Antibody-Drug Conjugates to Promote Immune Surveillance: Lessons Learned from Breast Cancer
Abstract
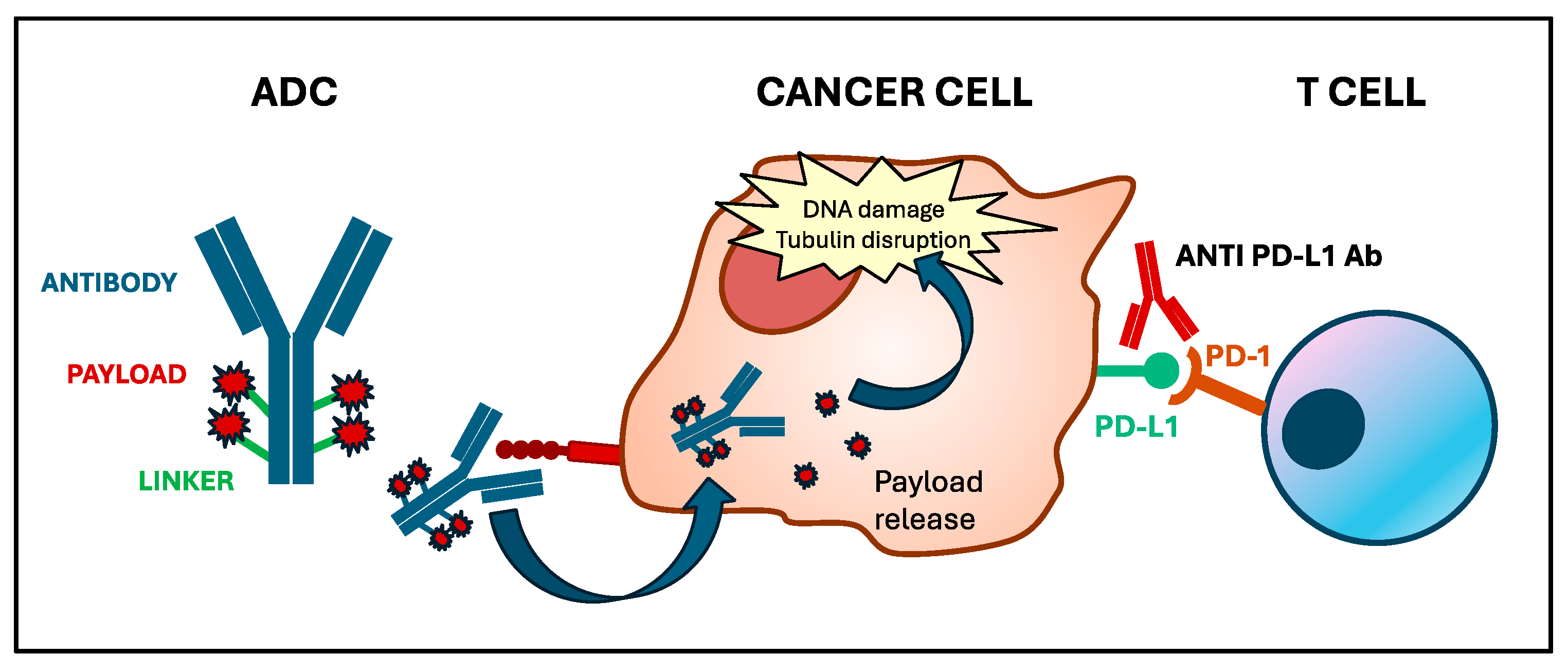
:1. Introduction
2. ADCs and Immunotherapy: Current Approvals for BC Patients
3. Preclinical Evidence: Immunogenicity of ADCs
4. Translational Evidence: Biomarkers of Response to ADCs Alone or in Combination with Immunotherapeutic Agents
4.1. Target Antigen Expression and Oncogenic Pathways
4.2. Immune Biomarkers in ADCs: Tumor-Infiltrating Lymphocytes and Neutrophil-to-Lymphocyte Ratio
4.3. Predictive Biomarkers for ADC-Immunotherapy Combinations
5. Clinical Evidence: Trials of ADCs and Immunotherapy Combinations
5.1. Evidence from Clinical Trials
| ADC | ICI | Trial Name | Phase | Study Design | Study Population (n° Enrolled) | Primary End Point | Efficacy Outcomes | Toxicity Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| T-DM1 | Atezolizumab | Hamilton et al. (Cohort 1B) | Ib | T-DM1 + Atezolizumab | mBC, HER2+ (6) | Safety and tolerability | ORR 35% | Any grade 100% G3–G4 50% sAE 33% | [24] |
| Hamilton et al. (Cohort 2C) | Ib | T-DM1 + Atezolizumab | mBC, HER2+ (14) | Safety and tolerability | ORR 35% | Any grade 100% G3–G4 71% sAE 57% | [24] | ||
| Hamilton et al. (Cohort 2B) | Ib | T-DM1 + Atezolizumab (Neodjuvant) | eBC, HER2+ (20) | Safety and tolerability | pCR 70% | Any grade 100% G3–G4 80% sAE 20% | [24] | ||
| KATE 2 | II | T-DM1 + Atezolizumab vs. T-DM1 + placebo | mBC, HER2+ (202) | PFS | PFS 8.2 vs. 6.8 months | sAE 19% G5 1 | [25] | ||
| IMpassion050 | III | T-DM1 + Atezolizumab vs. T-DM1 + placebo (post-neodjuvant phase) | eBC, HER2+ (454) | pCR (neoadjuvant phase) in ITT and PD-L1+ | NA | Any grade 96.6% G3–G4 25.9% sAE 8.6% | [48] | ||
| Pembrolizumab | Waks et al. | Ib | T-DM1 + Pembrolizumab | mBC, HER2+ (20) | Safety and tolerability | ORR 20% CBR 50% PFS 9.6 months DOR 10.1 months | Any grade 85% G3 20% sAE 10% | [23] | |
| T-DXd | Durvalumab | BEGONIA (Arm 6) | Ib/II | T-DXd + Durvalumab | mTNBC (56) | Safety and tolerability | ORR 57% PFS 12.6 months | Any grade 36% sAE 9% | [44] |
| Nivolumab | DS8201-A-U105 | Ib | T-DXd + Nivolumab | mBC, HER2+ (48) | ORR | ORR 65.6% ORR (HER2-low) 50% PFS 11.6 months PFS (HER2-low) 7 months | G3–G4 50% | [43] | |
| Dato-DXd | Durvalumab | BEGONIA (arm 7) | Ib/II | Dato-DXd + Durvalumab | mTNBC (47) | Safety and tolerability | ORR 79% | Any grade 36% sAE 15% | [44] |
| Ladiratuzumab vedotin | Pembrolizumab | Han et al. | Ib/II | Ladiratuzumab-vedotin + Pembrolizumab | a/mBC, HR−/HER2− (51) | Safety, tolerability, and activity | ORR 54% | Any grade 86% | [47] |
| ADC | ICI | Trial Name | NCT | Phase | Study Design | Study Population (Expected Enrollment) | Primary End Point |
|---|---|---|---|---|---|---|---|
| T-DM1 | Atezolizumab | KATE 3 | NCT04740918 | III | T-DM1 + Atezolizumab vs. T-DM1 + placebo | mBC, HER2+, PD-L1+ (96) | PFS, OS |
| ASTEFANIA | NCT04873362 | III | T-DM1 + Atezolizumab vs. T-DM1 + placebo | eBC, HER2+ without pCR after NAT (1700) | IDFS | ||
| T-DXd | Pembrolizumab | NA | NCT04042701 | Ib | T-DXd + Pembrolizumab | mBC, HER2+ (115 *) | DLT/MTD; ORR |
| Durvalumab | DESTINY-Breast07 | NCT04538742 | Ib/II | T-DXd + Durvalumab (Module 1) | mBC, HER2+ (244 in total) | Safety | |
| DESTINY-Breast08 | NCT04556773 | Ib | T-DXd + Durvalumab + Paclitaxel (Module 2) | HER2-low mBC (139 in total) | Safety | ||
| TRUDI | NCT05795101 | II | T-DXd + Durvalumab | eBC HER2-expressing, neoadjuvant (63) | pCR | ||
| SG | Pembrolizumab | SACI-IO | NCT04468061 | II | SG + Pembrolizumab vs. SG | mTNBC (110) | PFS |
| ASCENT-05 OptimICE-RD | NCT05633654 | III | SG + Pembrolizumab vs. Pembrolizumab ± Capecitabine | TNBC without pCR after NAT (1514) | IDFS | ||
| Atezolizumab | ASPRIA | NCT04434040 | II | SG + Atezolizumab | TNBC without pCR after NAT (40) | ctDNA clearence at 18 weeks | |
| Avelumab | InCITe | NCT03971409 | II | SG+ Avelumab (Arm B) | mTNBC (150 in total) | ORR | |
| Dato-DXd | Durvalumab | I-SPY 2 | NCT01042379 | II | Dato-DXd + Durvalumab (multiarm, adaptive trial) | eBC (5000 in total) | pCR |
| TROPION-03 | NCT05629585 | III | Dato-DXd + Durvalumab vs. Dato-DXd vs. Pembrolizumab and/or Capecitabine | TNBC without pCR after NAT (1075) | IDFS | ||
| TROPION-04 | NCT06112379 | III | Dato-DXd + Durvalumab vs. Pembrolizumab + CHT | TNBC; HR-low/HER2−, neoadjuvant (1728) | pCR; EFS | ||
| NA | NCT06103864 | III | Dato-DXd ± Durvalumab vs. CHT + Pembrolizumab | mTNBC; PD-L1+ (625) | PFS |
5.2. Toxicity Profile of ADC and Immunotherapy Combination
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-Drug Conjugates Come of Age in Oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antrás, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody-Drug Conjugates: In Search of Partners of Choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining Antibody-Drug Conjugates with Immunotherapy in Solid Tumors: Current Landscape and Future Perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.S.; Gokmen-Polar, Y.; Hoersch, S.; Xu, J.; Ruschoff, J.; Haas, S.d.; Verma, S. Role of Tumor Infiltrating Lymphocytes (TILs) in HER2+ Metastatic Breast Cancers (MBC) Treated with Trastuzumab Emtansine (T-DM1) or Lapatinib plus Capecitabine (L+C) (EMILIA Trial). J. Clin. Oncol. 2016, 34, 607. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results from DESTINY-Breast03, a Randomised, Open-Label, Phase 3 Trial. The Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Pavone, G.; Motta, L.; Martorana, F.; Motta, G.; Vigneri, P. A New Kid on the Block: Sacituzumab Govitecan for the Treatment of Breast Cancer and Other Solid Tumors. Molecules 2021, 26, 7294. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab Govitecan in Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-Line Atezolizumab plus Nab-Paclitaxel for Unresectable, Locally Advanced, or Metastatic Triple-Negative Breast Cancer: IMpassion130 Final Overall Survival Analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary Results from IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel with or without Atezolizumab for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Placebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic Complete Response (pCR) to Neoadjuvant Treatment with or without Atezolizumab in Triple-Negative, Early High-Risk and Locally Advanced Breast Cancer: NeoTRIP Michelangelo Randomized Study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; McArthur, H.L.; Schmid, P.; Cortés, J.; Harbeck, N.; Telli, M.L.; Cescon, D.W.; O’Shaughnessy, J.; Fasching, P.; Shao, Z.; et al. LBA21 KEYNOTE-756: Phase III Study of Neoadjuvant Pembrolizumab (Pembro) or Placebo (Pbo) + Chemotherapy (Chemo), Followed by Adjuvant Pembro or Pbo + Endocrine Therapy (ET) for Early-Stage High-Risk ER+/HER2− Breast Cancer. Ann. Oncol. 2023, 34, S1260–S1261. [Google Scholar] [CrossRef]
- Loi, S.; Curigliano, G.; Salgado, R.F.; Romero Diaz, R.I.; Delaloge, S.; Rojas, C.; Kok, M.; Saura Manich, C.; Harbeck, N.; Mittendorf, E.A.; et al. LBA20 A Randomized, Double-Blind Trial of Nivolumab (NIVO) vs. Placebo (PBO) with Neoadjuvant Chemotherapy (NACT) Followed by Adjuvant Endocrine Therapy (ET) ± NIVO in Patients (Pts) with High-Risk, ER+ HER2− Primary Breast Cancer (BC). Ann. Oncol. 2023, 34, S1259–S1260. [Google Scholar] [CrossRef]
- Spring, L.M.; Tolaney, S.M.; Fell, G.; Bossuyt, V.; Abelman, R.O.; Wu, B.; Maheswaran, S.; Trippa, L.; Comander, A.; Mulvey, T.; et al. Response-Guided Neoadjuvant Sacituzumab Govitecan for Localized Triple-Negative Breast Cancer: Results from the NeoSTAR Trial. Ann. Oncol. 2024, 35, 293–301. [Google Scholar] [CrossRef]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of Baseline Neutrophil-to-Lymphocyte Ratio for Progression-Free Survival of Patients with HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ai, L.; Jia, L.; Zhang, L.; Lei, B.; Zhang, Q. High Score of LDH plus dNLR Predicts Poor Survival in Patients with HER2-Positive Advanced Breast Cancer Treated with Trastuzumab Emtansine. BMC Cancer 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Keenan, T.E.; Li, T.; Tayob, N.; Wulf, G.M.; Richardson, E.T.; Attaya, V.; Anderson, L.; Mittendorf, E.A.; Overmoyer, B.; et al. Phase Ib Study of Pembrolizumab in Combination with Trastuzumab Emtansine for Metastatic HER2-Positive Breast Cancer. J. Immunother. Cancer 2022, 10, e005119. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.P.; Kaklamani, V.; Falkson, C.; Vidal, G.A.; Ward, P.J.; Patre, M.; Chui, S.Y.; Rotmensch, J.; Gupta, K.; Molinero, L.; et al. Impact of Anti-HER2 Treatments Combined with Atezolizumab on the Tumor Immune Microenvironment in Early or Metastatic Breast Cancer: Results from a Phase Ib Study. Clin. Breast Cancer 2021, 21, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Wysocki, P.J.; Ma, C.X.; Park, Y.H.; Fernandes, R.; Lord, S.; Baird, R.D.; Prady, C.; Jung, K.H.; Asselah, J.; et al. 379MO Datopotamab Deruxtecan (Dato-DXd) + Durvalumab (D) as First-Line (1L) Treatment for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer (a/mTNBC): Updated Results from BEGONIA, a Phase Ib/II Study. Ann. Oncol. 2023, 34, S337. [Google Scholar] [CrossRef]
- Torres, E.T.R.; Emens, L.A. Emerging Combination Immunotherapy Strategies for Breast Cancer: Dual Immune Checkpoint Modulation, Antibody-Drug Conjugates and Bispecific Antibodies. Breast Cancer Res. Treat. 2022, 191, 291–302. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Menzel, U.; Prummer, M.; Müller, P.; Buchi, M.; Kashyap, A.; Haessler, U.; Yermanos, A.; Gébleux, R.; Briendl, M.; et al. A Novel Anti-HER2 Anthracycline-Based Antibody-Drug Conjugate Induces Adaptive Anti-Tumor Immunity and Potentiates PD-1 Blockade in Breast Cancer. J. Immunother. Cancer 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ulrich, M.; Jonas, M.; Stone, I.J.; Linares, G.; Zhang, X.; Westendorf, L.; Benjamin, D.R.; Law, C.-L. Tumor-Associated Macrophages Can Contribute to Antitumor Activity through FcγR-Mediated Processing of Antibody-Drug Conjugates. Mol. Cancer Ther. 2017, 16, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, T.M.; Rossi, D.L.; Zalath, M.B.; Liu, D.; Arrojo, R.; Sharkey, R.M.; Chang, C.-H.; Goldenberg, D.M. Predictive Biomarkers for Sacituzumab Govitecan Efficacy in Trop-2-Expressing Triple-Negative Breast Cancer. Oncotarget 2020, 11, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 9674. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Lewis Phillips, G.D.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; De Haas, S.L.; Pegram, M.D. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wildiers, H.; Krop, I.E.; Smitt, M.; Yu, R.; Lysbet De Haas, S.; Gonzalez-Martin, A. Relationship between Tumor Biomarkers and Efficacy in TH3RESA, a Phase III Study of Trastuzumab Emtansine (T-DM1) vs. Treatment of Physician’s Choice in Previously Treated HER2-positive Advanced Breast Cancer. Int. J. Cancer 2016, 139, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab Deruxtecan in Metastatic Breast Cancer with Variable HER2 Expression: The Phase 2 DAISY Trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results from the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Raghav, K.; Masuishi, T.; Yamaguchi, K.; Nishina, T.; Elez, E.; Rodriguez, J.; Chau, I.; Di Bartolomeo, M.; Kawakami, H.; et al. 386O Exploratory Biomarker Analysis of DESTINY-CRC01, a Phase II, Multicenter, Open-Label Study of Trastuzumab Deruxtecan (T-DXd, DS-8201) in Patients (Pts) with HER2-Expressing Metastatic Colorectal Cancer (mCRC). Ann. Oncol. 2021, 32, S532. [Google Scholar] [CrossRef]
- Bardia, A.; Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Kalinsky, K.; Cortés, J.; Shaughnessy, J.O.; et al. Final Results from the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J. Clin. Oncol. 2024, 42, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Abstract GS1-11: Sacituzumab Govitecan (SG) vs Treatment of Physician’s Choice (TPC): Efficacy by Trop-2 Expression in the TROPiCS-02 Study of Patients (Pts) with HR+/HER2− Metastatic Breast Cancer (mBC). Cancer Res. 2023, 83 (Suppl. S5), GS1-11. [Google Scholar] [CrossRef]
- Bardia, A.; Rugo, H.S.; Cortés, J.; Tolaney, S.M.; Schmid, P.; Motwani, M.; Yoon, O.K.; Boice, J.; Zhuo, L.; Marmé, F. Trop-2 mRNA Expression and Association with Clinical Outcomes with Sacituzumab Govitecan (SG) in Patients with HR+/HER2− Metastatic Breast Cancer (mBC): Biomarker Results from the Phase 3 TROPiCS-02 Study. J. Clin. Oncol. 2023, 41, 1082. [Google Scholar] [CrossRef]
- Loi, S.; Schneeweiss, A.; Song, E.; Harries, M.; De Laurentiis, M.; Li, Y.; Wiese, C.; Poppe, R.; Emens, L.A. 329TiP KATE3: A Phase III Study of Trastuzumab Emtansine (T-DM1) in Combination with Atezolizumab or Placebo in Patients with Previously Treated HER2-Positive and PD-L1–Positive Locally Advanced or Metastatic Breast Cancer. Ann. Oncol. 2021, 32, S509. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Bachelot, T.; Bianchini, G.; Harbeck, N.; Loi, S.; Park, Y.H.; Prat, A.; Gilham, L.; Boulet, T.; Gochitashvili, N.; et al. ASTEFANIA: Adjuvant Ado-Trastuzumab Emtansine and Atezolizumab for High-Risk, HER2-Positive Breast Cancer. Future Oncol. 2022, 18, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.P.; Shapiro, C.L.; Boni, V.; Martin Jimenez, M.; Del Conte, G.; Cortés, J.; Agrawal, L.; Arkenau, H.-T.; Tan, A.R.; Debruyne, P.R.; et al. 162O Primary Analysis from DS8201-A-U105: A 2-Part, Open Label, Phase Ib Trial Assessing Trastuzumab Deruxtecan (T-DXd) with Nivolumab (Nivo) in Patients (Pts) with HER2-Expressing Advanced Breast Cancer. Ann. Oncol. 2022, 33, S196. [Google Scholar] [CrossRef]
- Schmid, P.; Wysocki, P.; Park, Y.H.; Jassem, J.; Jung, K.H.; Lord, S.; Huisden, R.; Stewart, R.; Vuković, P.; Nunes, A.T.; et al. Abstract PD11-08: PD11-08 Trastuzumab Deruxtecan (T-DXd) + Durvalumab (D) as First-Line (1L) Treatment for Unresectable Locally Advanced/Metastatic Hormone Receptor-Negative (HR−), HER2-Low Breast Cancer: Updated Results from BEGONIA, a Phase 1b/2 Study. Cancer Res. 2023, 83, PD11-08. [Google Scholar] [CrossRef]
- Schmid, P.; Wysocki, P.; Ma, C.; Park, Y.H.; Fernandes, R.; Lord, S.; Baird, R.D.; Prady, C.; Jung, K.H.; Asselah, J.; et al. Abstract PD11-09: PD11-09 Datopotamab Deruxtecan (Dato-DXd) + Durvalumab (D) as First-Line (1L) Treatment for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer (a/mTNBC): Updated Results from BEGONIA, a Phase 1b/2 Study. Cancer Res. 2023, 83 (Suppl. S5), PD11-09. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Keenan, T.E.; Li, T.; Lange, P.; Callahan, C.; Guerriero, J.; Tayob, N.; Anderson, L.; Yam, C.; Daniel, B.R.; et al. Saci-IO TNBC: Randomized Phase II Trial of Sacituzumab Govitecan (SG) +/− Pembrolizumab in PD-L1– Metastatic Triple-Negative Breast Cancer (mTNBC). J. Clin. Oncol. 2021, 39, TPS1106. [Google Scholar] [CrossRef]
- Han, H.; Diab, S.; Alemany, C.; Basho, R.; Brown-Glaberman, U.; Meisel, J.; Pluard, T.; Cortes, J.; Dillon, P.; Ettl, J.; et al. Abstract PD1-06: Open Label Phase 1b/2 Study of Ladiratuzumab Vedotin in Combination with Pembrolizumab for First-Line Treatment of Patients with Unresectable Locally-Advanced or Metastatic Triple-Negative Breast Cancer. Cancer Res. 2020, 80, PD1-06. [Google Scholar] [CrossRef]
- Huober, J.; Barrios, C.H.; Niikura, N.; Jarząb, M.; Chang, Y.-C.; Huggins-Puhalla, S.L.; Pedrini, J.; Zhukova, L.; Graupner, V.; Eiger, D.; et al. Atezolizumab with Neoadjuvant Anti–Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J. Clin. Oncol. 2022, 40, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical Toxicity of Antibody Drug Conjugates: A Meta-Analysis of Payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Im, S.A.; Armstrong, A.; Park, Y.H.; Chung, W.P.; Nowecki, Z.; Lord, S.; Wysocki, P.J.; Lu, Y.S.; Dry, H.; et al. BEGONIA: Phase 1b/2 Study of Durvalumab (D) Combinations in Locally Advanced/Metastatic Triple-Negative Breast Cancer (TNBC)—Initial Results from Arm 1, D+paclitaxel (P), and Arm 6, D+trastuzumab Deruxtecan (T-DXd). J. Clin. Oncol. 2021, 39, 1023. [Google Scholar] [CrossRef]
- Müller, P.; Martin, K.; Theurich, S.; Schreiner, J.; Savic, S.; Terszowski, G.; Lardinois, D.; Heinzelmann-Schwarz, V.A.; Schlaak, M.; Kvasnicka, H.-M.; et al. Microtubule-Depolymerizing Agents Used in Antibody-Drug Conjugates Induce Antitumor Immunity by Stimulation of Dendritic Cells. Cancer Immunol. Res. 2014, 2, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Genta, S.; Coburn, B.; Cescon, D.W.; Spreafico, A. Patient-Derived Cancer Models: Valuable Platforms for Anticancer Drug Testing. Front. Oncol. 2022, 12, 976065. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Chaby, G.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Moreira, A.; et al. Impact of the Corticosteroid Indication and Administration Route on Overall Survival and the Tumor Response after Immune Checkpoint Inhibitor Initiation. Ther. Adv. Med. Oncol. 2021, 13, 175883592199665. [Google Scholar] [CrossRef] [PubMed]


| Biomarker | Source | Treatment | Setting | Results | Refs. | |
|---|---|---|---|---|---|---|
| ADC single agent | TILs | Tissue | T-DM1 | 95 patients HER2+ mBC |
| [4] |
| TILs | Tissue | SG | 50 patients eTNBC |
| [20] | |
| NLR | Blood | T-DM1 | 53 patients HER2+ mBC |
| [21] | |
| NLR | Blood | T-DM1 | 51 patients HER2+ mBC |
| [22] | |
| ADC-ICI combos | PD-L1 and TILs | Tissue | T-DM1 + Pembrolizumab | 20 patients HER2+ mBC |
| [23] |
| PD-L1, TILs and TCR-Seq | Tissue | T-DM1 + Atezolizumab | 73 patients HER2+ early/mBC |
| [24] | |
| PD-L1, TILs and CD8 | Tissue | T-DM1 + Atezolizumab | 330 patients HER2+ mBC |
| [25] | |
| PD-L1 | Tissue | Dato-DXd + Durvalumab | 62 patients mTNBC |
| [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nucera, S.; Conti, C.; Martorana, F.; Wilson, B.; Genta, S. Antibody-Drug Conjugates to Promote Immune Surveillance: Lessons Learned from Breast Cancer. Biomedicines 2024, 12, 1491. https://doi.org/10.3390/biomedicines12071491
Nucera S, Conti C, Martorana F, Wilson B, Genta S. Antibody-Drug Conjugates to Promote Immune Surveillance: Lessons Learned from Breast Cancer. Biomedicines. 2024; 12(7):1491. https://doi.org/10.3390/biomedicines12071491
Chicago/Turabian StyleNucera, Sabrina, Chiara Conti, Federica Martorana, Brooke Wilson, and Sofia Genta. 2024. "Antibody-Drug Conjugates to Promote Immune Surveillance: Lessons Learned from Breast Cancer" Biomedicines 12, no. 7: 1491. https://doi.org/10.3390/biomedicines12071491






