Recovery from Acute Kidney Injury and Long-Term Prognosis following Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Follow-Up and Outcome
2.3. Data Collection and Definitions
2.4. Study Strata and Groups
2.5. Statistical Analysis
3. Results
3.1. Study Population and Strata
3.2. Baseline Characteristics in Accordance with Status of Death
3.3. Baseline Characteristics in Accordance with Status of Death
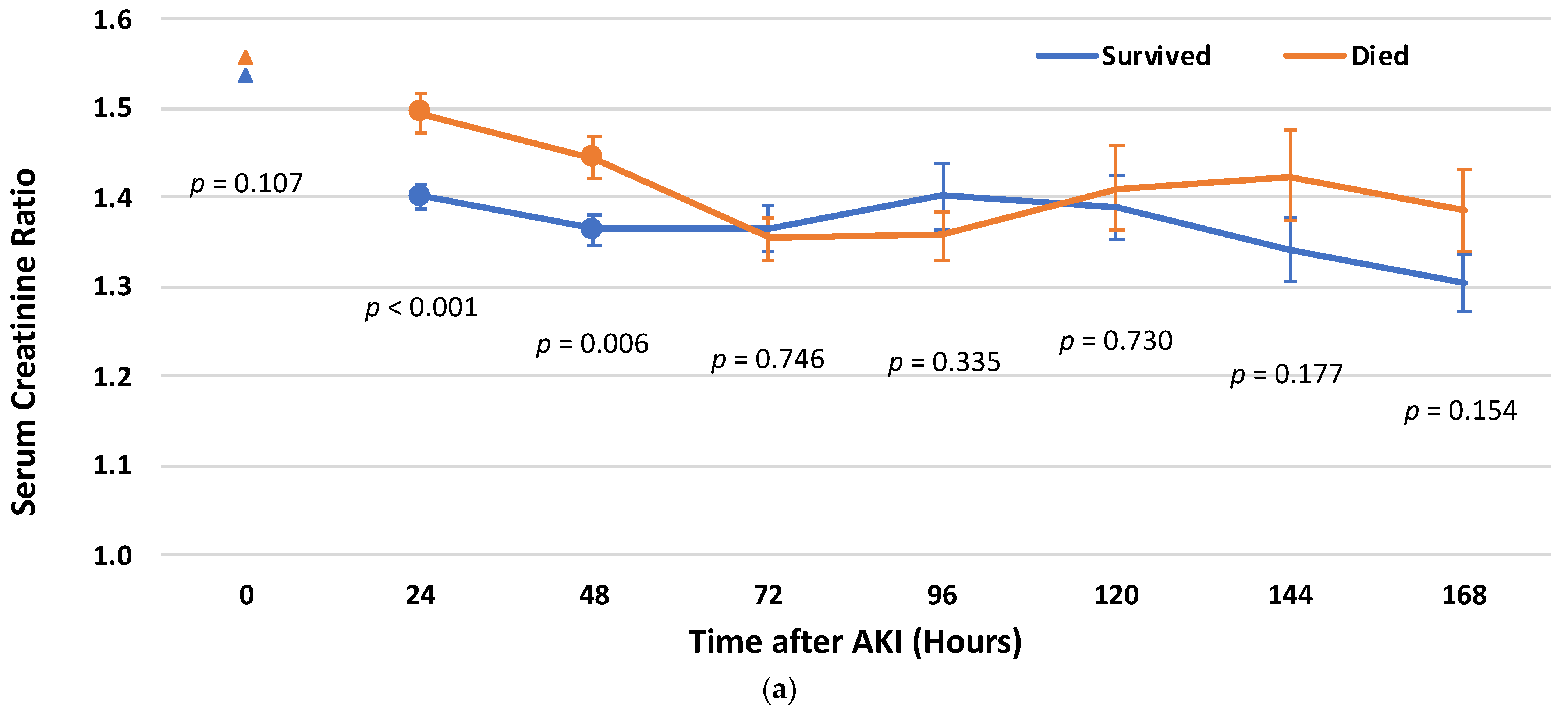
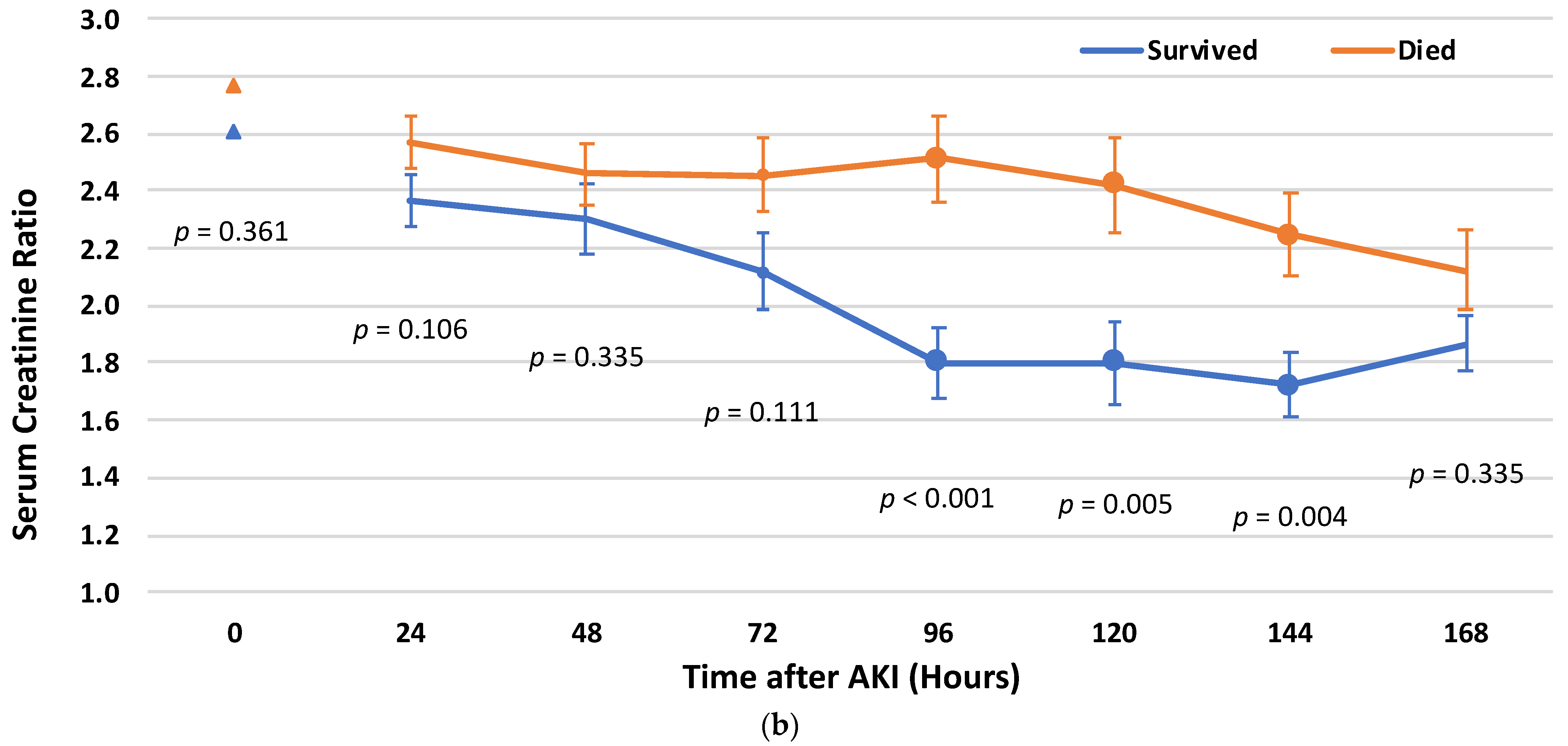
3.4. Cr Rate Cut-Off Points for Post-AKI Recovery
3.5. Survival Analysis
3.6. Post-AKI Recovery and the Risk for Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Bruetto, R.G.; Torres, U.S.; Otaviano, A.P.; Zanetta, D.M.T.; Burdmann, E.A. Incidence and Mortality of Acute Kidney Injury after Myocardial Infarction: A Comparison between KDIGO and RIFLE Criteria. PLoS ONE 2013, 8, e69998. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, E.; Chalikias, G.; Tziakas, D. The Incidence and the Prognostic Impact of Acute Kidney Injury in Acute Myocardial Infarction Patients: Current Preventive Strategies. Cardiovasc. Drugs Ther. 2018, 32, 3281–3298. [Google Scholar] [CrossRef]
- Skalsky, K.; Shiyovich, A.; Steinmetz, T.; Kornowski, R. Chronic Renal Failure and Cardiovascular Disease: A Comprehensive Appraisal. J. Clin. Med. 2022, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Muntner, P.; Chen, A.Y.; Alexander, K.P.; Roe, M.T.; Wiviott, S.D. Short-Term Outcomes of Acute Myocardial Infarction in Patients With Acute Kidney Injury: A Report From the National Cardiovascular Data Registry. Circulation 2012, 125, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Liu, B.C.; Zou, Y.; Pan, J.R.; Tao, Y.; Yang, M. Risk factors of acute kidney injury after acute myocardial infarction. Ren. Fail. 2016, 38, 1353–1358. [Google Scholar] [CrossRef]
- Chalikias, G.; Serif, L.; Kikas, P.; Thomaidis, A.; Stakos, D.; Makrygiannis, D.; Chatzikyriakou, S.; Papoulidis, N.; Voudris, V.; Lantzouraki, A.; et al. Long-term impact of acute kidney injury on prognosis in patients with acute myocardial infarction. Int. J. Cardiol. 2019, 283, 48–54. [Google Scholar] [CrossRef]
- Pickering, J.W.; Blunt, I.R.H.; Than, M.P. Acute Kidney Injury and mortality prognosis in Acute Coronary Syndrome patients: A meta-analysis. Nephrology 2018, 23, 237–246. [Google Scholar] [CrossRef]
- Parikh, C.R.; Coca, S.G.; Wang, Y.; Masoudi, F.A.; Krumholz, H.M. Long-term Prognosis of Acute Kidney Injury After Acute Myocardial Infarction. Arch. Intern. Med. 2008, 168, 987–995. [Google Scholar] [CrossRef]
- Itach, T.; Banai, A.; Paran, Y.; Zahler, D.; Merdler, I.; Eliashiv, D.; Banai, S.; Shacham, Y. Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients. J. Clin. Med. 2022, 11, 2169. [Google Scholar] [CrossRef]
- Goldberg, A.; Kogan, E.; Hammerman, H.; Markiewicz, W.; Aronson, D. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009, 76, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, R.; Shiyovich, A.; Gilutz, H.; Azab, A.N.; Plakht, Y. Short and long-term prognosis following acute myocardial infarction according to the country of origin. Soroka acute myocardial infarction II (SAMI II) project. Int. J. Cardiol. 2018, 259, 227–233. [Google Scholar] [CrossRef]
- Plakht, Y.; Abu Eid, A.; Gilutz, H.; Shiyovich, A. Trends of Cardiovascular Risk Factors in Patients With Acute Myocardial Infarction: Soroka Acute Myocardial Infarction II (SAMI II) Project. Angiology 2019, 70, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, K.; Levi, A.; Bental, T.; Vaknin-Assa, H.; Assali, A.; Steinmetz, T.; Kornowski, R.; Perl, L. The Definition of “Acute Kidney Injury” Following Percutaneous Coronary Intervention and Cardiovascular Outcomes. Am. J. Cardiol. 2021, 56, 39–43. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal recovery after acute kidney injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef]
- Ismail, Y.; Kasmikha, Z.; Green, H.L.; McCullough, P.A. Cardio-Renal Syndrome Type 1: Epidemiology, Pathophysiology, and Treatment. Semin. Nephrol. 2012, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions. JACC Cardiovasc. Interv. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after Acute Kidney Injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.y.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef] [PubMed]

| (a) | ||||
|---|---|---|---|---|
| Group | Survived | Died | Total | p |
| n | 433 | 402 | 835 | |
| Demographics | ||||
| Age, Years, Mean (SD) | 62.05 (10.51) | 71.95 (10.63) | 66.82 (11.66) | <0.001 |
| <65 | 273 (63) | 111 (27.6) | 384 (46.0) | <0.001 |
| 65–75 | 108 (24.9) | 129 (32.1) | 237 (28.4) | |
| ≥75 | 52 (12.0) | 162 (40.3) | 214 (25.6) | |
| Sex, Male | 367 (84.8) | 293 (72.9) | 660 (79.0) | <0.001 |
| Ethnicity, Arab/Other | 74 (17.1) | 72 (17.9) | 146 (17.5) | 0.755 |
| Cardiac diseases | ||||
| Cardiomegaly | 46 (10.6) | 65 (16.2) | 111 (13.3) | 0.018 |
| Supraventricular arrhythmias | 44 (10.2) | 113 (28.1) | 157 (18.8) | <0.001 |
| Congestive heart failure | 77 (17.8) | 149 (37.1) | 226 (27.1) | <0.001 |
| Pulmonary heart disease | 21 (4.8) | 53 (13.2) | 74 (8.9) | <0.001 |
| Chronic ischemic heart disease | 410 (94.7) | 345 (85.8) | 755 (90.4) | <0.001 |
| Previous myocardial infarction | 65 (15.0) | 120 (29.9) | 185 (22.2) | <0.001 |
| Previous percutaneous coronary intervention | 85 (19.6) | 101 (25.1) | 186 (22.3) | 0.057 |
| Previous coronary artery bypass graft | 22 (5.1) | 55 (13.7) | 77 (9.2) | <0.001 |
| Atrioventricular block | 19 (4.4) | 17 (4.2) | 36 (4.3) | 0.910 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 178 (41.1) | 230 (57.2) | 408 (48.9) | <0.001 |
| Dyslipidemia | 376 (86.8) | 326 (81.1) | 702 (84.1) | 0.023 |
| Hypertension | 267 (61.7) | 297 (73.9) | 564 (67.5) | <0.001 |
| Obesity | 112 (25.9) | 103 (25.6) | 215 (25.7) | 0.936 |
| Smoking | 207 (47.8) | 151 (37.6) | 358 (42.9) | 0.003 |
| Peripheral vascular disease | 39 (9.0) | 101 (25.1) | 140 (16.8) | <0.001 |
| Family history of ischemic heart disease | 64 (14.8) | 13 (3.2) | 77 (9.2) | <0.001 |
| Other disorders | ||||
| Chronic obstructive pulmonary disease | 17 (3.9) | 70 (17.4) | 87 (10.4) | <0.001 |
| Neurological disorders | 52 (12.0) | 93 (23.1) | 145 (17.4) | <0.001 |
| Malignancy | 9 (2.1) | 33 (8.2) | 42 (5.0) | <0.001 |
| Anemia | 265 (61.2) | 269 (66.9) | 534 (64.0) | 0.086 |
| Gastrointestinal bleeding | 14 (3.2) | 24 (6.0) | 38 (4.6) | 0.058 |
| Schizophrenia/Psychosis | 8 (1.8) | 15 (3.7) | 23 (2.8) | 0.097 |
| Alcohol/Drug addiction | 7 (1.6) | 16 (4.0) | 23 (2.8) | 0.037 |
| History of malignancy | 18 (4.2) | 25 (6.2) | 43 (5.1) | 0.178 |
| Administrative characteristics of the hospitalization | ||||
| Admitted/transposed to ICCU | 345 (79.7) | 244 (60.7) | 589 (70.5) | <0.001 |
| Length of hospital stay, days, Mean (SD) | 16.46 (10.04) | 18.55 (14.87) | 17.47 (12.64) | 0.017 |
| ≥7 | 375 (86.6) | 335 (83.3) | 710 (85.0) | 0.185 |
| Clinical characteristics of the hospitalization | ||||
| Type of AMI, STEMI | 234 (54.0) | 154 (38.3) | 388 (46.5) | <0.001 |
| Results of echocardiography | ||||
| Echocardiography performance | 365 (84.3) | 309 (76.9) | 674 (80.7) | 0.007 |
| Severe left ventricular dysfunction | 53 (14.5) | 76 (24.6) | 129 (19.1) | 0.001 |
| Left ventricular hypertrophy | 13 (3.6) | 22 (7.1) | 35 (5.2) | 0.038 |
| Mitral regurgitation | 17 (4.7) | 37 (12.0) | 54 (8.0) | <0.001 |
| Tricuspid regurgitation | 7 (1.9) | 22 (7.1) | 29 (4.3) | 0.001 |
| Pulmonary hypertension | 17 (4.7) | 52 (16.8) | 69 (10.2) | <0.001 |
| Results of angiography | ||||
| Angiography performance | 357 (82.4) | 251 (62.4) | 608 (72.8) | <0.001 |
| Measure of coronary artery disease | ||||
| No/non-significant | 4 (1.1) | 5 (2.0) | 9 (1.5) | 0.175 |
| One vessel | 48 (13.4) | 24 (9.6) | 72 (11.8) | |
| Two vessels | 73 (20.4) | 43 (17.1) | 116 (19.1) | |
| Three vessels/Left main artery | 232 (65.0) | 179 (71.3) | 411 (67.6) | |
| Type of treatment | ||||
| Noninvasive | 11 (2.5) | 110 (27.4) | 121 (14.5) | <0.001 |
| Percutaneous coronary intervention | 142 (32.8) | 140 (34.8) | 282 (33.8) | |
| Coronary artery bypass graft | 280 (64.7) | 152 (37.8) | 432 (51.7) | |
| Kidney function | ||||
| eGFR (first), Mean (SD) | 84.30 (20.00) | 80.80 (18.09) | 82.62 (19.17) | 0.008 |
| Creatinine at AKI, Mean (SD) | 1.20 (0.25) | 1.27 (0.25) | 1.23 (0.25) | <0.001 |
| (b) | ||||
| Group | Survived | Died | Total | p |
| n | 87 | 147 | 234 | |
| Demographics | ||||
| Age, Years, Mean (SD) | 62.33 (11.70) | 71.39 (11.39) | 68.02 (12.29) | <0.001 |
| <65 | 48 (55.2) | 38 (25.9) | 86 (36.8) | <0.001 |
| 65–75 | 28 (32.2) | 49 (33.3) | 77 (32.9) | |
| ≥75 | 11 (12.6) | 60 (40.8) | 71 (30.3) | |
| Sex, Male | 59 (67.8) | 77 (52.4) | 136 (58.1) | 0.021 |
| Ethnicity, Arab/Other | 12 (13.8) | 22 (15.0) | 34 (14.5) | 0.806 |
| Cardiac diseases | ||||
| Cardiomegaly | 8 (9.2) | 29 (19.7) | 37 (15.8) | 0.033 |
| Supraventricular arrhythmias | 16 (18.4) | 47 (32.0) | 63 (26.9) | 0.024 |
| Congestive heart failure | 17 (19.5) | 60 (40.8) | 77 (32.9) | 0.001 |
| Pulmonary heart disease | 6 (6.9) | 28 (19.0) | 34 (14.5) | 0.011 |
| Chronic ischemic heart disease | 81 (93.1) | 111 (75.5) | 192 (82.1) | 0.001 |
| Previous myocardial infarction | 15 (17.2) | 44 (29.9) | 59 (25.2) | 0.031 |
| Previous percutaneous coronary intervention | 16 (18.4) | 27 (18.4) | 43 (18.4) | 0.996 |
| Previous coronary artery bypass graft | 3 (3.4) | 20 (13.6) | 23 (9.8) | 0.012 |
| Atrioventricular block | 4 (4.6) | 8 (5.4) | 12 (5.1) | 0.777 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 37 (42.5) | 90 (61.2) | 127 (54.3) | 0.006 |
| Dyslipidemia | 71 (81.6) | 118 (80.3) | 189 (80.8) | 0.802 |
| Hypertension | 51 (58.6) | 108 (73.5) | 159 (67.9) | 0.019 |
| Obesity | 19 (21.8) | 39 (26.5) | 58 (24.8) | 0.422 |
| Smoking | 42 (48.3) | 48 (32.7) | 90 (38.5) | 0.018 |
| Peripheral vascular disease | 12 (13.8) | 39 (26.5) | 51 (21.8) | 0.023 |
| Family history of ischemic heart disease | 8 (9.2) | 6 (4.1) | 14 (6.0) | 0.111 |
| Other disorders | ||||
| Chronic obstructive pulmonary disease | 6 (6.9) | 31 (21.1) | 37 (15.8) | 0.004 |
| Neurological disorders | 14 (16.1) | 49 (33.3) | 63 (26.9) | 0.004 |
| Malignancy | 3 (3.4) | 8 (5.4) | 11 (4.7) | 0.486 |
| Anemia | 47 (54.0) | 113 (76.9) | 160 (68.4) | <0.001 |
| Gastrointestinal bleeding | 4 (4.6) | 9 (6.1) | 13 (5.6) | 0.772 |
| Schizophrenia/Psychosis | 1 (1.1) | 4 (2.7) | 5 (2.1) | 0.422 |
| Alcohol/Drug addiction | 1 (1.1) | 1 (0.7) | 2 (0.9) | 0.706 |
| History of malignancy | 3 (3.4) | 6 (4.1) | 9 (3.8) | 0.808 |
| Administrative characteristics of the hospitalization | ||||
| Admitted/transposed to ICCU | 76 (87.4) | 91 (61.9) | 167 (71.4) | <0.001 |
| Length of hospital stay, days, Mean (SD) | 19.37 (32.82) | 32.82 (28.18) | 27.82 (24.46) | <0.001 |
| ≥7 | 81 (93.1) | 139 (94.6) | 220 (94.0) | 0.650 |
| Clinical characteristics of the hospitalization | ||||
| Type of AMI, STEMI | 42 (48.3) | 52 (35.4) | 94 (40.2) | 0.052 |
| Results of echocardiography | ||||
| Echocardiography performance | 73 (83.9) | 112 (76.2) | 185 (79.1) | 0.161 |
| Severe left ventricular dysfunction | 13 (17.8) | 35 (31.3) | 48 (25.9) | 0.041 |
| Left ventricular hypertrophy | 4 (5.5) | 8 (7.1) | 12 (6.5) | 0.653 |
| Mitral regurgitation | 3 (4.1) | 8 (7.1) | 11 (5.9) | 0.394 |
| Tricuspid regurgitation | 2 (2.7) | 9 (8.0) | 11 (5.9) | 0.205 |
| Pulmonary hypertension | 4 (5.5) | 15 (13.4) | 19 (10.3) | 0.083 |
| Results of angiography | ||||
| Angiography performance | 74 (85.1) | 88 (59.9) | 162 (69.2) | <0.001 |
| Measure of coronary artery disease | ||||
| No/non-significant | 2 (2.7) | 4 (4.5) | 6 (3.7) | 0.214 |
| One vessel | 16 (21.6) | 10 (11.4) | 26 (16.0) | |
| Two vessels | 16 (21.6) | 17 (19.3) | 33 (20.4) | |
| Three vessels/Left main artery | 40 (54.1) | 57 (64.8) | 97 (59.9) | |
| Type of treatment | ||||
| Noninvasive | 3 (3.4) | 49 (33.3) | 52 (22.2) | <0.001 |
| Percutaneous coronary intervention | 30 (34.5) | 49 (33.3) | 79 (33.8) | |
| Coronary artery bypass graft | 54 (62.1) | 49 (33.3) | 103 (44.0) | |
| Kidney function | ||||
| eGFR (first), Mean (SD) | 113.34 (109.81) | 91.98 (44.88) | 99.92 (76.29) | 0.086 |
| Creatinine at AKI, Mean (SD) | 1.71 (1.00) | 1.82 (1.15) | 1.78 (1.10) | 0.434 |
| (a) | ||||
|---|---|---|---|---|
| Parameter | B (SE) | AdjHR | (95% CI) | p |
| Recovery group *: | ||||
| Rapid recovery | 1 (ref.) | |||
| No rapid recovery | 0.341 (0.132) | 1.407 | (1.086–1.824) | 0.010 |
| Missing values of serum Creatinine | −0.023 (0.184) | 0.978 | (0.681–1.403) | 0.903 |
| Age (years): ≥75 vs. <75 | 0.771 (0.113) | 2.162 | (1.731–2.700) | <0.001 |
| Supraventricular arrhythmias | 0.379 (0.116) | 1.461 | (1.163–1.836) | 0.001 |
| Congestive heart failure | 0.387 (0.111) | 1.473 | (1.186–1.831) | <0.001 |
| Previous myocardial infarction | 0.383 (0.116) | 1.467 | (1.169–1.840) | <0.001 |
| Previous coronary artery bypass graft | 0.376 (0.153) | 1.457 | (1.079–1.968) | 0.014 |
| Diabetes mellitus | 0.470 (0.108) | 1.600 | (1.296–1.976) | <0.001 |
| Chronic obstructive pulmonary disease | 0.938 (0.140) | 2.554 | (1.940–3.362) | <0.001 |
| Malignancy | 0.657 (0.190) | 1.929 | (1.330–2.797) | <0.001 |
| Schizophrenia/Psychosis | 0.746 (0.271) | 2.109 | (1.241–3.584) | 0.006 |
| Alcohol/Drug addiction | 1.084 (0.262) | 2.955 | (1.770–4.934) | <0.001 |
| Type of treatment: | ||||
| Type of AMI: NSTEMI vs. STEMI | 0.200 (0.109) | 1.221 | (0.986–1.513) | 0.067 |
| Noninvasive | 1 (ref.) | |||
| Percutaneous coronary intervention | −0.947 (0.138) | 0.338 | (0.296–0.509) | <0.001 |
| Coronary artery bypass graft | −1.326 (0.139) | 0.265 | (0.202–0.349) | <0.001 |
| (b) | ||||
| Parameter | B (SE) | AdjHR | (95% CI) | p |
| Recovery group *: | ||||
| Early recovery | 1 (ref.) | |||
| No early recovery | 0.555 (0.242) | 1.742 | (1.085–2.797) | 0.022 |
| Missing values of serum Creatinine | −0.022 (0.189) | 0.978 | (0.676–1.416) | 0.908 |
| Age (years): ≥75 vs. <75 | 0.969 (0.179) | 2.636 | (1.856–3.743) | <0.001 |
| Cardiomegaly | 1.137 (0.225) | 3.117 | (2.007–4.841) | <0.001 |
| Diabetes mellitus | 0.817 (0.190) | 2.263 | (1.560–3.282) | <0.001 |
| Neurological disorders | 0.472 (0.182) | 1.603 | (1.123–2.290) | 0.009 |
| Malignancy | 0.830 (0.375) | 2.294 | (1.099–4.788) | 0.027 |
| Type of treatment: | ||||
| Noninvasive | 1 (ref.) | |||
| Percutaneous coronary intervention | −1.039 (0.214) | 0.354 | (0.233–0.538) | <0.001 |
| Coronary artery bypass graft | −1.777 (0.226) | 0.169 | (0.109–0.263) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalsky, K.; Shiyovich, A.; Shechter, A.; Gilutz, H.; Plakht, Y. Recovery from Acute Kidney Injury and Long-Term Prognosis following Acute Myocardial Infarction. Biomedicines 2024, 12, 1490. https://doi.org/10.3390/biomedicines12071490
Skalsky K, Shiyovich A, Shechter A, Gilutz H, Plakht Y. Recovery from Acute Kidney Injury and Long-Term Prognosis following Acute Myocardial Infarction. Biomedicines. 2024; 12(7):1490. https://doi.org/10.3390/biomedicines12071490
Chicago/Turabian StyleSkalsky, Keren, Arthur Shiyovich, Alon Shechter, Harel Gilutz, and Ygal Plakht. 2024. "Recovery from Acute Kidney Injury and Long-Term Prognosis following Acute Myocardial Infarction" Biomedicines 12, no. 7: 1490. https://doi.org/10.3390/biomedicines12071490






