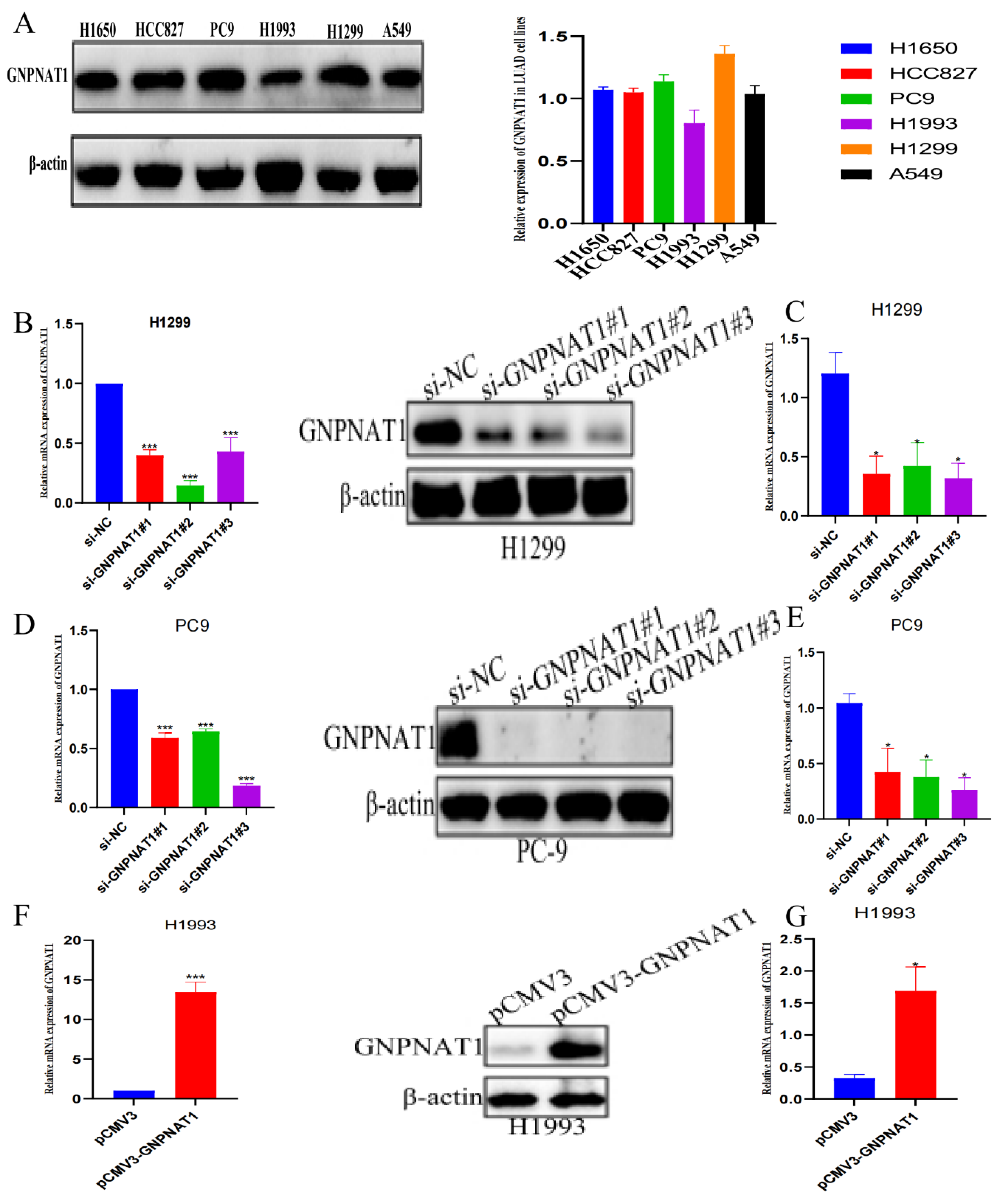
3.1. GNPNAT1 was Highly Expressed in LUAD
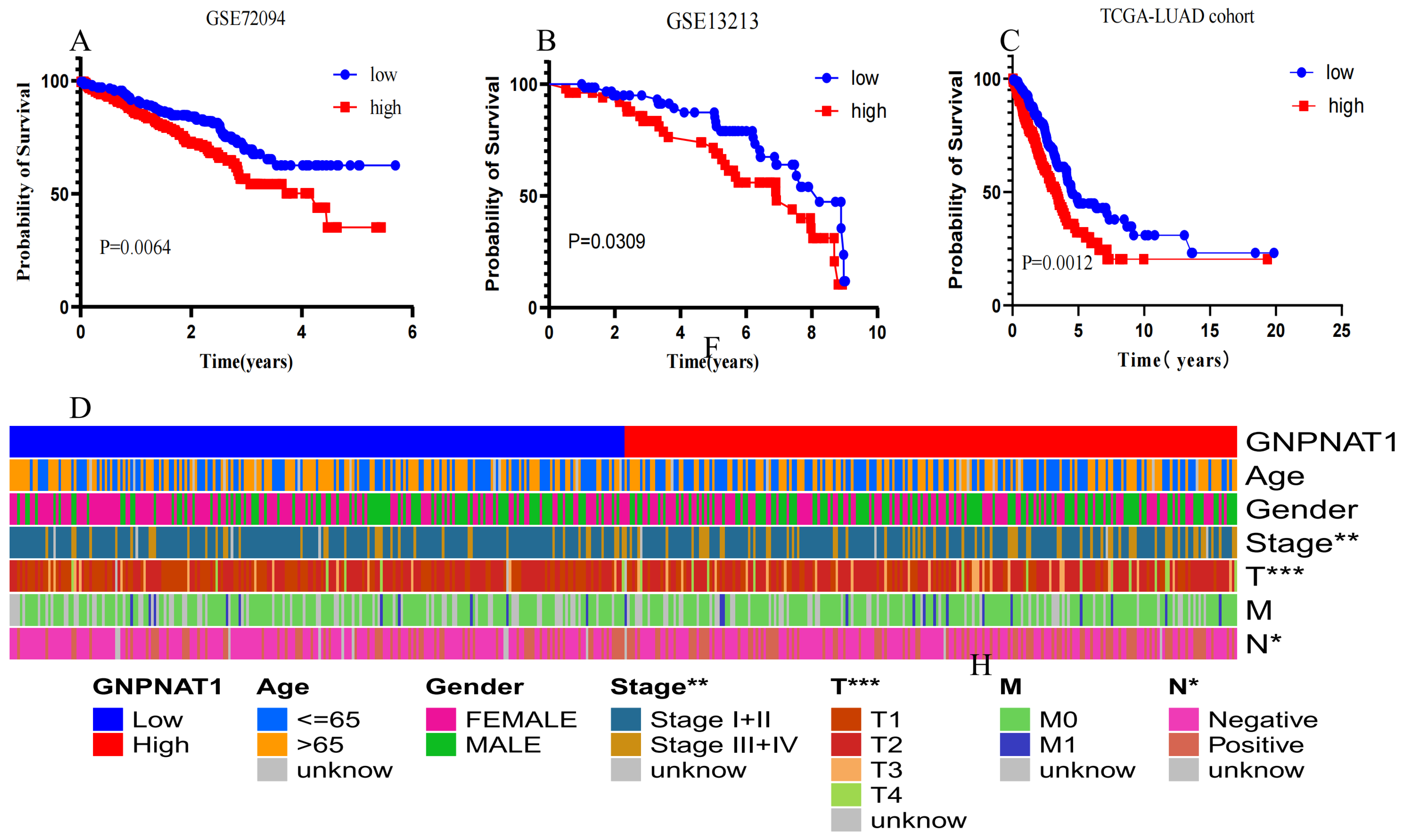
3.2. GNPNAT1 Was Associated with Poor Prognosis in LUAD
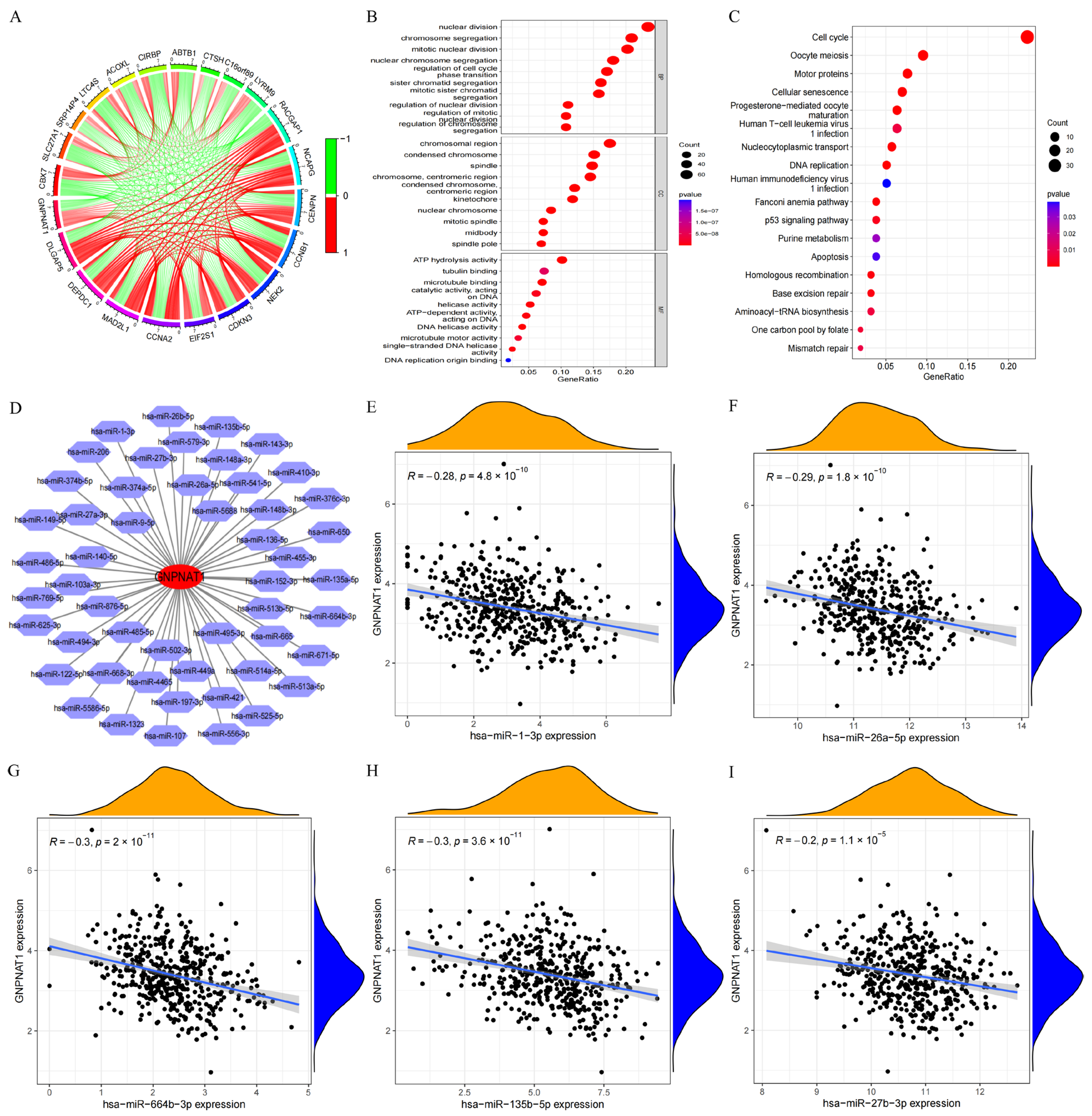
3.3. Has-miR-1-3p and Has-miR-26a-5p Were the miRNA Targets of GNPNAT1
3.4. Correlation of GNPNAT1 Expression with Immune Infiltration in LUAD
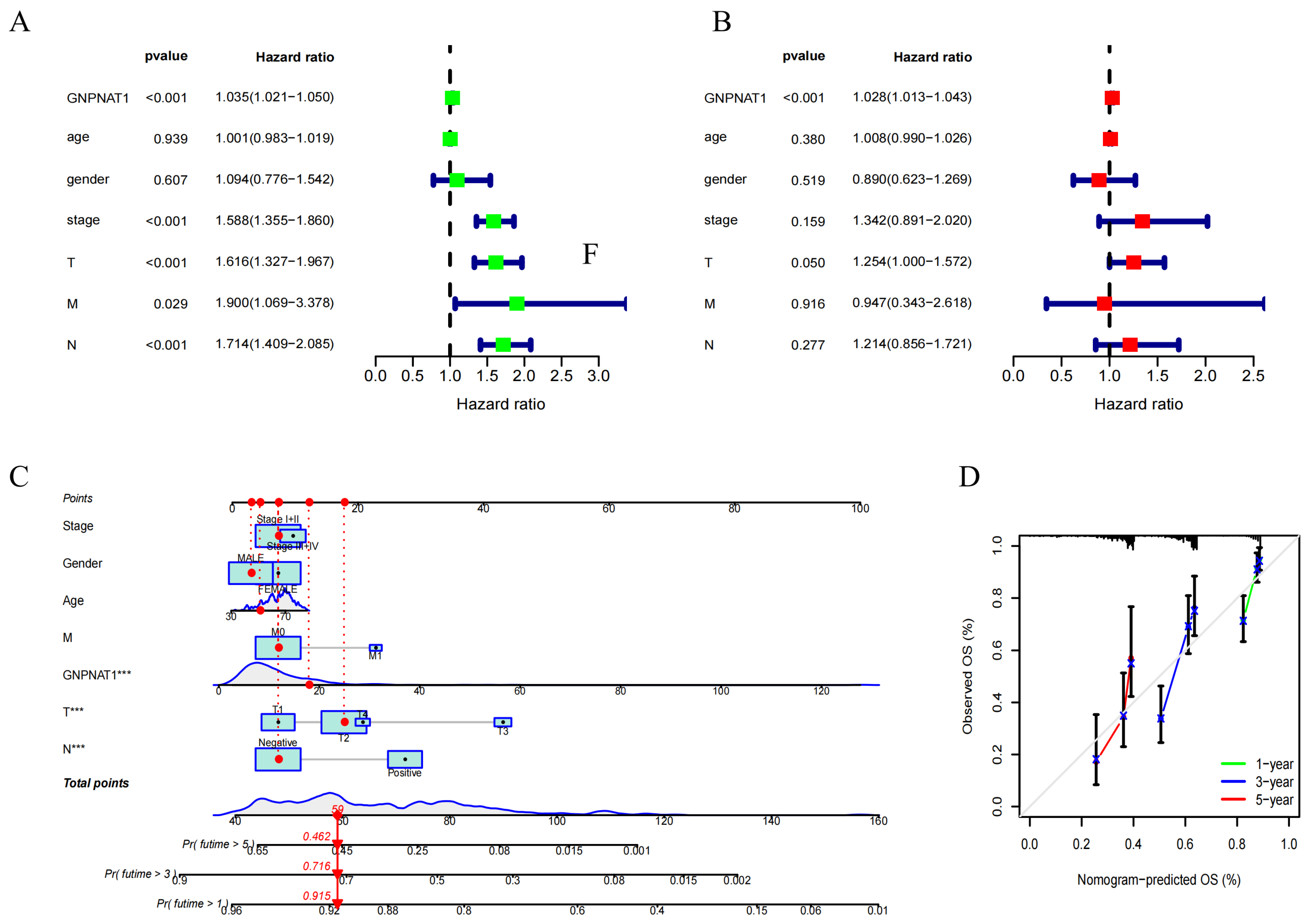
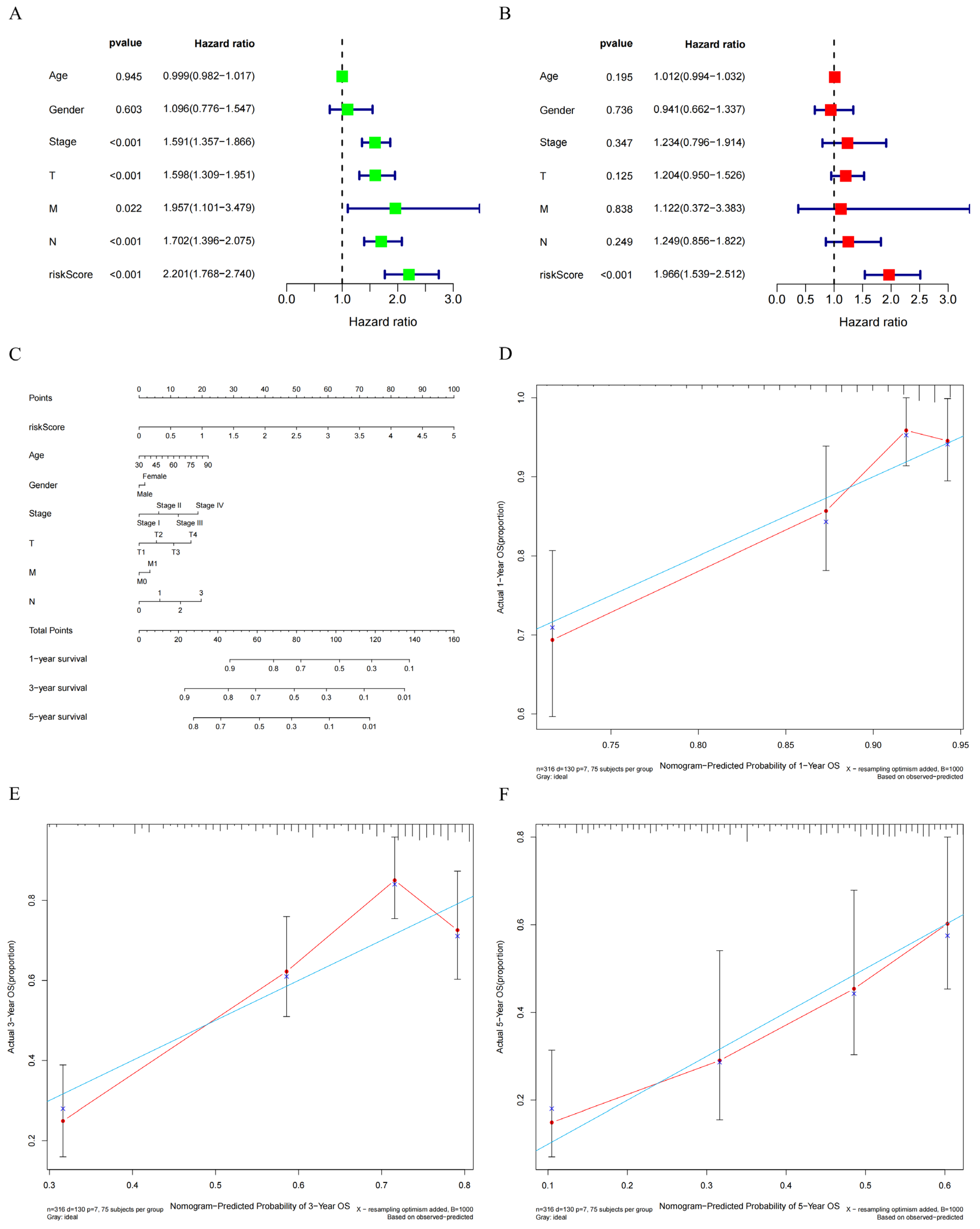
3.5. Construction of a Prognostic Signature Based on GNPNAT1-Associated Immunomodulator Genes
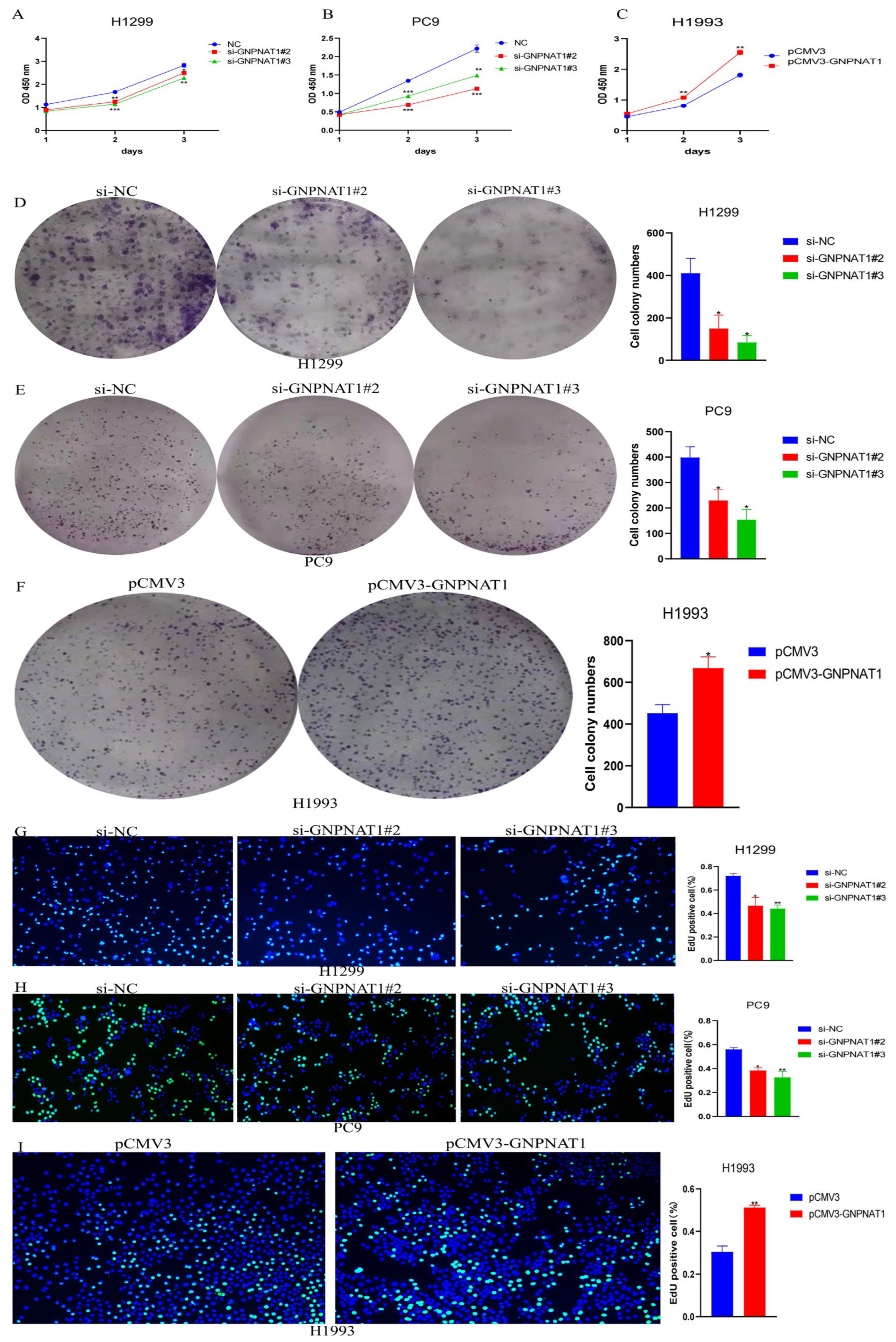
3.6. GNPNAT1 Promoted LUAD Cell Proliferation
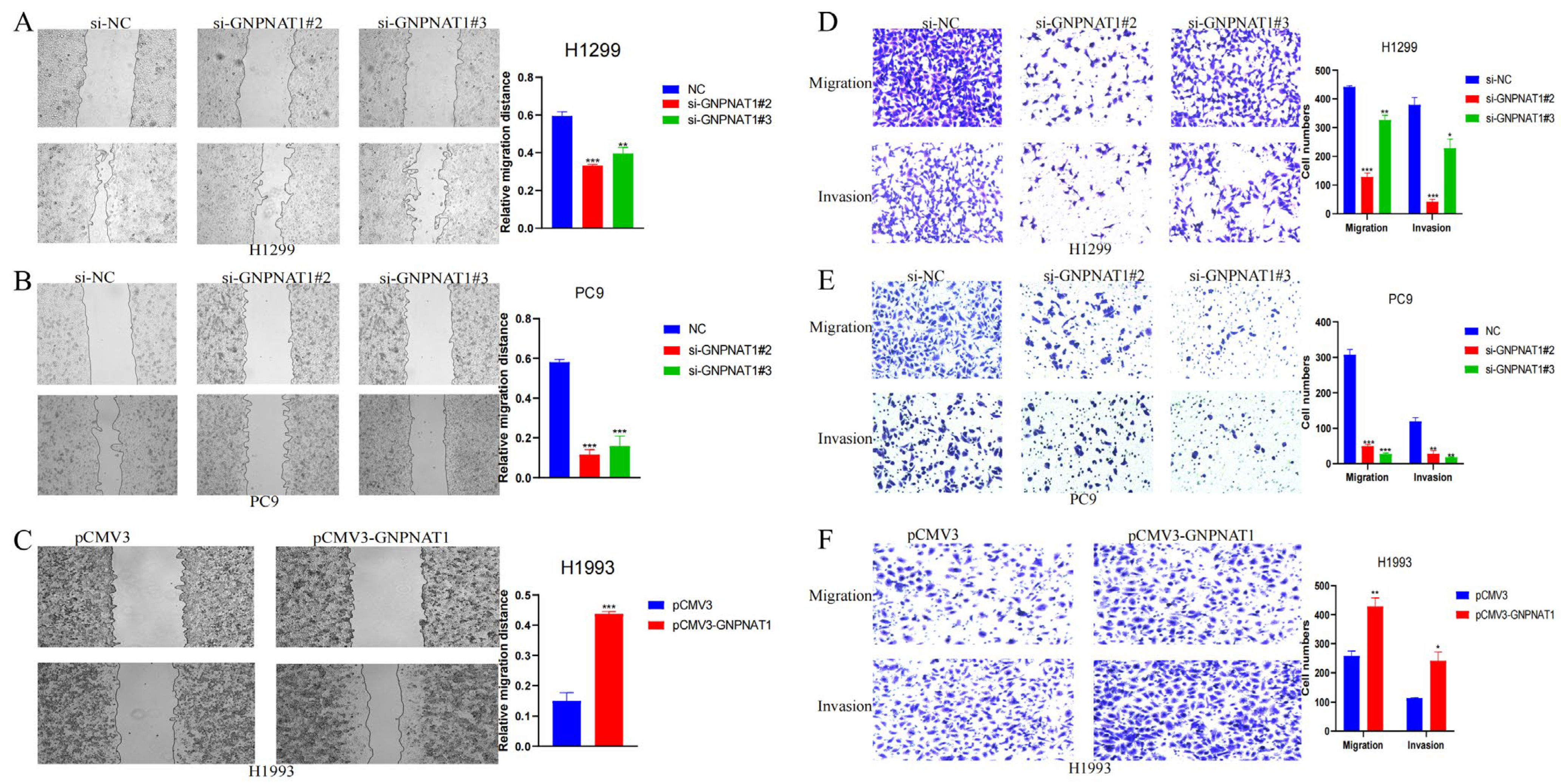
3.7. GNPNAT1 Promoted LUAD Cells Migration and Invasion
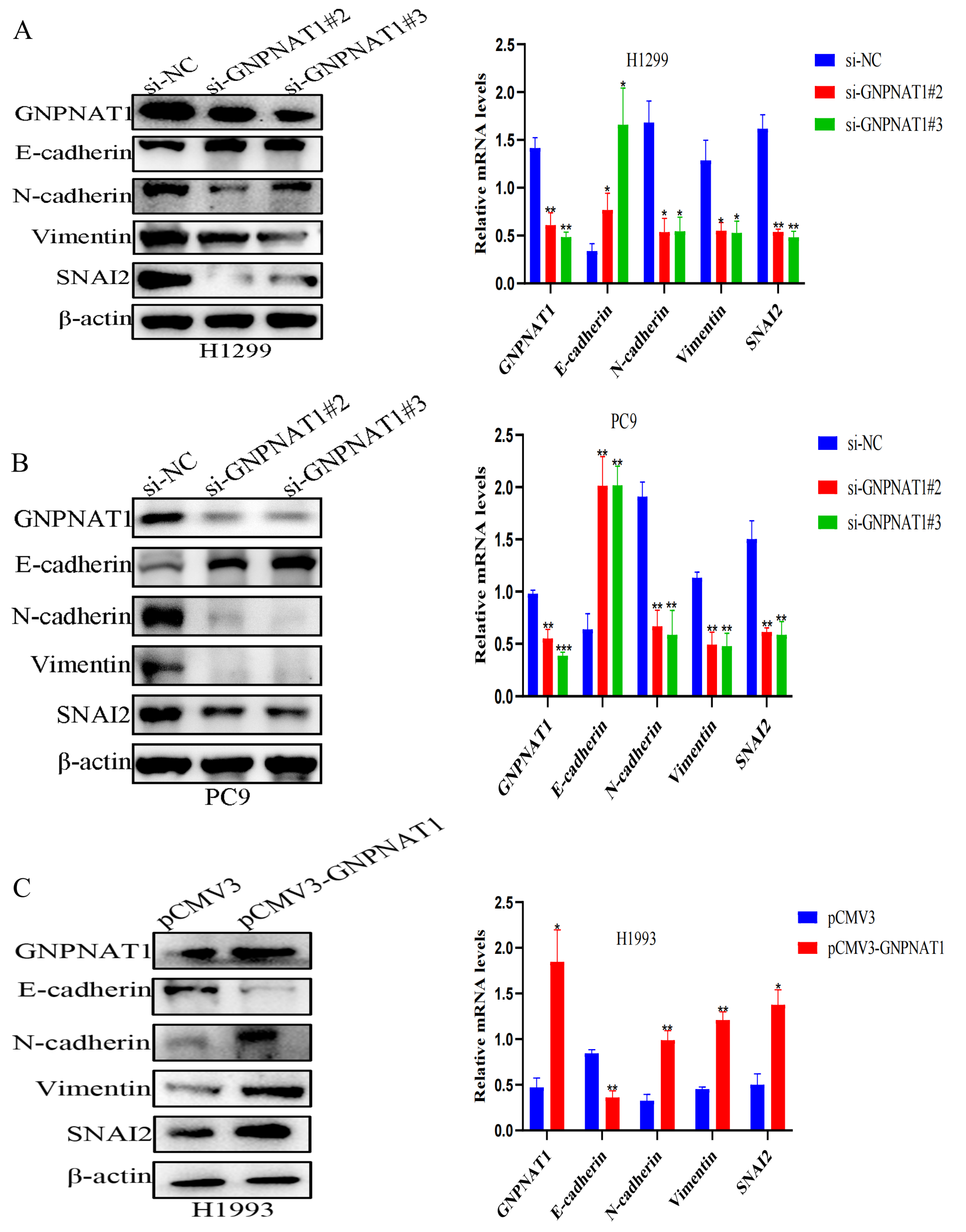
3.8. GNPNAT1 Induced EMT Process of LUAD Cell
3.9. Snai2 Was Required in GNPNAT1-Mediated Migration, Invasion, and EMT Processes
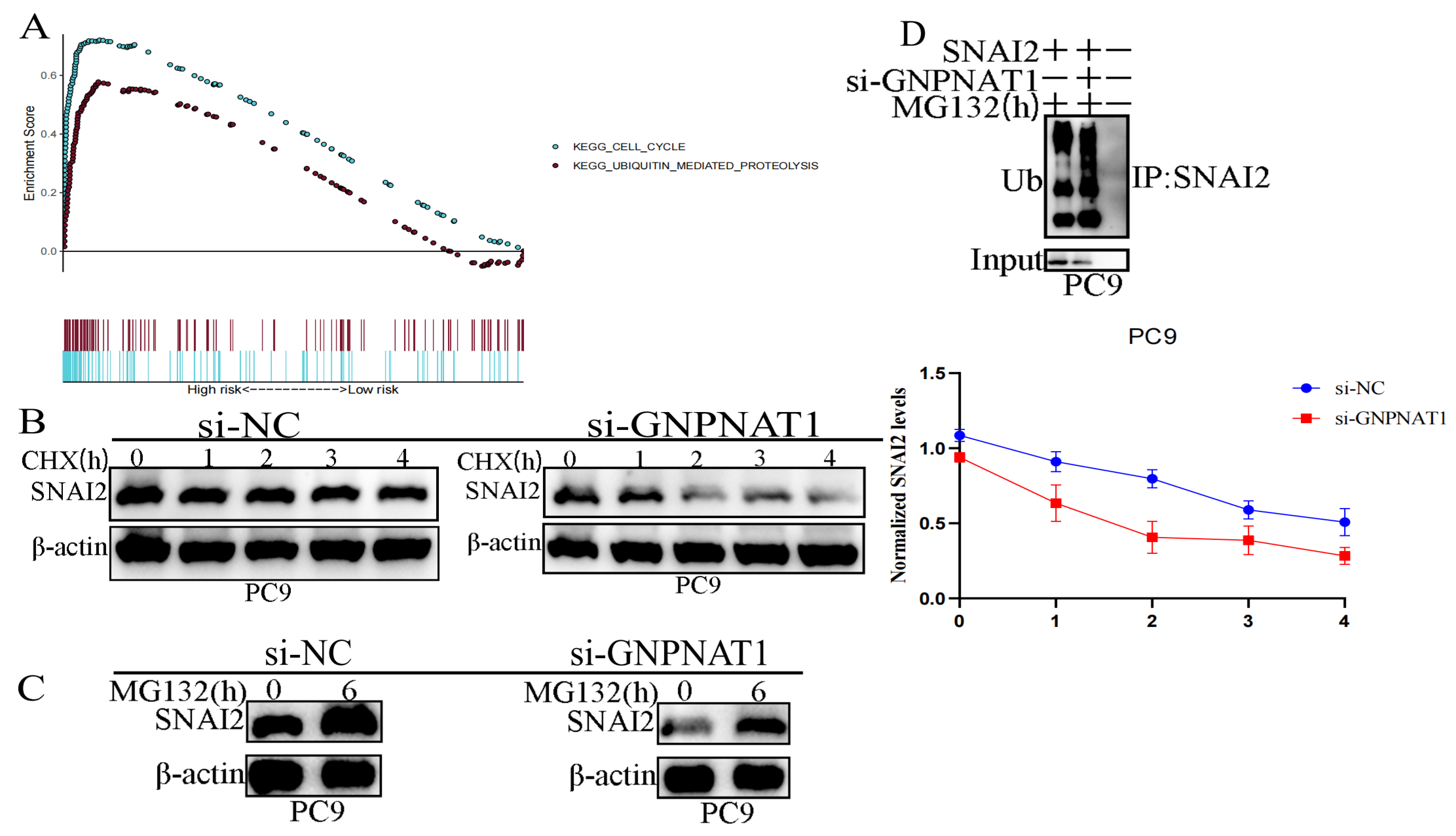
3.10. GNPNAT1 Promoted Cancer Cell Metastasis through Stabilization of Snai2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef]
- Spira, A.; Ettinger, D.S. Multidisciplinary Management of Lung Cancer. N. Engl. J. Med. 2004, 350, 379–392. [Google Scholar] [CrossRef]
- Fennell, D.A.; Summers, Y.; Cadranel, J.; Benepal, T.; Christoph, D.C.; Lal, R.; Das, M.; Maxwell, F.; Visseren-Grul, C.; Ferry, D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treatt. Rev. 2016, 44, 42–50. [Google Scholar] [CrossRef]
- Kasinski, A.L.; Slack, F.J. miRNA-34 Prevents Cancer Initiation and Progression in a Therapeutically Resistant K-ras and p53-Induced Mouse Model of Lung Adenocarcinoma. Cancer Res. 2012, 72, 5576–5587. [Google Scholar] [CrossRef]
- Song, Y.H.; Zhang, C.Q.; Chen, F.F.; Lin, X.Y. Upregulation of Neural Precursor Cell Expressed Developmentally Downregulated 4-1 is Associated with Poor Prognosis and Chemoresistance in Lung Adenocarcinoma. Chin. Med. J.-Peking 2018, 131, 16–24. [Google Scholar] [CrossRef]
- Copur, M.S.; Crockett, D.; Gauchan, D.; Ramaekers, R.; Mleczko, K. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Targeted Therapy. J. Clin. Oncol. 2018, 36, 2006. [Google Scholar] [CrossRef]
- Lam, C.; Low, J.Y.; Tran, P.T.; Wang, H. The hexosamine biosynthetic pathway and cancer: Current knowledge and future therapeutic strategies. Cancer Lett. 2021, 503, 11–18. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liang, Y.H.; Li, L.F.; Su, X.D. Acceptor substrate binding revealed by crystal structure of human glucosamine-6-phosphate N-acetyltransferase 1. FEBS Lett. 2008, 582, 2973–2978. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Shojaie, A.; Panzitt, K.; Sonavane, R.; Venghatakrishnan, H.; Manikkam, M.; Zaslavsky, A.; Putluri, V.; Vasu, V.T.; Zhang, Y.; et al. Inhibition of the hexosamine biosynthetic pathway promotes castration-resistant prostate cancer. Nat. Commun. 2016, 7, 11612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Li, H.; Guo, J.; Wang, J.; Zhang, L. Potential role of glucosamine-phosphate N-acetyltransferase 1 in the development of lung adenocarcinoma. Aging 2021, 13, 7430–7453. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Ma, C.; Zhang, J.; Zhang, Y.; Fu, Z.; Luo, H. Independent Prognostic Potential of GNPNAT1 in Lung Adenocarcinoma. Biomed. Res. Int. 2020, 2020, 8851437. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, K.; Wang, J.; Mei, T.; Zhao, M.; Huang, D. Upregulation of GNPNAT1 Predicts Poor Prognosis and Correlates with Immune Infiltration in Lung Adenocarcinoma. Front. Mol. Biosci. 2021, 8, 605754. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, S.; Huang, H.; Zhong, C.; Liu, Z.; Zhang, X.; Wang, W.; Xie, S.; Wu, K.; Lu, T.; et al. Upregulation of glucosamine-phosphate N-acetyltransferase 1 is a promising diagnostic and predictive indicator for poor survival in patients with lung adenocarcinoma. Oncol. Lett. 2021, 21, 488. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, Y.; Wang, Y.; Chen, H.; Zhang, R.; Hu, Z.; Chai, C.; Chen, T. GNPNAT1 is a potential biomarker correlated with immune infiltration and immunotherapy outcome in breast cancer. Front. Immunol. 2023, 14, 1152678. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Z.W.; Hu, S.; Xiang, Y.; Deng, Y.; Wan, F.J.; Zhang, T.C.; Yang, Z.Y.; Liao, X.H.; Hu, H.; et al. GNPNAT1 promotes the stemness of breast cancer and serves as a potential prognostic biomarker. Oncol. Rep. 2023, 50, 157. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Peng, B.; Li, G.; Sun, X.; Wang, G. Glucosamine-phosphate N-acetyltransferase 1 and its DNA methylation can be biomarkers for the diagnosis and prognosis of lung cancer. J. Clin. Lab. Anal. 2022, 36, e24628. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, N.; Ren, Y. GNPNAT1 Predicts Poor Prognosis and Cancer Development in Non-Small Cell Lung Cancer. Cancer Manag. Res. 2022, 14, 2419–2428. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Ma, Y.; Gong, H.; Yang, S.; Fang, Q.; Hu, Z. Nanoparticle abraxane possesses impaired proliferation in A549 cells due to the underexpression of glucosamine 6-phosphate N-acetyltransferase 1 (GNPNAT1/GNA1). Int. J. Nanomed. 2017, 12, 1685–1697. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, W.; Ai, H.; Tang, J.; Shen, C. Gene Expression Analysis of Lung Adenocarcinoma and Matched Adjacent Non-tumor Lung Tissue. Tumori J. 2014, 100, 338–345. [Google Scholar]
- Zhang, Y.; Foreman, O.; Wigle, D.A.; Kosari, F.; Vasmatzis, G.; Salisbury, J.L.; van Deursen, J.; Galardy, P.J. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J. Clin. Investig. 2012, 122, 4362–4374. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.; Rodriguez-Canales, J.; Behrens, C.; Thompson, D.M.; Botros, I.W.; Tang, H.; Xie, Y.; Rekhtman, N.; Travis, W.D.; Wistuba, I.I.; et al. An Expression Signature as an Aid to the Histologic Classification of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 4880–4889. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, W.; Li, C.; Tang, K.; Zhang, J.; Lei, Y.; He, W.; Peng, S.; Kuang, M.; Zhang, H.; et al. Development and Validation of a Novel Signature to Predict Overall Survival in “Driver Gene–negative” Lung Adenocarcinoma (LUAD): Results of a Multicenter Study. Clin. Cancer Res. 2019, 25, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Tomida, S.; Takeuchi, T.; Shimada, Y.; Arima, C.; Matsuo, K.; Mitsudomi, T.; Yatabe, Y.; Takahashi, T. Relapse-Related Molecular Signature in Lung Adenocarcinomas Identifies Patients with Dismal Prognosis. J. Clin. Oncol. 2009, 27, 2793–2799. [Google Scholar] [CrossRef]
- Schabath, M.B.; Welsh, E.A.; Fulp, W.J.; Chen, L.; Teer, J.K.; Thompson, Z.J.; Engel, B.E.; Xie, M.; Berglund, A.E.; Creelan, B.C.; et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene 2016, 35, 3209–3216. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, G.; Zhou, Z.; Zhang, X.; Wang, Y.; Song, G.; Ding, E.; Sun, X.; Zhong, L.; Li, S.; et al. RBBP6, a RING finger-domain E3 ubiquitin ligase, induces epithelial–mesenchymal transition and promotes metastasis of colorectal cancer. Cell Death Dis. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Xu, S.; Zhang, H.; Wang, Y.; Xiao, C.; Jiang, T.; Wu, L.; Zhang, T.; Sun, X.; Zhong, L.; et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J. Exp. Clin. Cancer Res. 2016, 35, 148. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Adcock, I.M.; Garssen, J.; Mortaz, E.; Varahram, M.; Mirsaeidi, M.; Velayati, A. The roles of miRNAs as potential biomarkers in lung diseases. Eur. J. Pharmacol. 2016, 791, 395–404. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, Y.; Liang, L.; Wu, J.; Cao, L.; Zhou, X.; Song, Z.; Ye, Z.; Zhao, Z.; Feng, H.; et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/ miR-1-3p axis in bladder cancer. J. Exp. Clin. Cancer Res. 2022, 41, 228. [Google Scholar] [CrossRef]
- Zhou, S.; Han, H.; Yang, L.; Lin, H. MiR-1-3p targets CENPF to repress tumor-relevant functions of gastric cancer cells. BMC Gastroenterol. 2022, 22, 145. [Google Scholar] [CrossRef]
- Du, G.; Yu, X.; Chen, Y.; Cai, W. MiR-1-3p Suppresses Colorectal Cancer Cell Proliferation and Metastasis by Inhibiting YWHAZ-Mediated Epithelial–Mesenchymal Transition. Front. Oncol. 2021, 11, 634596. [Google Scholar] [CrossRef]
- Li, S.M.; Wu, H.L.; Yu, X.; Tang, K.; Wang, S.G.; Ye, Z.Q.; Hu, J. The putative tumour suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1. J. Exp. Clin. Cancer Res. 2018, 37, 219. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Yang, L.; Li, L.; Gao, X.; Ni, T.; Yang, X.; Fan, Q.; Sun, X.; Wang, Y. Loss of exosomal miR-26a-5p contributes to endometrial cancer lymphangiogenesis and lymphatic metastasis. Clin. Transl. Med. 2022, 12, e846. [Google Scholar] [CrossRef]
- Miyamoto, K.; Seki, N.; Matsushita, R.; Yonemori, M.; Yoshino, H.; Nakagawa, M.; Enokida, H. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br. J. Cancer 2016, 115, 354–363. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rosivatz, E.; Becker, I.; Specht, K.; Fricke, E.; Luber, B.; Busch, R.; Höfler, H.; Becker, K.-F. Differential Expression of the Epithelial-Mesenchymal Transition Regulators Snail, SIP1, and Twist in Gastric Cancer. Am. J. Pathol. 2002, 161, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, C.; Comijn, J.; De Craene, B.; Vermassen, P.; Bruyneel, E.; Andersen, H.; Tulchinsky, E.; Van Roy, F.; Berx, G. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell–cell junctions. Nucleic Acids Res. 2005, 33, 6566–6578. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Zhau, H.E.; Odero-Marah, V.A.; Osunkoya, A.O.; Kimbro, K.S.; Tighiouart, M.; Liu, T.; Simons, J.W.; O’Regan, R.M. Insulin-like Growth Factor-I–Dependent Up-regulation of ZEB1 Drives Epithelial-to-Mesenchymal Transition in Human Prostate Cancer Cells. Cancer Res. 2008, 68, 2479–2488. [Google Scholar] [CrossRef]
- Shih, J.-Y.; Yang, P.-C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011, 32, 1299–1304. [Google Scholar] [CrossRef]
- Alves, C.C.; Carneiro, F.; Hoefler, H.; Becker, K.-F. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front. Biosci.-Landmrk 2009, 14, 3035–3050. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, A.; Wirtz, R.W.; Werner, D.; Steinmetz, K.; Claas, S.; Brueckl, W.M.; Jäger, E.; Al-Batran, S.-E. SNAI2/SLUG and estrogen receptor mRNA expression are inversely correlated and prognostic of patient outcome in metastatic non-small cell lung cancer. BMC Cancer 2015, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zheng, Y.; Dai, M.; Wu, J.; Yu, B.; Zhang, H.; Kong, W.; Wu, H.; Yu, X. Snail2 induced E-cadherin suppression and metastasis in lung carcinoma facilitated by G9a and HDACs. Cell Adhes. Migr. 2019, 13, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wang, W.L.; Chang, Y.L.; Wu, C.T.; Chao, Y.C.; Kao, S.H.; Yuan, A.; Lin, C.W.; Yang, S.C.; Chan, W.K.; et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol. 2009, 11, 694–704. [Google Scholar] [CrossRef]
- Jiang, H.; Li, T.; Qu, Y.; Wang, X.; Li, B.; Song, J.; Sun, X.; Tang, Y.; Wan, J.; Yu, Y.; et al. Long non-coding RNA SNHG15 interacts with and stabilizes transcription factor Slug and promotes colon cancer progression. Cancer Lett. 2018, 425, 78–87. [Google Scholar] [CrossRef]
- Liu, S.; Shi, L.; Wang, Y.; Ye, D.; Ju, H.; Ma, H.; Yang, W.; Wang, Y.; Hu, J.; Deng, J.; et al. Stabilization of Slug by NF-κB is Essential for TNF-α -Induced Migration and Epithelial-Mesenchymal Transition in Head and Neck Squamous Cell Carcinoma Cells. Cell. Physiol. Biochem. 2018, 47, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Kim, C.K.; Hwang, K.R.; Park, H.Y.; Koh, J.; Chung, D.H.; Lee, C.W.; Ha, G.H. Pellino-1 promotes lung carcinogenesis via the stabilization of Slug and Snail through K63-mediated polyubiquitination. Cell Death Differ. 2017, 24, 469–480. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, F.; Jing, Z.; Ren, X.; Jia, D.; Zeng, Y.; Yu, Y. GNPNAT1 Serves as a Prognostic Biomarker Correlated with Immune Infiltration and Promotes Cancer Cell Metastasis through Stabilization of Snai2 in Lung Adenocarcinoma. Biomedicines 2024, 12, 1477. https://doi.org/10.3390/biomedicines12071477
He J, Li F, Jing Z, Ren X, Jia D, Zeng Y, Yu Y. GNPNAT1 Serves as a Prognostic Biomarker Correlated with Immune Infiltration and Promotes Cancer Cell Metastasis through Stabilization of Snai2 in Lung Adenocarcinoma. Biomedicines. 2024; 12(7):1477. https://doi.org/10.3390/biomedicines12071477
Chicago/Turabian StyleHe, Jinqi, Faxiang Li, Zihan Jing, Xingmei Ren, Dexin Jia, Yuan Zeng, and Yan Yu. 2024. "GNPNAT1 Serves as a Prognostic Biomarker Correlated with Immune Infiltration and Promotes Cancer Cell Metastasis through Stabilization of Snai2 in Lung Adenocarcinoma" Biomedicines 12, no. 7: 1477. https://doi.org/10.3390/biomedicines12071477



