Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments
Abstract
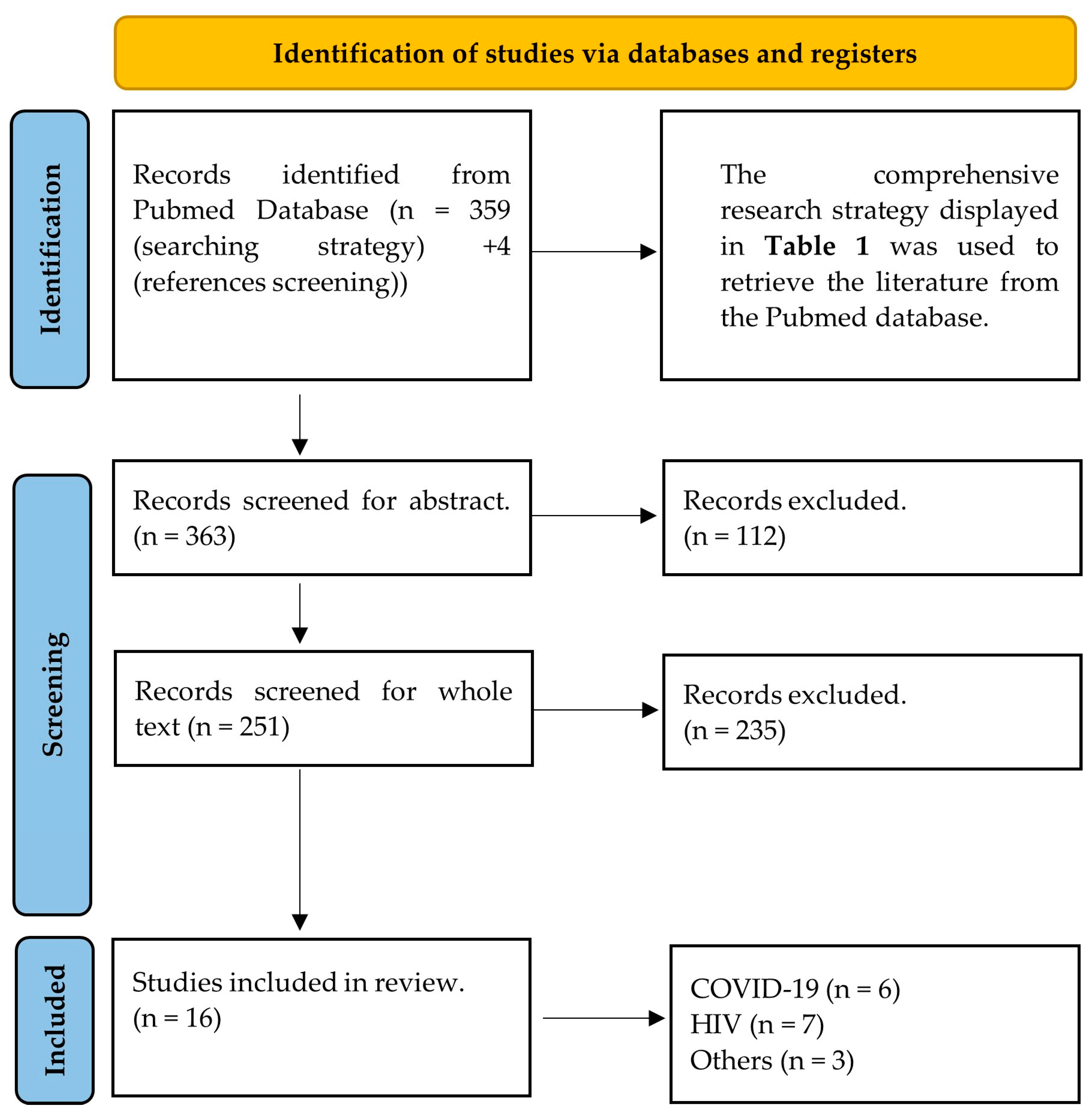
:1. Introduction
2. Methods
2.1. Identification and Protocol
2.2. Eligibility Criteria
3. Results
4. Coronavirus Disease 2019
5. Human Immunodeficiency Virus
6. Other Viral Infections
7. Discussion
7.1. Viral Impact on Retinal Microvasculature
7.2. Viral Infections and Inflammation: Impacts on Vascular Stability
7.3. Methodological Discrepancies in Retinal Microvasculature Assessment
7.4. Retinal Vessel Diameters as Biomarkers of Health Outcomes
8. Summary
9. Limitations
10. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 14 January 2024).
- Roul, H.; Mary-Krause, M.; Ghosn, J.; Delaugerre, C.; Pialoux, G.; Cuzin, L.; Launay, O.; Lacombe, J.-M.; Menard, A.; De Truchis, P.; et al. CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS 2018, 32, 2605. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Bidwell Goetz, M.; Leaf, D.; Oursler, K.A.; et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Duncan, M.S.; Alcorn, C.; Chang, C.H.; Kundu, S.; Mumpuni, A.; Smith, E.K.; Loch, S.; Bedigian, A.; Vittinghoff, E.; et al. HIV Infection and the Risk of World Health Organization–Defined Sudden Cardiac Death. J. Am. Heart Assoc. 2021, 10, e021268. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017, 2, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef]
- Goswami, N.; Fredriksen, P.M.; Lundin, K.E.A.; Agu, C.; Elias, S.O.; Motaung, K.S.; Brix, B.; Cvirn, G.; Sourij, H.; Stelzl, E.; et al. COVID-19 and its effects on endothelium in HIV-positive patients in sub-Saharan Africa: Cardiometabolic risk, thrombosis and vascular function (ENDOCOVID STUDY). BMC Infect. Dis. 2021, 21, 719. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Kamau, F.M.; Everson, F.; Kgokane, B.; De Boever, P.; Goswami, N.; Webster, I.; Strijdom, H. HIV and Antiretroviral Therapy Are Independently Associated with Cardiometabolic Variables and Cardiac Electrical Activity in Adults from the Western Cape Region of South Africa. J. Clin. Med. 2021, 10, 4112. [Google Scholar] [CrossRef] [PubMed]
- Marincowitz, C.; Genis, A.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Strijdom, H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019, 286, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Swart, C.; Fourie, C.; Lammertyn, L.; Roux, S.B.-L.; Fourie, C.; Lammertyn, L.; Roux, S.B.-L.; Strijdom, H.; Kamau, F.; De Boever, P.; et al. Comparison of endothelial function and cardiometabolic profiles of people living with HIV in two South African regions: The EndoAfrica study. Cardiovasc. J. Afr. 2022, 33, 15–20. [Google Scholar] [CrossRef]
- Botha-Le Roux, S.; Elvstam, O.; De Boever, P.; Goswami, N.; Magnusson, M.; Nilsson, P.M.; Strijdom, H.; Björkman, P.; Fourie, C.M.T. Cardiovascular Profile of South African Adults with Low-Level Viremia during Antiretroviral Therapy. J. Clin. Med. 2022, 11, 2812. [Google Scholar] [CrossRef]
- COVID-Coronavirus Statistics–Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 15 January 2024).
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Thomas, M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, e256294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der, N.C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Gille-Johnson, P. Microvascular Dysfunction in Patients with Critical Covid-19, a Pilot Study. Shock 2021, 56, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Kessler, H.H.; Brodmann, M. Case Report: Changes of Vascular Reactivity and Arterial Stiffness in a Patient with Covid-19 Infection. Front. Cardiovasc. Med. 2021, 8, 671669. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Ruzzenenti, G.; Maloberti, A.; Giani, V.; Biolcati, M.; Leidi, F.; Monticelli, M.; Grasso, E.; Cartella, I.; Palazzini, M.; Garatti, L.; et al. Covid and Cardiovascular Diseases: Direct and Indirect Damages and Future Perspective. High Blood Press. Cardiovasc. Prev. 2021, 28, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Saloň, A.; Neshev, R.; Teraž, K.; Šimunič, B.; Peskar, M.; Marušič, U.; Pišot, S.; Šlosar, L.; Gasparini, M.; Pišot, R.; et al. A pilot study: Exploring the influence of COVID-19 on cardiovascular physiology and retinal microcirculation. Microvasc. Res. 2023, 150, 104588. [Google Scholar] [CrossRef] [PubMed]
- Saloň, A.; Vladic, N.; Schmid-Zalaudek, K.; Steuber, B.; Hawliczek, A.; Urevc, J.; Bergauer, A.; Pivec, V.; Shankhwar, V.; Goswami, N. Sex Variations in Retinal Microcirculation Response to Lower Body Negative Pressure. Biology 2023, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Saloň, A.; Steuber, B.; Neshev, R.; Schmid-Zalaudek, K.; De Boever, P.; Bergmann, E.; Picha, R.; Fredriksen, P.M.; Nkeh-Chungag, B.N.; Goswami, N. Vascular Responses following Light Therapy: A Pilot Study with Healthy Volunteers. J. Clin. Med. 2023, 12, 2229. [Google Scholar] [CrossRef] [PubMed]
- Dinevski, D.; Lučovnik, M.; Žebeljan, I.; Guzelj, D.; Dinevski, I.V.; Salon, A.; De Boever, P.; Goswami, N. Analysis of Retinal Blood Vessel Diameters in Pregnant Women Practicing Yoga: A Feasibility Study. Healthcare 2022, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.; Stradner, M.; Roessler, A.; Brix, B.; Lackner, A.; Salon, A.; Goswami, N. A Pilot Study: Hypertension, Endothelial Dysfunction and Retinal Microvasculature in Rheumatic Autoimmune Diseases. J. Clin. Med. 2021, 10, 4067. [Google Scholar] [CrossRef] [PubMed]
- Saloň, A.; Çiftci, G.M.; Zubac, D.; Šimunič, B.; Pišot, R.; Narici, M.; Fredriksen, P.M.; Nkeh-Chungag, B.N.; Sourij, H.; Šerý, O.; et al. Retinal venular vessel diameters are smaller during ten days of bed rest. Sci. Rep. 2023, 13, 19258. [Google Scholar] [CrossRef] [PubMed]
- Hosák, L.; Zeman, T.; Studnička, J.; Stepanov, A.; Ustohal, L.; Michalec, M.; Lochman, J.; Jurečka, T.; Sadykov, E.; Goswami, N.; et al. Retinal arteriolar and venular diameters are widened in patients with schizophrenia. Psychiatry Clin. Neurosci. 2020, 74, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; Spruit, M.A.; Theunis, J.; Goswami, N.; Vanfleteren, L.E.; Franssen, F.M.E.; Wouters, E.F.M.; De Boever, P. Looking into the eye of patients with chronic obstructive pulmonary disease: An opportunity for better microvascular profiling of these complex patients. Acta Ophthalmol. 2018, 96, 539–549. [Google Scholar] [CrossRef]
- Hanssen, H.; Streese, L.; Vilser, W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog. Retin. Eye Res. 2022, 91, 101095. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Klein, B.E.K.; Hubbard, L.D.; Nieto, F.J.; the Atherosclerosis Risk in Communities Study. Retinal Arteriolar Diameter and Risk for Hypertension. Ann. Intern. Med. 2004, 140, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Cheung, N.; Wang, J.J.; Klein, R.; Klein, B.E.; Cotch, M.F.; Sharrett, A.R.; Shea, S.; Islam, F.A.; Wong, T.Y. Retinal vessel diameters and risk of hypertension: The Multiethnic Study of Atherosclerosis. J. Hypertens. 2009, 27, 2386. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Wang, J.J.; Wong, T.Y.; Rochtchina, E.; Klein, R.; Leeder, S.R.; Mitchell, P. Retinal Arteriolar Narrowing Is Associated With 5-Year Incident Severe Hypertension. Hypertension 2004, 44, 442–447. [Google Scholar] [CrossRef]
- Wong, T.Y.; Shankar, A.; Klein, R.; Klein, B.E.K.; Hubbard, L.D. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ 2004, 329, 79. [Google Scholar] [CrossRef]
- Ikram, M.K.; Witteman, J.C.M.; Vingerling, J.R.; Breteler, M.M.B.; Hofman, A.; de Jong, P.T.V.M. Retinal Vessel Diameters and Risk of Hypertension. Hypertension 2006, 47, 189–194. [Google Scholar] [CrossRef]
- Wang, J.J.; Taylor, B.; Wong, T.Y.; Chua, B.; Rochtchina, E.; Klein, R.; Mitchell, P. Retinal Vessel Diameters and Obesity: A Population-Based Study in Older Persons. Obesity 2006, 14, 206–214. [Google Scholar] [CrossRef]
- Wong, T.Y.; Duncan, B.B.; Golden, S.H.; Klein, R.; Couper, D.J.; Klein, B.E.K.; Hubbard, L.D.; Sharrett, A.R.; Schmidt, M.I. Associations between the Metabolic Syndrome and Retinal Microvascular Signs: The Atherosclerosis Risk in Communities Study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lovern, C.; Lycett, K.; He, M.; Wake, M.; Wong, T.Y.; Burgner, D.P. The association between markers of inflammation and retinal microvascular parameters: A systematic review and meta-analysis. Atherosclerosis 2021, 336, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.M.A.; Nguyen, T.T.; Wang, J.J.; Tai, E.S.; Shankar, A.; Saw, S.M.; Aung, T.; Lim, S.C.; Mitchell, P.; Wong, T.Y. Quantitative retinal vascular calibre changes in diabetes and retinopathy: The Singapore Malay eye study. Eye 2009, 23, 1719–1724. [Google Scholar] [CrossRef]
- Kifley, A.; Wang, J.J.; Cugati, S.; Wong, T.Y.; Mitchell, P. Retinal Vascular Caliber, Diabetes, and Retinopathy. Am. J. Ophthalmol. 2007, 143, 1024–1026. [Google Scholar] [CrossRef]
- Tikellis, G.; Wang, J.J.; Tapp, R.; Simpson, R.; Mitchell, P.; Zimmet, P.Z.; Shaw, J.; Wong, T.Y. The relationship of retinal vascular calibre to diabetes and retinopathy: The Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia 2007, 50, 2263–2271. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wang, J.J.; Islam, F.M.A.; Mitchell, P.; Tapp, R.J.; Zimmet, P.Z.; Simpson, R.; Shaw, J.; Wong, T.Y. Retinal Arteriolar Narrowing Predicts Incidence of Diabetes: The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes 2008, 57, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Schmidt, M.I.; Pankow, J.S.; Couper, D.J.; Klein, B.E.K.; Hubbard, L.D.; Duncan, B.B.; the ARIC Investigators. Retinal Arteriolar Narrowing and Risk of Diabetes Mellitus in Middle-aged Persons. JAMA 2002, 287, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Shankar, A.; Klein, R.; Klein, B.E.K.; Hubbard, L.D. Retinal Arteriolar Narrowing, Hypertension, and Subsequent Risk of Diabetes Mellitus. Arch. Intern. Med. 2005, 165, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Schram, M.T.; Berendschot, T.T.J.M.; Webers, C.A.B.; Kroon, A.A.; van der Kallen, C.J.H.; Henry, R.M.A.; Schaper, N.C.; Huang, F.; Dashtbozorg, B.; et al. Type 2 diabetes and HbA1c are independently associated with wider retinal arterioles: The Maastricht study. Diabetologia 2020, 63, 1408–1417. [Google Scholar] [CrossRef]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Sharrett, A.R.; Klein, B.E.K.; Wang, J.J.; Chambless, L.E.; Wong, T.Y. Risk Prediction of Coronary Heart Disease Based on Retinal Vascular Caliber (from the Atherosclerosis Risk In Communities [ARIC] Study). Am. J. Cardiol. 2008, 102, 58–63. [Google Scholar] [CrossRef]
- Wang, J.J.; Liew, G.; Klein, R.; Rochtchina, E.; Knudtson, M.D.; Klein, B.E.K.; Wong, T.Y.; Burlutsky, G.; Mitchell, P. Retinal vessel diameter and cardiovascular mortality: Pooled data analysis from two older populations. Eur. Heart J. 2007, 28, 1984–1992. [Google Scholar] [CrossRef]
- Wong, T.Y.; Kamineni, A.; Klein, R.; Sharrett, A.R.; Klein, B.E.; Siscovick, D.S.; Cushman, M.; Duncan, B.B. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons: The Cardiovascular Health Study. Arch. Intern. Med. 2006, 166, 2388–2394. [Google Scholar] [CrossRef]
- Chandra, A.; Seidelmann, S.B.; Claggett, B.L.; Klein, B.E.; Klein, R.; Shah, A.M.; Solomon, S.D. The association of retinal vessel calibres with heart failure and long-term alterations in cardiac structure and function: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Heart Fail. 2019, 21, 1207–1215. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef]
- Mutlu, U.; Ikram, M.K.; Wolters, F.J.; Hofman, A.; Klaver, C.C.W.; Ikram, M.A. Retinal Microvasculature Is Associated With Long-Term Survival in the General Adult Dutch Population. Hypertension 2016, 67, 281–287. [Google Scholar] [CrossRef]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef]
- Invernizzi, A.; Schiuma, M.; Parrulli, S.; Torre, A.; Zicarelli, F.; Colombo, V.; Marini, S.; Villella, E.; Bertoni, A.; Antinori, S.; et al. Retinal vessels modifications in acute and post-COVID-19. Sci. Rep. 2021, 11, 19373. [Google Scholar] [CrossRef] [PubMed]
- Aşıkgarip, N.; Temel, E.; Hızmalı, L.; Örnek, K.; Sezgin, F.M. Retinal Vessel Diameter Changes in COVID-19 Infected Patients. Ocul. Immunol. Inflamm. 2021, 29, 645–651. [Google Scholar] [CrossRef]
- Gündoğan, M.; Vural, E.; Bayram, N.; Altunel, O.; Gündoğan, F.; Göktaş, S. Change in retinal vessel diameter and choroidal thickness in patients with severe COVID-19. Photodiagnosis Photodyn. Ther. 2022, 37, 102674. [Google Scholar] [CrossRef]
- Kuchler, T.; Günthner, R.; Ribeiro, A.; Hausinger, R.; Streese, L.; Wöhnl, A.; Kesseler, V.; Negele, J.; Assali, T.; Carbajo-Lozoya, J.; et al. Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis 2023, 26, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Leung, I.Y.-F.; Lai, S.; Ren, S.; Kempen, J.; Klein, R.; Tso, M.O.M.; Lai, H.C. Early retinal vascular abnormalities in African-American cocaine users. Am. J. Ophthalmol. 2008, 146, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gangaputra, S.; Kalyani, P.S.; Fawzi, A.A.; Van Natta, M.L.; Hubbard, L.D.; Danis, R.P.; Thorne, J.E.; Holland, G.N. Studies of the Ocular Complications of AIDS Research Group Retinal vessel caliber among people with acquired immunodeficiency syndrome: Relationships with disease-associated factors and mortality. Am. J. Ophthalmol. 2012, 153, 434–444.e1. [Google Scholar] [CrossRef]
- Tan, P.B.; Hee, O.K.; Cheung, C.; Yeo, T.K.; Agrawal, R.; Ng, J.; Lim, T.H.; Wong, T.Y.; Teoh, S.C. Retinal Vascular Parameter Variations in Patients With Human Immunodeficiency Virus. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7962–7967. [Google Scholar] [CrossRef]
- Pathai, S.; Weiss, H.A.; Lawn, S.D.; Peto, T.; D’Costa, L.M.; Cook, C.; Wong, T.Y.; Gilbert, C.E. Retinal arterioles narrow with increasing duration of anti-retroviral therapy in HIV infection: A novel estimator of vascular risk in HIV? PLoS ONE 2012, 7, e51405. [Google Scholar] [CrossRef]
- Edwar, L.; Karim, B.; Wijaya, I.P.; Ariyanto, I.; Tanudjaja, S.A.; Estiasari, R.; Sitompul, R.; Price, P. Factors Affecting the Health of Retinal Vessels in Human Immunodeficiency Virus Patients Beginning Anti-Retroviral Therapy. AIDS Res. Hum. Retroviruses 2019, 35, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.N.; Sayin Kutlu, S.; Parca, O.; Kutlu, M.; Pekel, G. The Thicknesses of Choroid, Macular Segments, Peripapillary Retinal Nerve Fiber Layer, And Retinal Vascular Caliber in Hiv-1-Infected Patients without Infectious Retinitis. Retina 2019, 39, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Tan, P.; Hee, O.; Agrawal, R.; Lim, T.-H.; Wong, T.-Y.; Teoh, S.C. Brief Report: Retinal Microvasculature and Immune Restoration among South Eastern Asian Patients with HIV/AIDS Over a 9-Month Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2022, 90, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, H.K.; Bhai, S.; John, M.; Xavier, J. Ocular manifestations of dengue fever in an East Indian epidemic. Can. J. Ophthalmol. 2006, 41, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Yalinbas, D.; Komurluoglu, A.; Bozali, E. Increased Retinal Vessel Tortuosity Associated With Crimean-Congo Hemorrhagic Fever in Children. Pediatr. Infect. Dis. J. 2021, 40, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Fitt, C.; Luong, T.V.; Cresp, D.; Hutchinson, A.; Lim, K.; Hodgson, L.; Colville, D.; Savige, J. Increased retinal venular calibre in acute infections. Sci. Rep. 2021, 11, 17280. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Derada Troletti, C.; Fontijn, R.D.; Gowing, E.; Charabati, M.; van Het Hof, B.; Didouh, I.; van der Pol, S.M.A.; Geerts, D.; Prat, A.; van Horssen, J.; et al. Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis. 2019, 10, 45. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19): Corticosteroids, Including Dexamethasone. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-dexamethasone (accessed on 9 June 2024).
- Nguyen, T.T.; Wang, J.J.; Sharrett, A.R.; Islam, F.M.A.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Wong, T.Y. Relationship of Retinal Vascular Caliber With Diabetes and Retinopathy: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008, 31, 544–549. [Google Scholar] [CrossRef]
- Serre, K.; Sasongko, M.B. Modifiable Lifestyle and Environmental Risk Factors Affecting the Retinal Microcirculation. Microcirculation 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Louwies, T.; Int Panis, L.; Alders, T.; Bonné, K.; Goswami, N.; Nawrot, T.S.; Dendale, P.; De Boever, P. Microvascular reactivity in rehabilitating cardiac patients based on measurements of retinal blood vessel diameters. Microvasc. Res. 2019, 124, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Imhof, K.; Zahner, L.; Schmidt-Trucksäss, A.; Faude, O.; Hanssen, H. Influence of physical fitness and activity behavior on retinal vessel diameters in primary schoolchildren. Scand. J. Med. Sci. Sports 2016, 26, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Pressler, A.; Hanssen, H.; Dimitrova, M.; Krumm, M.; Halle, M.; Scherr, J. Acute and chronic effects of marathon running on the retinal microcirculation. Atherosclerosis 2011, 219, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Panis, L.I.; Kicinski, M.; De Boever, P.; Nawrot, T.S. Retinal microvascular responses to short-term changes in particulate air pollution in healthy adults. Environ. Health Perspect. 2013, 121, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Provost, E.B.; Int Panis, L.; Saenen, N.D.; Kicinski, M.; Louwies, T.; Vrijens, K.; De Boever, P.; Nawrot, T.S. Recent versus chronic fine particulate air pollution exposure as determinant of the retinal microvasculature in school children. Environ. Res. 2017, 159, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Tham, Y.C.; Hsu, W.; Lee, M.L.; Klein, R.; Klein, B.; Ikram, M.K.; Wong, T.Y.; Cheung, C.Y.-L. Comparison of Common Retinal Vessel Caliber Measurement Software and a Conversion Algorithm. Transl. Vis. Sci. Technol. 2016, 5, 11. [Google Scholar] [CrossRef]
- French, C.; Heitmar, R. Comparison of Static Retinal Vessel Caliber Measurements by Different Commercially Available Platforms. Optom. Vis. Sci. 2021, 98, 1104–1112. [Google Scholar] [CrossRef]
| Category | Specific Category | Keywords | Strategy |
|---|---|---|---|
| Population | Inclusion and Exclusion Criteria | (“Animals” [Mesh] NOT (“Animals” [Mesh] AND “Humans” [Mesh])) | #1 |
| review [Publication Type] OR “systematic review” [Publication Type] OR “systematic literature review” [Publication Type] OR “meta-analysis” [Publication Type] OR “meta analysis” [Publication Type] OR “meta-analytic review” [Publication Type] | #2 | ||
| Search #1 OR #2 | #3 | ||
| Interest | Viral Infections | “viruses” [Mesh] OR Virus* [tiab] OR “viral particle*” [tiab] OR “enteric virus*” [tiab] OR “viral contamination*” [tiab] | #4 |
| Control | N/A | N/A | |
| Outcome | Retinal Microcirculation Diameter Investigations | “retinal vessels” [Mesh] OR “retinal vessel*“ [tiab] OR “retinal vessel diameter*“ [tiab] OR “retinal arter*“ [tiab] OR “retinal vein*“ [tiab] OR “retinal vascular calibre*“ [tiab] OR “retinal vascular caliber*“ [tiab] OR “retinal arteriolar calibre*“ [tiab] OR “retinal arteriolar caliber“ [tiab] OR “retinal venular calibre*“ [tiab] OR “retinal venular caliber*“ [tiab] OR “arteriovenous ratio*“ [tiab] OR “retinal arteriolar dilatation*“ [tiab] OR “retinal venular dilatation*“ [tiab] OR “retinal vascular change*“ [tiab] OR “retinal blood vessel*“ [tiab] OR “arteriolar narrowing*“ [tiab] OR “retinal arteriolar narrowing*“ [tiab] OR “retinal microcirculation*“ [ tiab] OR “retinal vasculature*“ [tiab] | #5 |
| Search strategy | #4 AND #5 NOT #3 | ||
| Title | Study Details | Study Design | Target Group | Imaging Tool | Purpose | Summary of Findings | |
|---|---|---|---|---|---|---|---|
| Coronavirus Disease 2019 (COVID-19) | |||||||
| Retinal findings in patients with COVID-19: Results from the SERPICO-19 study | Alessandro Invernizzi et al. (2020), Italy [54] | Cross-sectional (SERPICO-19) | 23–82 years (n = 54 + 133; COVID-19 patients + unexposed subjects) | Digital Retinography System (DRS) fundus camera (CenterVue, Padua, Italy) | The investigation of the presence of retinal alterations in patients with COVID-19 and subjects unexposed to the virus by using fundus photographs. | Diameters of both, arteries, and veins were higher in COVID-19 patients compared to unexposed subjects. The diameter of veins was positively associated with COVID-19 both in severe and non-severe cases compared to unexposed subjects. Moreover, the diameter of the veins in COVID-19 patients negatively correlated with the time from symptom onset and positively correlated with disease severity. | COVID-19: ↑ CRAE; ↑ CRVE |
| Retinal vessels modifications in acute and post-COVID-19 | Alessandro Invernizzi et al. (2021), Italy [55] | Cross-sectional, longitudinal study (SERPICO-19) | 24–72 years (n = 32 + 53; COVID-19 patients + unexposed subjects) | Digital Retinography System (DRS) fundus camera (CenterVue, Padua, Italy) | Investigation (at baseline and 6 months later) of alterations of the retina and its vasculature in patients with COVID-19 within 30 days of onset of symptoms. | At baseline, arteriolar and venular diameters were significantly higher in COVID-19 patients compared to unexposed subjects. Both significantly decreased in COVID-19 patients at follow-up. Vessel diameter remained significantly higher in severe COVID-19 patients compared to unexposed subjects after 6 months. | COVID-19: ↑ CRAE; ↑ CRVE |
| Retinal Vessel Diameter Changes in COVID-19 Infected Patients | Nazife Aşıkgarip et al. (2021), Turkey [56] | Prospective study | 9–78 years, (n = 25 + 25; COVID-19 patients + healthy controls) | Optical coherence tomography (OCT) | To assess longitudinal changes of retinal vessel diameters measured in patients with COVID-19. | While the baseline diameters of the vessels in COVID-19 patients were increased compared to controls, their diameters decreased after remission in all quadrants in comparison to baseline measurements. | COVID-19: ↑ CRAE; ↑ CRVE |
| Change in retinal vessel diameter and choroidal thickness in patients with severe COVID-19: Change In Retinal Parameters In Patients With Severe COVID-19 | Gündoğan M et al. (2022), Turkey [57] | Prospective, cross-sectional study | 29–65 years (n = 30 + 30; COVID-19 patients + healthy controls) | Spectralis OCT+HRA with infrared reflectance (IR) images | To compare the differences in retinal vascular structure and choroidal thickness between the active disease and post-recovery periods in COVID-19 patients and healthy controls. | The study did not find changes in lumen diameter in either artery or vein. | - |
| Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation | T. Kuchler et al. (2023), Germany [58] | Observational prospective cohort study (“All Eyes on PCS”) | 41 COVID-19 patients (42.2 y ± 12.2) and 41 healthy controls (41.8 y ± 13.7) | Static Vessel Analyzer (IMEDOS Systems, Jena, Germany based on TRC-NW8 non-mydriatic retinal camera; Topcon, Tokyo, Japan) | Investigation of the endothelial function through evaluation of retinal microcirculation in patients with post-COVID-19 syndrome (PCS) compared to an age- and gender-matched healthy cohort. | Narrower central retinal artery equivalent (CRAE; 178.1 [167.5–190.2] vs. 189.1 [179.4–197.2], p = 0.01) and lower arteriolar–venular ratio (AVR; (0.84 [0.8–0.9] vs. 0.88 [0.8–0.9], p = 0.007) were observed. Additionally, significantly reduced CRAE (183.5 [177.4–197.0] vs. 174.0 [161.5–181.0], p = 0.03) and AVR (0.88 [0.82–0.91] vs. 0.82 [0.77–0.86], p = 0.02) in PCS patients with CFS were observed. | PCS: ↓ CRAE; ↓ AVR |
| A pilot study: Exploring the influence of COVID-19 on cardiovascular physiology and retinal microcirculation | A. Saloň et al. (2023), Slovenia [24] | Pilot, longitudinal study, as a part of a larger project | 35 COVID-19 patients (60 ± 10 years) | Retinal camera Optomed Aurora (Optomed Oy, Oulu, Finland) | Assessment of cardiovascular changes in patients post-COVID-19 hospital discharge, examining both microvascular and macrovascular parameters. Measurements were taken at two post-discharge time points: on the day of discharge or day 10 post-hospitalization and 60 days post-hospitalization. | A significantly narrower CRVE (from 240.94 μm, SD: 16.05, to 198.05 μm, SD: 17.36, p = 0.013) and trend of decreasing CRAE (from 138.87 μm, SD: 12.19, to 136.77 μm, SD: 13.19, p = 0.068) were recorded when two measurement time points have been compared. | COVID-19: ↑ CRAE; ↑ CRVE |
| Title | Study Details | Study Design | Target Group | Imaging Tool | Purpose | Summary of Findings | |
|---|---|---|---|---|---|---|---|
| HIV (Human Immunodeficiency Virus) | |||||||
| Early retinal vascular abnormalities in African-American cocaine users | Ivan Y-F Leung (2008), USA [59] | Population-based cross-sectional study | 29–45 years (n = 68, out of which 42 HIV-positive patients) | Digital fundus camera (Zeiss FF-series) | Examination of the potential association between cocaine use and early retinal vascular abnormalities. | No significant associations were observed between HIV infection and any retinal vascular parameters. | - |
| Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with disease-associated factors and mortality | Sapna Gangaputra et al. (2012), USA [60] | Longitudinal Study of the Ocular Complications of AIDS (LSOCA) | 38–48 years (n = 1250; HIV-positive patients) | Wide-angle fundus camera | Assessment of the associations between retinal vessel calibers, factors related to AIDS, and mortality. | Narrower retinal arterioles and venules were related to a history of highly active antiretroviral therapy (ART); and larger CRAE with lower CD4+ T-lymphocyte count. There was a 12% increase in mortality risk per quartile of decreasing AVR. | ↑ HIV ART: ↓ CRAE; ↓ CRVE, ↑ CRAE: ↓ CD4+, ↓ AVR: ↑ Mortality |
| Retinal vascular parameter variations in patients with human immunodeficiency virus | Petrina B. Tan et al. (2013), Singapore [61] | Case-control study (SEED program) | 26–64 years (n = 85 + 251; HIV-positive patients + healthy controls) | 45° retinal camera (Canon CR-DGi; Canon, Tokyo, Japan) with a digital camera back (10D SLR; Canon) | The study compares the retinal vascular parameters in patients with HIV infection with healthy controls and determines the relationship between these parameters and HIV-related blood biomarkers. | No direct differences in retinal vascular calibers were observed between the groups. Increased viral loads in HIV patients were associated with decreased retinal arteriolar caliber and decreased arteriolar-venular ratio. | ↑ viral load: ↓ CRAE and ↓ AVR |
| Retinal arterioles narrow with increasing duration of anti-retroviral therapy in HIV infection: a novel estimator of vascular risk in HIV? | Sophia Pathai et al. (2013), South Africa [62] | Case-control study | 35–48 years (n = 242 + 249; HIV-positive patients + healthy controls) | Fundus camera Canon CF-2 | Investigation of the relationship between retinal vessel calibers and clinical and demographic characteristics in HIV-infected individuals in South Africa. | Unadjusted arteriolar diameters tended to widen and unadjusted venular diameters tended to narrow in HIV patients compared with healthy controls. Age as a factor modified diameters of vessels; narrower diameters in HIV patients but not in healthy controls. In HIV patients, retinal arteriolar diameters narrowed with increasing duration of HIV ART, independently of age, and with an HIV viral load >10,000 copies/mL while on HIV ART. HIV-related venular changes were not detected. | HIV: ↑ CRAE and ↓ CRVE, ↑ HIV ART duration and ↑ HIV viral load: ↓ CRAE |
| Factors Affecting the Health of Retinal Vessels in Human Immunodeficiency Virus Patients Beginning Anti-Retroviral Therapy | Lukman Edwar et al. (2019), Indonesia [63] | Comprehensive, longitudinal study (JakCCANDO) | 19–48 years (n = 79 + 17; HIV-positive patients + healthy controls) | Nikon D70s (Tokyo, Japan) 6 megapixel camera | The study assesses the effects of HIV, ART, and cytomegalovirus (CMV) on the diameter of retinal arteries, as a non-invasive approach to measure vasculopathy in HIV patients beginning ART. | Patients with HIV had narrower retinal arteries and higher levels of CMV antibodies than healthy controls. The diameter of the retinal arteries decreased over twelve months of ART. Right arterial diameter correlated with CMV antibodies and left arterial diameter at 3rd month, correlated with carotid Intima-Media Thickness (cIMT). Smoking and alcohol consumption were the strongest predictors of retinal arterial diameter after adjustment for HIV RNA. While the diameters of patients who confirmed smoking were wider compared to non-smokers, those who confirmed alcohol consumption were smaller. | HIV: ↓ CRAE, ↑ HIV ART duration: ↓ CRAE |
| THE THICKNESSES OF CHOROID, MACULAR SEGMENTS, PERIPAPILLARY RETINAL NERVE FIBER LAYER, AND RETINAL VASCULAR CALIBER IN HIV-1-INFECTED PATIENTS WITHOUT INFECTIOUS RETINITIS | Cetin, Ebru N. et al. (2019), Turkey [64] | Cross-sectional study | 17–75 years (n = 45 + 47; HIV-positive patients + healthy controls) | Spectral-domain optical coherence tomography (SD-OCT) | To evaluate choroidal, macular, and peripapillary retinal nerve fiber layer thicknesses and retinal vascular caliber alterations in HIV-1–infected patients without opportunistic infections. | The differences in retinal vascular caliber were not significant between the groups. | - |
| Brief Report: Retinal Microvasculature and Immune Restoration Among South Eastern Asian Patients With HIV/AIDS Over a 9-Month Antiretroviral Therapy | Li, Ling-Jun et al. (2022), Singapore [65] | Prospective cohort study | 45.6 (SD 10.2) years (n = 100; HIV) | 45° retinal camera (Canon CR-DGi; Canon, Tokyo, Japan) with a digital camera back (10D SLR; Canon) | This study aims to investigate whether retinal vascular abnormalities, indicative of real-time immune dysfunction, correlate with immune restoration in 100 HIV/AIDS patients undergoing a 9-month ART regimen. | Narrower arteriolar caliber (per 10 μm decrease), and wider venular caliber (per 10 μm increase) in the retina assessed at baseline were significantly associated with 9-month reductions in CD4+ T-cell count by 52.97 cells/μL (P = 0.006) and 33.55 cells/μL (P = 0.01) accordingly. | ↓ CRAE and ↑ CRVE: ↓ CD4+ |
| Title | Study Details | Study Design | Target Group | Imaging Tool | Purpose | Summary of Findings |
|---|---|---|---|---|---|---|
| Other Viral Infections | ||||||
| Ocular manifestations of dengue fever in an East Indian epidemic | Harpreet K Kapoor et al. (2006), India [66] | Observational study | 13–65 years (n = 134; dengue fever) | - | To document the range of ocular manifestations observed in patients with dengue fever and to determine if there are any notable associations with specific laboratory parameters. | Two (1.5%) patients had dilatation and tortuosity of vessels as the only finding in both eyes. |
| Increased Retinal Vessel Tortuosity Associated with Crimean-Congo Hemorrhagic Fever in Children | Duygu Yalinbas et al. (2021), Turkey [67] | Prospective study | 12.4 ± 3.6 years (n = 24; Crimean-Congo Hemorrhagic Fever) | Slit-lamp biomicroscopy, and dilated fundus examination | Children diagnosed with Crimean–Congo Hemorrhagic Fever underwent a complete ophthalmologic examination. | The fundus examination showed, that two (8.3%), out of twenty-four children, were found with dilatation of the retinal vein. |
| Increased retinal venular calibre in acute infections | Cara Fitt et al. (2021), Australia [68] | Observational study | 65.7 ± 18.4 years (n = 43; infections) | Non-mydriatic retinal camera (Canon CR5-45, Tokyo) | To investigate the effect of acute infections (participants with infections and elevated CRP levels (>100 mg/L)) on retinal arteriolar and venular calibres, and to determine whether changes in calibres occur as infections resolve (before and after antibiotic treatment). Additionally, the study aims to explore the relationship between changes in retinal vessel calibre and inflammatory markers, particularly CRP levels. | The mean venular calibre (CRVE) of participants decreased from 240.9 ± 26.9 μm to 233.4 ± 23.5 μm (p = 0.0017). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saloň, A.; De Boever, P.; Goswami, N. Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments. Biomedicines 2024, 12, 1488. https://doi.org/10.3390/biomedicines12071488
Saloň A, De Boever P, Goswami N. Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments. Biomedicines. 2024; 12(7):1488. https://doi.org/10.3390/biomedicines12071488
Chicago/Turabian StyleSaloň, Adam, Patrick De Boever, and Nandu Goswami. 2024. "Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments" Biomedicines 12, no. 7: 1488. https://doi.org/10.3390/biomedicines12071488
APA StyleSaloň, A., De Boever, P., & Goswami, N. (2024). Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments. Biomedicines, 12(7), 1488. https://doi.org/10.3390/biomedicines12071488







