Newcastle Disease Virus Virotherapy: Unveiling Oncolytic Efficacy and Immunomodulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Virus Titration in SL-27 Cells
2.3. Preparation of Tumor Cell Lysate
2.4. In Silico Oncolytic Activity of NDV on HCT-116 and HT-29 Cells
2.4.1. MTT Assay
2.4.2. Cell Apoptosis Evaluation Using an Annexin V Kit
2.5. In Vivo Protocol
2.5.1. Mice
2.5.2. Ethical Approval
2.5.3. Experimental Design
Lethal Toxic Dose-50 (LTD50)
Animal Inoculation
- Experiment 1: Assessment of Oncolytic Activity
- Experiment 2: Assessment of Immunomodulation
2.6. Statistical Analysis
3. Results
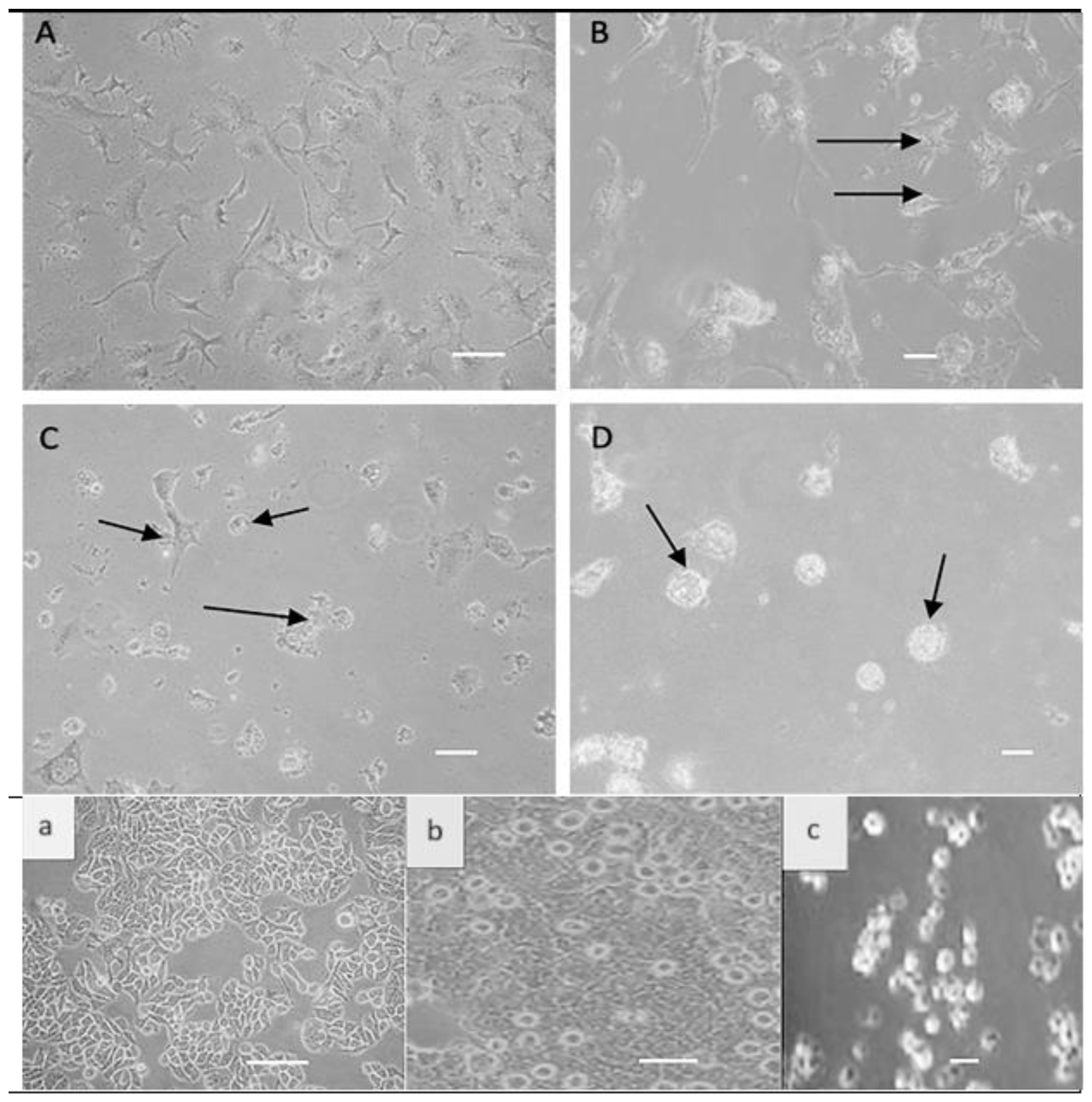
3.1. Direct Effect of NDV on Colon Cancer Cells
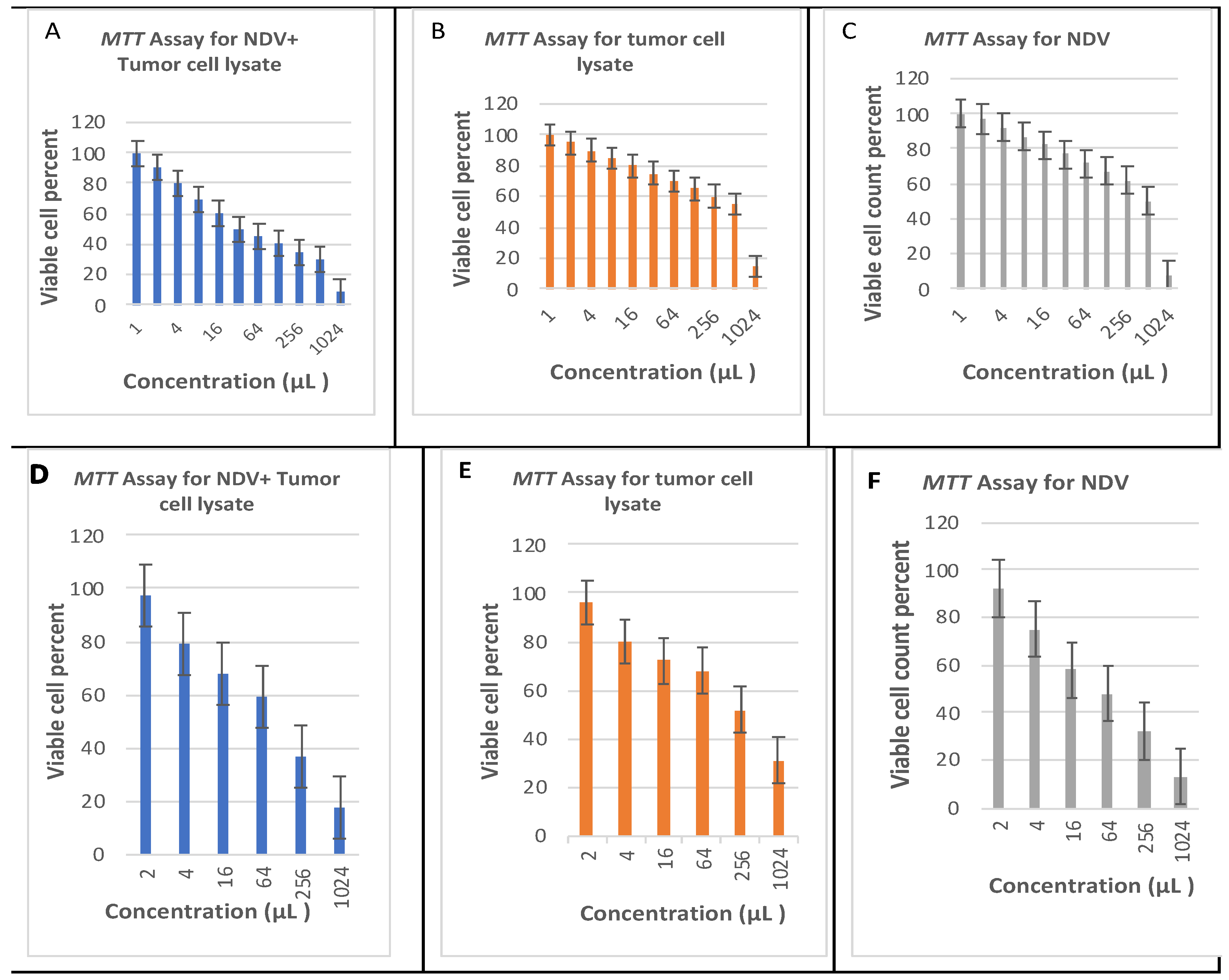
3.2. Estimated Oncolytic Activity of NDV by MTT Assay
3.3. Oncolytic NDV Induces Apoptosis in Colon Cancer Cells
3.4. In Vivo Mice Model Assessment
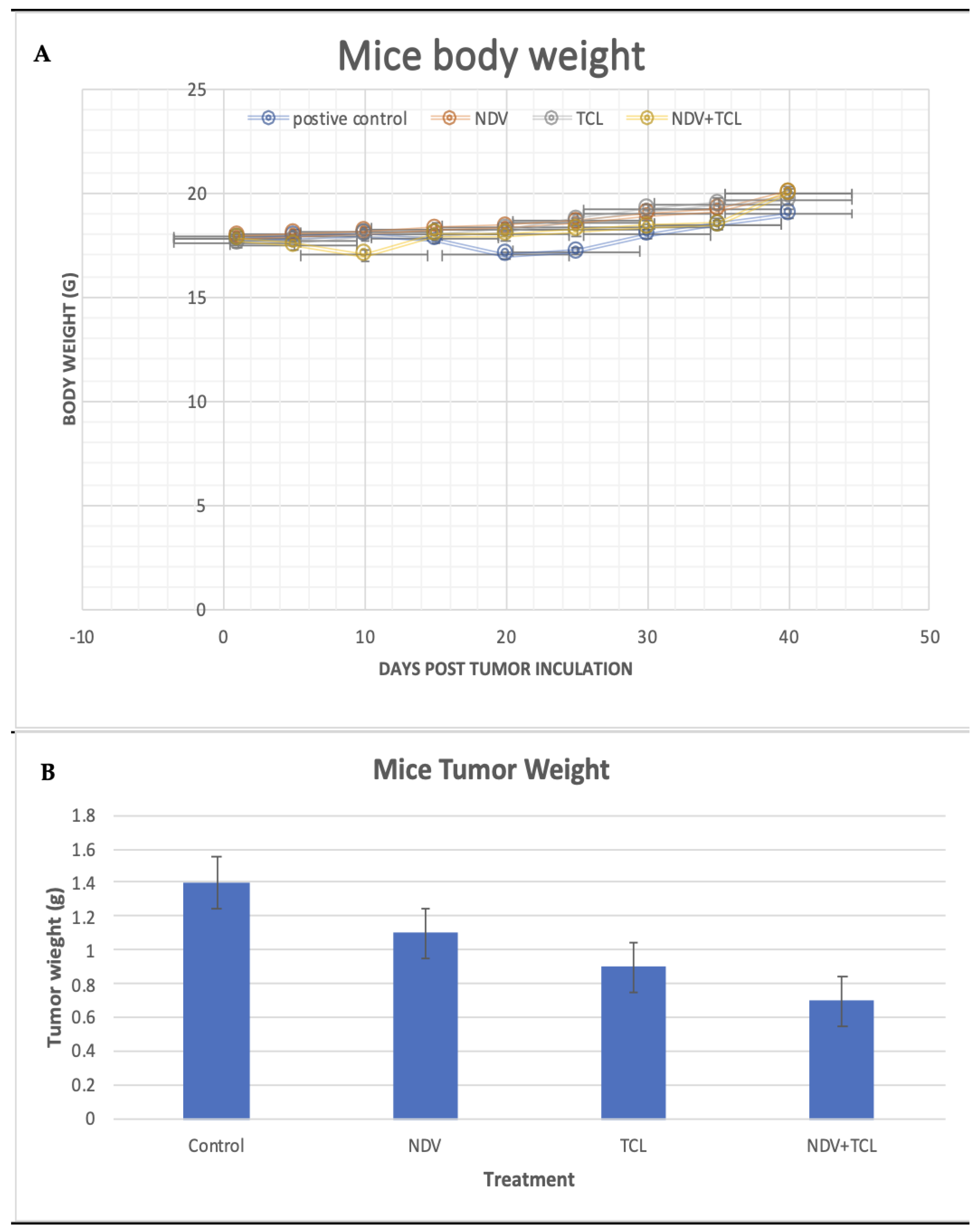
3.4.1. Tumor Induction and Treatment Effect on Body Weight, Tumor Size, and Volume
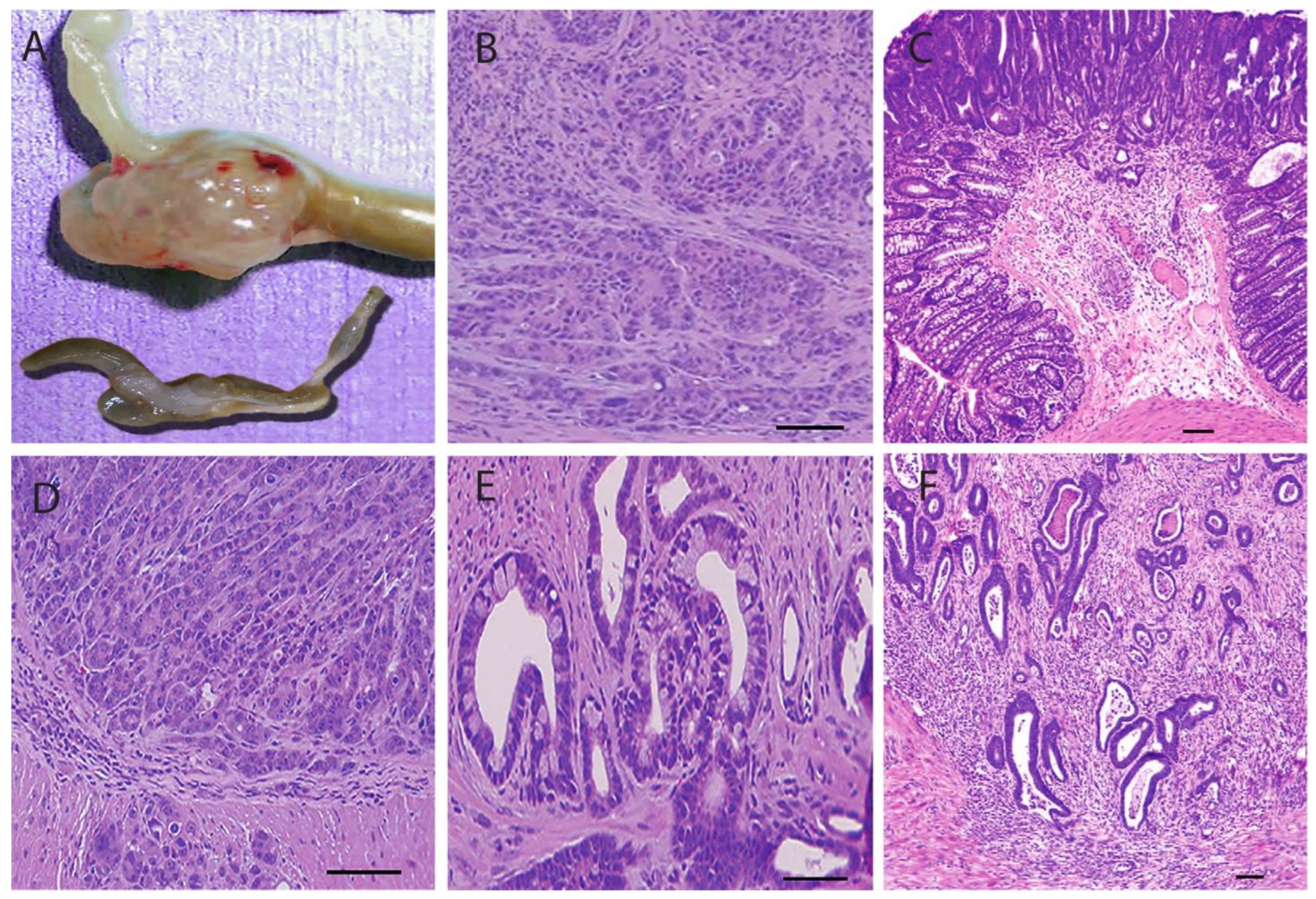
3.4.2. Pathological Sections
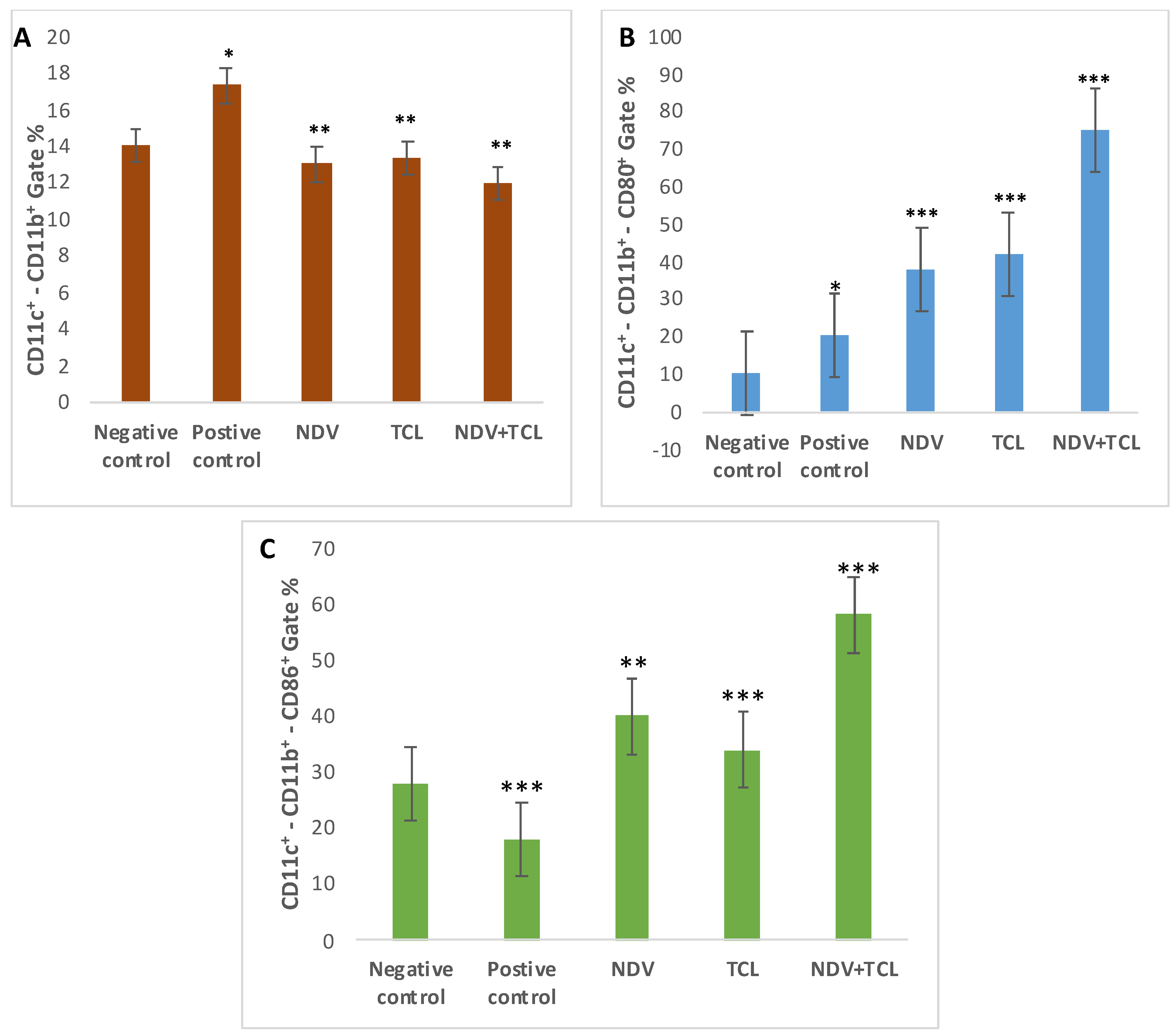
3.4.3. Assessment of Treatment on Tumor-Induced Mice Using Flow Cytometry
3.4.4. ELISA Assessment of Serum and Spleen Tissue Homogenate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breitbach, C.J.; Lichty, B.D.; Bell, J.C. Oncolytic Viruses: Therapeutics with an Identity Crisis. EBioMedicine 2016, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Hazini, A.; Seymour, L.W. Tackling HLA Deficiencies Head on with Oncolytic Viruses. Cancers 2021, 13, 719. [Google Scholar] [CrossRef]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Microbiol. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Microbiol. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Pikor, L.A.; Bell, J.C.; Diallo, J.S. Oncolytic Viruses: Exploiting Cancer’s Deal with the Devil. Trends Cancer 2015, 1, 266–277. [Google Scholar] [CrossRef]
- Russell, L.; Peng, K.W.; Russell, S.J.; Diaz, R.M. Oncolytic Viruses: Priming Time for Cancer Immunotherapy. BioDrugs 2019, 33, 485–501. [Google Scholar] [CrossRef]
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Woller, N.; Gürlevik, E.; Ureche, C.I.; Schumacher, A.; Kühnel, F. Oncolytic viruses as anticancer vaccines. Front. Oncol. 2014, 4, 188. [Google Scholar] [CrossRef]
- Fábián, Z.; Szeberenyi, C.J.; Csatary, L.K. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J. Virol. 2007, 81, 2817–2830. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Zakay-Rones, J.M.; Gomori, E.; Linetsky, L.; Rasooly, E.; Greenbaum, S.; Rozenman-Yair, A.; Panet, E.; Libson, C.S.; Irving, E.; et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lorence, R.M.; Rood, P.A.; Kelley, K.W. Newcastle disease virus as an antineoplastic agent: Induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J. Natl. Cancer Inst. 1988, 80, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Sinkovics, J.G.; Horvath, J.C. Newcastle disease virus (NDV): Brief history of its oncolytic strains. J. Clin. Virol. 2000, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef]
- Yaacov, B.; Eliahoo, E.; Lazar, I.; Ben-Shlomo, M.; Greenbaum, I.; Panet, A.; Zakay-Rones, Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008, 15, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Fournier, P. Newcastle disease virus: A promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol. Biol. 2009, 542, 565–605. [Google Scholar] [PubMed]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, S.; Wang, T.; Li, Y.; Jiang, K.; Lin, G.; Ma, Y.; Barr, M.P.; Song, F.; Zhang, G.; et al. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am. J. Cancer Res. 2015, 5, 3612–3623. [Google Scholar] [PubMed]
- Ye, T.; Jiang, K.; Wei, L.; Barr, M.P.; Xu, Q.; Zhang, G.; Ding, C.; Meng, S.; Piao, H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am. J. Cancer Res. 2018, 8, 1514–1527. [Google Scholar]
- Shao, X.; Wang, X.; Guo, X.; Jiang, K.; Ye, T.; Chen, J.; Fang, J.; Gu, L.; Wang, S.; Zhang, G.; et al. STAT3 Contributes to Oncolytic Newcastle Disease Virus-Induced Immunogenic Cell Death in Melanoma Cells. Front. Oncol. 2019, 9, 436. [Google Scholar] [CrossRef]
- Beutler, B.; Cerami, A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 1986, 320, 584–588. [Google Scholar] [CrossRef] [PubMed]
- von Hoegen, P.; Zawatzky, R.; Schirrmacher, V. Modification of tumor cells by a low dose of Newcastle disease virus. III. Potentiation of tumor-specific cytolytic T cell activity via induction of interferon-alpha/beta. Cell Immunol. 1990, 126, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.; von Hoegen, P.; Schirrmacher, V. Modification of tumor cells by a low dose of Newcastle disease virus. II. Augmented tumor-specific T cell response as a result of CD4+ and CD8+ immune T cell cooperation. Cancer Immunol. Immunother. 1989, 28, 22–28. [Google Scholar] [CrossRef]
- Earl, P.L.; Cooper, N.; Wyatt, L.S.; Moss, B.; Carroll, M.W. Preparation of cell cultures and vaccinia virus stocks. Curr. Protoc. Protein Sci. 2001, Chapter 5, Unit5.12. [Google Scholar]
- Alaniz, L.; Rizzo, M.M.; Mazzolini, G. Pulsing dendritic cells with whole tumor cell lysates. Methods Mol. Biol. 2014, 1139, 27–31. [Google Scholar]
- Céspedes, M.V.; Espina, C.; García-Cabezas, M.A.; Trias, M.; Boluda, A.; Gómez del Pulgar, M.T.; Sancho, F.J.; Nistal, M.; Lacal, J.C.; Mangues, R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am. J. Pathol. 2007, 170, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Gurley, K.E.; Moser, R.D.; Kemp, C.J. Induction of Colon Cancer in Mice with 1,2-Dimethylhydrazine. Cold Spring Harb. Protoc. 2015, 2015, pdb.prot077453. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Gonçalves, E.; Mendes, B.A.L.; Silva-Reis, R.; Faustino-Rocha, A.I.; Gama, A.; Oliveira, P.A. Animal Models of Colorectal Cancer: From Spontaneous to Genetically Engineered Models and Their Applications. Vet. Sci. 2021, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiao, X.; Wang, J.; Yang, J.; Yang, C.; Qiao, Y.; Guan, Y.; Wen, A.; Jiang, L. Amelioration of DMH-induced colon cancer by eupafolin through the reprogramming of apoptosis-associated p53/Bcl2/Bax signaling in rats. Eur. J. Inflamm. 2022, 20, 20587392211069771. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Han, D.; Tang, B.; Ma, J. Delivery and Biosafety of Oncolytic Virotherapy. Front. Oncol. 2020, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Syed Najmuddin, S.U.F.; Amin, Z.M.; Tan, S.W.; Yeap, S.K.; Kalyanasundram, J.; Veerakumarasivam, A.; Chan, S.C.; Chia, S.L.; Yusoff, K.; Alitheen, N.B. Oncolytic effects of the recombinant Newcastle disease virus, rAF-IL12, against colon cancer cells in vitro and in tumor-challenged NCr-Foxn1nu nude mice. PeerJ 2020, 8, e9761. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Cao, Y.; Su, H.; Liu, T.; Tian, L.; Zhang, Y.; Yang, J.; Xiao, W.; Li, D. Newcastle disease virus expressing an angiogenic inhibitor exerts an enhanced therapeutic efficacy in colon cancer model. PLoS ONE 2022, 17, e0264896. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jadhav, A.C.; Xu, J.; Harris, A.L.; Nair, V.; Huang, W.E. Metabolic Reprogramming in Colon Cancer Cells Persistently Infected with Newcastle Disease Virus. Cancers 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Najmuddin, S.; Amin, Z.M.; Tan, S.W.; Yeap, S.K.; Kalyanasundram, J.; Ani, M.A.C.; Veerakumarasivam, A.; Chan, S.C.; Chia, S.L.; Yusoff, K.; et al. Cytotoxicity study of the interleukin-12-expressing recombinant Newcastle disease virus strain, rAF-IL12, towards CT26 colon cancer cells in vitro and in vivo. Cancer Cell Int. 2020, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, S.; Yang, S.; Qiu, X.; Meng, C.; Tan, L.; Song, C.; Liao, Y.; Liu, W.; Sun, Y.; et al. Newcastle Disease virus infection activates PI3K/Akt/mTOR and p38 MAPK/Mnk1 pathways to benefit viral mRNA translation via interaction of the viral NP protein and host eIF4E. PLoS Pathog. 2020, 16, e1008610. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tian, W.Y.; Huang, J.J.; Gao, L.X.; Fan, X.H. MicroRNA-204 plays a role as a tumor suppressor in Newcastle disease virus-induced oncolysis in lung cancer A549 cells. Oncol. Lett. 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Al-Ziaydi, A.G.; Al-Shammari, A.M.; Hamzah, M.I.; Kadhim, H.S.; Jabir, M.S. Newcastle disease virus suppress glycolysis pathway and induce breast cancer cells death. Virusdisease 2020, 31, 341–348. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Y.; Hong, X.; Liu, X.; Su, X.; Li, S.; Dong, X.; Zhao, G.; Li, Y. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci. Rep. 2018, 8, 11470. [Google Scholar] [CrossRef]
- Ch’ng, W.C.; Stanbridge, E.J.; Yusoff, K.; Shafee, N. The oncolytic activity of Newcastle disease virus in clear cell renal carcinoma cells in normoxic and hypoxic conditions: The interplay between von Hippel-Lindau and interferon-β signaling. J. Interf. Cytokine Res. 2013, 33, 346–354. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Gu, L.; Jiang, K.; Wang, S.; Chen, J.; Fang, J.; Guo, X.; Yuan, M.; Shi, J.; et al. Targeting STAT3 enhances NDV-induced immunogenic cell death in prostate cancer cells. J. Cell Mol. Med. 2020, 24, 4286–4297. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.E.; Meulen, V.T.; Henle, W. Studies on persistent infections of tissue culture. VI. Reversible changes in Newcastle disease virus populations as a result of passage in L cells or chick embryos. J. Virol. 1967, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 2009, 559, 19–32. [Google Scholar] [PubMed]
- Schirrmacher, V. Molecular Mechanisms of Anti-Neoplastic and Immune Stimulatory Properties of Oncolytic Newcastle Disease Virus. Biomedicines 2022, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; He, J.; Zhong, L.; Zhao, Y. Advances in the Study of Antitumour Immunotherapy for Newcastle Disease Virus. Int. J. Med. Sci. 2021, 18, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Peckert-Maier, K.; Royzman, D.; Langguth, P.; Marosan, A.; Strack, A.; Sadeghi Shermeh, A.; Steinkasserer, A.; Zinser, E.; Wild, A.B. Tilting the Balance: Therapeutic Prospects of CD83 as a Checkpoint Molecule Controlling Resolution of Inflammation. Int. J. Mol. Sci. 2022, 23, 732. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; Fox, T.A.; Booth, C.; Pesenacker, A.M.; Halliday, N.; et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat. Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef]
- Benvenuti, F.; Lagaudrière-Gesbert, C.; Grandjean, I.; Jancic, C.; Hivroz, C.; Trautmann, A.; Lantz, O.; Amigorena, S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 2004, 172, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.C.; Parnes, J.R. The roles of CD4 and CD8 in T cell activation. Semin. Immunol. 1991, 3, 133–141. [Google Scholar] [PubMed]
- Mittelbrunn, M.; Martínez del Hoyo, G.; López-Bravo, M.; Martín-Cofreces, N.B.; Scholer, A.; Hugues, S.; Fetler, L.; Amigorena, S.; Ardavín, C.; Sánchez-Madrid, F. Imaging of plasmacytoid dendritic cell interactions with T cells. Blood 2009, 113, 75–84. [Google Scholar] [CrossRef]
- Kato, Y.; Tanaka, Y.; Miyagawa, F.; Yamashita, S.; Minato, N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J. Immunol. 2001, 167, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; D’Asaro, M.; Caccamo, N.; Iovino, F.; Francipane, M.G.; Meraviglia, S.; Orlando, V.; La Mendola, C.; Gulotta, G.; Salerno, A.; et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J. Immunol. 2009, 182, 7287–7296. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.A.; Shea-Donohue, T.; Atamas, S.P. Regulation of inflammation by interleukin-4: A review of “alternatives”. J. Leukoc Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Bashyam, H. Interleukin-12: A master regulator. J. Exp. Med. 2007, 204, 969. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaher, K.A.; Alrahimi, J.S.; Basingab, F.S.; Aldahlawi, A.M. Newcastle Disease Virus Virotherapy: Unveiling Oncolytic Efficacy and Immunomodulation. Biomedicines 2024, 12, 1497. https://doi.org/10.3390/biomedicines12071497
Zaher KA, Alrahimi JS, Basingab FS, Aldahlawi AM. Newcastle Disease Virus Virotherapy: Unveiling Oncolytic Efficacy and Immunomodulation. Biomedicines. 2024; 12(7):1497. https://doi.org/10.3390/biomedicines12071497
Chicago/Turabian StyleZaher, Kawther A., Jehan S. Alrahimi, Fatemah S. Basingab, and Alia M. Aldahlawi. 2024. "Newcastle Disease Virus Virotherapy: Unveiling Oncolytic Efficacy and Immunomodulation" Biomedicines 12, no. 7: 1497. https://doi.org/10.3390/biomedicines12071497
APA StyleZaher, K. A., Alrahimi, J. S., Basingab, F. S., & Aldahlawi, A. M. (2024). Newcastle Disease Virus Virotherapy: Unveiling Oncolytic Efficacy and Immunomodulation. Biomedicines, 12(7), 1497. https://doi.org/10.3390/biomedicines12071497






