A Mini-Review of Diagnostic Methods for the Antigen and Antibody Detection of Rocky Mountain and Brazilian Spotted Fever
Abstract
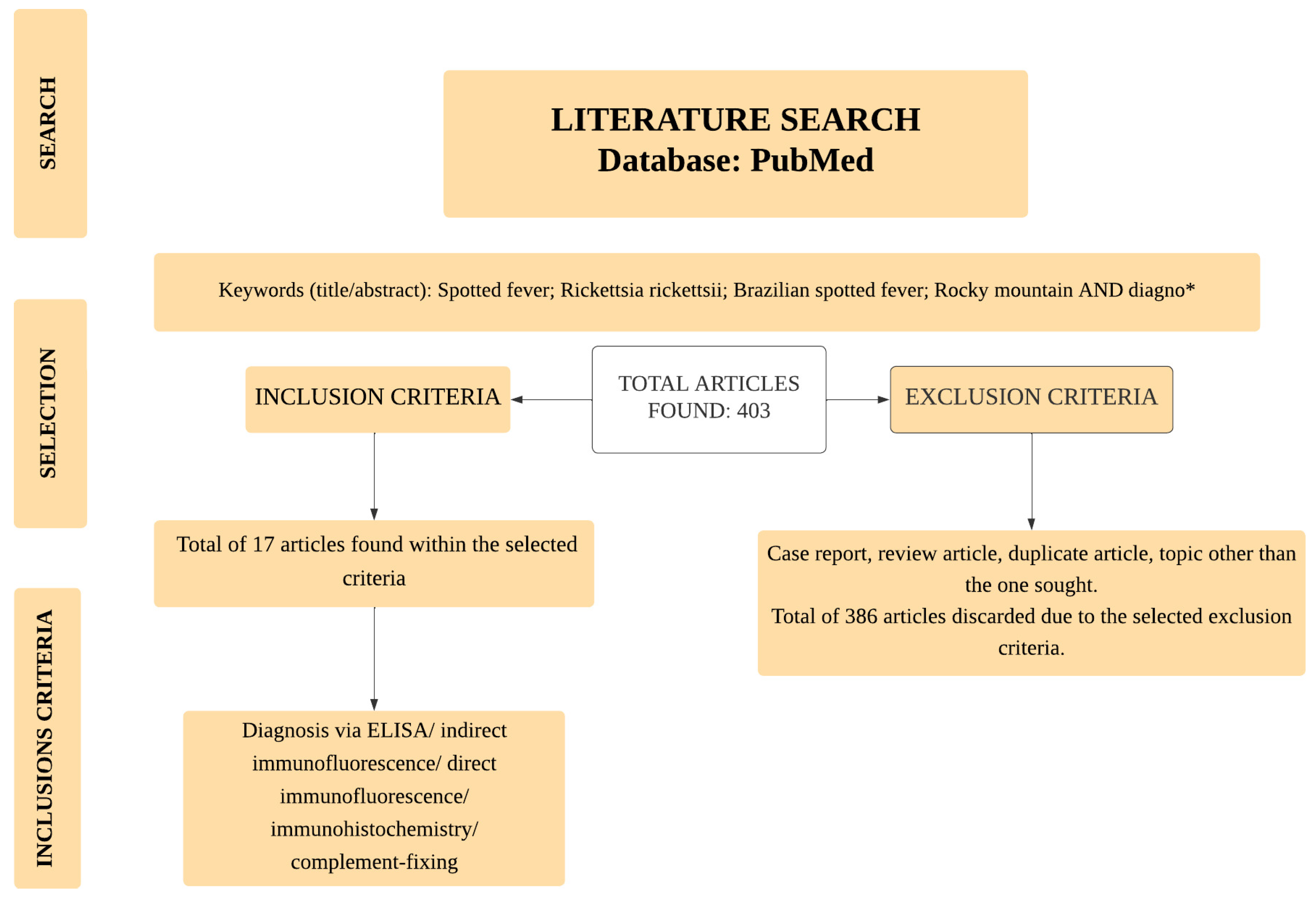
:1. Introduction
2. Materials and Methods
3. Antigen and Antibody Detection for Diagnosing RMSF and BSF Infections
3.1. Serological Tests Used to Detect RMSF and BSF
3.1.1. ELISA
3.1.2. Indirect Immunofluorescence Assay
3.1.3. Complement Fixation
3.2. Direct Immunofluorescence Assay
3.3. Immunohistochemistry
3.4. Comparative Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricketts, H.T. The study of rocky mountain spotted fever (tick fever?) by means of animal inoculations. a preliminary communication. J. Am. Med. Assoc. 1906, 47, 33–36. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Souza, C.E.; Angerami, R.N.; Samy, A.M. Mapping Brazilian spotted fever: Linking etiological agent, vectors, and hosts. Acta Trop. 2020, 207, 105496. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.R. Rocky Mountain Spotted Fever. J. Infect. Dis. 1929, 44, 257–276. Available online: http://www.jstor.org/stable/30083600 (accessed on 6 March 2024).
- Wood, W.W. Spotted fever as reported from Idaho; Report of the Surgeon General of the Army to the Secretary of War; U.S. Government Printing Office: Washington, DC, USA, 1896.
- Harden, V.A. Rocky Mountain Spotted Fever: History of A Twentieth-Century Disease; Johns Hopkins University Press: Baltimore, MD, USA, 1990; ISBN 978-0-8018-3905-4. [Google Scholar]
- Xiao, Y.; Beare, P.A.; Best, S.M.; Morens, D.M.; Bloom, M.E.; Taubenberger, J.K. Genetic sequencing of a 1944 Rocky Mountain spotted fever vaccine. Sci. Rep. 2023, 13, 4687. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.S. Rocky Mountain Spotted Fever. In One Health Triad; Unique Scientific Publishers: Faisalabad, Pakistan, 2023; pp. 53–59. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Brinton, L.P. Mechanisms of transovarial infection of spotted fever Rickettsiae in ticks. Ann. N. Y. Acad. Sci. 1975, 266, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.N.; Casper, E.A. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am. J. Trop. Med. Hyg. 1981, 30, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Berlese, A. Acari Austro-Americani quos collegit Alysius Balzam. Bull. Della Soc. Entomol. Italy 1888, 20, 171–222. [Google Scholar]
- Labruna, M.B.; Krawczak, F.S.; Gerardi, M.; Binder, L.C.; Barbieri, A.R.M.; Paz, G.F.; Rodrigues, D.S.; Araújo, R.N.; Bernardes, M.L.; Leite, R.C. Isolation of Rickettsia rickettsii from the tick Amblyomma sculptum from a Brazilian spotted fever-endemic area in the Pampulha Lake region, southeastern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2017, 8, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Luz, H.R.; Costa, F.B.; Benatti, H.R.; Ramos, V.N.; de A. Serpa, M.C.; Martins, T.F.; Acosta, I.C.L.; Ramirez, D.G.; Muñoz-Leal, S.; Ramirez-Hernandez, A.; et al. Epidemiology of capybara-associated Brazilian spotted fever. PLoS Negl. Trop. Dis. 2019, 13, e0007734. [Google Scholar] [CrossRef]
- Couto, D.V.; Medeiros, M.Z.; Filho, G.H.; Lima, A.M.; Barbosa, A.B.; Vicari, C.F. Brazilian spotted fever: The importance of dermatological signs for early diagnosis. An. Bras. Dermatol. 2015, 90, 248–250. [Google Scholar] [CrossRef]
- Guedes, E.; Leite, R.C.; Prata, M.C.A.; Pacheco, R.C.; Walker, D.H.; Labruna, M.B. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem. Inst. Oswaldo Cruz 2005, 100, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Hegarty, B.C.; Maggi, R.G.; Lantos, P.M.; Aslett, D.M.; Bradley, J.M. Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg. Infect. Dis. 2011, 17, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Long, B.; Koyfman, A. The Evaluation and Management of Rocky Mountain Spotted Fever in the Emergency Department: A Review of the Literature. J. Emerg. Med. 2018, 55, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.C.P.; Pereira, K.F.; Sabec-Pereira, D.K.; Daronco, A.; César, A.R.D.A. Epidemiology of rocky mountain spotted fever in Brazil, 2010–2020. Arq. Ciências Saúde 2023, 23, 1512–1527. [Google Scholar] [CrossRef]
- Piranda, E.M.; Faccini, J.L.; Pinter, A.; Saito, T.B.; Pacheco, R.C.; Hagiwara, M.K.; Labruna, M.B. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: Clinical and laboratory findings. Mem. Inst. Oswaldo Cruz 2008, 103, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Signs and Symptoms. 2019. Available online: https://www.cdc.gov/rmsf/healthcare-providers/signs-symptoms.html (accessed on 19 December 2023).
- Drexler, N.A.; Close, R.; Yaglom, H.D.; Traeger, M.; Parker, K.; Venkat, H.; Villarroel, L.; Brislan, J.; Pastula, D.M.; Armstrong, P.A. Morbidity and Functional Outcomes Following Rocky Mountain Spotted Fever Hospitalization-Arizona, 2002–2017. Open Forum Infect. Dis. 2022, 9, ofac506. [Google Scholar] [CrossRef] [PubMed]
- Archita, R. Rocky mountain spotted fever- A case series. Panacea J. Med. Sci. 2023, 13, 246–249. [Google Scholar]
- Chung, I.H.; Robinson, L.K.; Stewart-Juba, J.J.; Dasch, G.A.; Kato, C.Y. Analytically sensitive Rickettsia species detection for laboratory diagnosis. Am. J. Trop. Med. Hyg. 2022, 106, 51352. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Stewart, A.G.A. An Update on the Laboratory Diagnosis of Rickettsia spp. Infection. Pathogens 2021, 10, 1319. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Clinical and Laboratory Diagnosis. 2021. Available online: https://www.cdc.gov/rmsf/healthcare-providers/ClinLab-Diagnosis.html (accessed on 18 December 2023).
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Vaca, D.J.; Dobler, G.; Fischer, S.F.; Keller, C.; Konrad, M.; von Loewenich, F.D.; Orenga, S.; Sapre, S.U.; van Belkum, A.; Kempf, V.A.J. Contemporary diagnostics for medically relevant fastidious microorganisms belonging to the genera Anaplasma, Bartonella, Coxiella, Orientia and Rickettsia. FEMS Microbiol. Rev. 2022, 46, fuac013. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Bengtson, M.; Golverdingen, M.; Waling, L.; Dekker, C. Diagnosing point-of-care diagnostics for neglected tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009405. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, R.; Leinikki, P. Diagnostic Techniques: Serological and Molecular Approaches. In Encyclopedia of Virology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 29–37. [Google Scholar] [CrossRef]
- Ministério da Saúde. Febre Maculosa. 2022. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa (accessed on 17 December 2023).
- Tabatabaei, M.S.; Ahmed, M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol. Biol. 2022, 2508, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.V. Direct ELISA. Methods Mol. Biol. 2015, 1318, 61–67. [Google Scholar] [PubMed]
- Sakamoto, S.; Putalunm, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–34. [Google Scholar] [CrossRef]
- Radulovic, S.; Speed, R.; Feng, H.M.; Taylor, C.; Walker, D.H. EIA with species-specific monoclonal antibodies: A novel seroepidemiologic tool for determination of the etiologic agent of spotted fever rickettsiosis. J. Infect. Dis. 1993, 168, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Wiedeman, C.; Singleton, J.; Carpenter, L.R.; McElroy, K.; Mosites, E.; Chung, I.; Kato, C.; Morris, K.; Moncayo, A.C.; et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am. J. Trop. Med. Hyg. 2014, 91, 767. [Google Scholar] [CrossRef]
- Straily, A.; Stuck, S.; Singleton, J.; Brennan, S.; Marcum, S.; Condit, M.; Lee, C.; Kato, C.; Tonnetti, L.; Stramer, S.L.; et al. Antibody titers reactive with Rickettsia rickettsii in blood donors and implications for surveillance of spotted fever rickettsiosis in the United States. J. Infect. Dis. 2020, 221, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Bordet, J.; Octave, G. Sur l’existence de substances sensibilisatrices dans la plupart des sérums anti-microbiens. Ann. Inst. Pasteur 1901, 15, 289. [Google Scholar]
- Osler, A.G. Quantitative studies of complement fixation. Bacteriol. Rev. 1958, 22, 246–266. [Google Scholar] [CrossRef]
- Bengtson, I.A. Complement fixation in the rickettsial diseases: Technique of the test. In Public Health Reports (1896–1970); SAGE: Newcastle upon Tyne, UK, 1942; Volume 24, pp. 402–405. [Google Scholar]
- Eagle, H.A. Quantitative complement fixation technique. J. Infect. Dis. 1930, 1, 231–239. [Google Scholar] [CrossRef]
- Kent, J.F.; Fife, E.H., Jr. Precise standardization of reagents for complementary fixation. Am. J. Trop. Med. Hyg. 1963, 12, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Bier, O.G.; Siqueira, M.; Esteves, M.B. Quantitative studies of complement fixation. I–A simplified and accurate procedure based on 50 percent hemorrhagic end point. Rev. Inst. Med. Trop. São Paulo 1968, 10, 199–208. [Google Scholar]
- Belyavin, G. Influenza complement-fixation. A simple quantitative micro-method. J. Hyg. 1953, 51, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Gajdusek, D.C.; Robbins, M.L.; Robbins, F.C. Diagnosis of herpes simplex infections by the complement fixation test. Am. J. Med. 1952, 149, 235–240. [Google Scholar] [CrossRef]
- Bengtson, I.A. Applications of the complement-fixation test in the study of rickettsial diseases. Am. J. Public. Health 1945, 35, 701–707. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Williams, W.W.; Laubaugh, E.E. A study of complement fixation in syphilis with treponema antigens. J. Med. Res. 1913, 28, 345. [Google Scholar]
- Pappas, M.G.; Cannon, L.T., Sr.; Hockmeyer, W.T.; Smith, D.H. Evaluation of complement fixation procedures for the diagnosis of visceral leishmaniasis. Ann. Trop. Med. Parasitol. 1985, 79, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Chaffee, E.F.; Fife, E.H., Jr.; Kent, J.F. Diagnosis of Trypanosoma cruzi infection by complement fixation. Am. J. Trop. Med. Hyg. 1956, 5, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, D.L.; Thorpe, B.D.; Haskell, C.D. Infectious diseases in wild animals in Utah. VI. Experimental infection of birds with Rickettsia rickettsii. J. Bacteriol. 1966, 91, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.C.; Redus, M.A.; Tzianabos, T.; Warfield, D.T. Recent experience with the complement fixation test in the laboratory diagnosis of rickettsial diseases in the United States. J. Clin. Microbiol. 1976, 4, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Blanton, L.S.; Walker, D.H. Rickettsiae as emerging infectious agents. Clin. Lab. Med. 2017, 37, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Aoki, V.; Sousa, J.X., Jr.; Fukumori, L.M.; Périgo, A.M.; Freitas, E.L.; Oliveira, Z.N. Direct and indirect immunofluorescence. An. Bras. Dermatol. 2010, 85, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.C.; Bagley, L.R. Identification of Rickettsia rickettsii in formalin-fixed, paraffin-embedded tissues by immunofluorescence. J. Clin. Microbiol. 1978, 8, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Cain, B.G. A method for specific diagnosis of Rocky Mountain spotted fever on fixed, paraffin-embedded tissue by immunofluorescence. J. Infect. Dis. 1978, 137, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, G.; Lennette, E.T.; Honig, P. Diagnosis of Rocky Mountain spotted fever by immunofluorescent identification of Rickettsia rickettsii in skin biopsy tissue. J. Pediatr. 1979, 95, 63–65. [Google Scholar] [CrossRef]
- Davidson, M.G.; Breitschwerdt, E.B.; Walker, D.H.; Nasisse, M.P.; Sussman, W.E. Identification of Rickettsiae in Cutaneous i Biopsy Specimens From Dogs With Experimental Rocky Mountain Spotted Fever. J. Vet. Intern. Med. 1989, 3, 8–11. [Google Scholar] [CrossRef]
- Melles, H.H.; Colombo, S.; de Lemos, E.R. Isolamento de Rickettsia em cultura de células vero [Isolation of Rickettsia in vero cell culture]. Rev. Soc. Bras. Med. Trop. 1999, 32, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Amadeu Borges, F. Imunohistoquímica; Creative Commons: Mountain View, CA, USA, 2014. [Google Scholar]
- Polak, J.M.; Van Noorden, S. Introduction to Immunocytochemistry; BIOS Scientific Publishers: Oxford, UK, 2003. [Google Scholar]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Greer, P.W.; Ferebee, T.L.; Singleton, J., Jr.; McKechnie, D.B.; Treadwell, T.A.; Krebs, J.W.; Clarke, M.J.; Holman, R.C.; Olson, J.G.; et al. Hidden mortality attributable to Rocky Mountain spotted fever: Immunohistochemical detection of fatal, serologically unconfirmed disease. J. Infect. Dis. 1999, 179, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.N.; Casper, E.A.; MacCormack, J.N.; Sexton, D.; Thomas, L.A.; Anacker, R.L.; Burgdorfer, W.; Vick, S. A comparison of serologic methods for diagnosis of Rocky Mountain spotted fever. Am. J. Epidemiol. 1977, 105, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Zubair, M.; Tadi, P. Weil Felix Test. In StatPearls [Internet]; [Updated 2023 Feb 15]; StatPearls Publishing: Treasure Island, FL, USA, January 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559225/ (accessed on 15 March 2024).
- Hechemy, K. Laboratory diagnosis of Rocky Mountain spotted fever. N. Engl. J. Med. 1979, 300, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Burday, M.S.; Folds, J.D. Laboratory diagnosis of Rocky Mountain spotted fever. South Med. J. 1980, 73, 1443–1446, 1449. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.L.; Dumler, J.S.; Fiset, P.; Wisseman, C.L., Jr.; Snyder, M.J.; Levine, M.M. Serodiagnosis of Rocky Mountain spotted fever: Comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J. Infect. Dis. 1983, 148, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Hechemy, K.E.; Michaelson, E.E.; Anacker, R.L.; Zdeb, M.; Sasowski, S.J.; Kleeman, K.T.; Joseph, J.M.; Patel, J.; Kudlac, J.; Elliott, L.B.; et al. Evaluation of latex-Rickettsia rickettsii test for Rocky Mountain spotted fever in 11 laboratories. J. Clin. Microbiol. 1983, 18, 938–946. [Google Scholar] [CrossRef] [PubMed]
- White, W.L.; Patrick, J.D.; Miller, L.R. Evaluation of Immunoperoxidase Techniques to Detect Rickettsia rickeltsii in Fixed Tissue Sections. Am. J. Clin. Pathol. 1994, 101, 747–752. [Google Scholar] [CrossRef]
- Prata, J.A.; Souza, C.E.; Angerami, R.N.; Barbosa, T.M.; Santos, F.C.; Colombo, S.; Guercio, V.M.; Donalísio, M.R. Antibodies for Rickettsia spp. in patients with negative serology for dengue virus, leptospirosis, and meningococcal disease in municipalities of São Paulo State, Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 567–571. [Google Scholar] [CrossRef]
- Del Fiol, F.S.; Junqueira, F.M.; Rocha, M.C.P.; Toledo, M.I.; Filho, S.B. A febre maculosa no Brasil. Rev. Panam. Salud Publica 2010, 27, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Salje, J.; Weitzel, T.; Newton, P.N.; Varghese, G.M.; Day, N. Rickettsial infections: A blind spot in our view of neglected tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009353. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. DATASUS. 2023. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/febremaculosabr.def (accessed on 5 February 2024).
- Sidiq, Z.; Hanif, M.; Dwivedi, K.K.; Chopra, K.K. Benefits and limitations of serological assays in COVID-19 infection. Indian J. Tuberc. 2020, 67, S163–S166. [Google Scholar] [CrossRef] [PubMed]
- Prado, I.C.; Mendes, V.G.; Souza, A.L.A.; Dutra, R.F.; De-Simone, S.G. Electrochemical immunosensor for differential diagnostic of Wuchereria bancrofti using a synthetic peptide. Biosens. Bioelectron. 2018, 113, 9–15. [Google Scholar] [CrossRef]
- Higa, L.O.S.; Csordas, B.G.; Garcia, M.V.; Oshiro, L.M.; Duarte, P.O.; Barros, J.C.; Andreotti, R. Spotted fever group Rickettsia and Borrelia sp. cooccurrence in Amblyomma sculptum in the Midwest region of Brazil. Exp. Appl. Acarol. 2020, 81, 441–455. [Google Scholar] [CrossRef]
| Disease Stage | Infected Host | Sample Used | Method | Results | Author/Country |
|---|---|---|---|---|---|
| Acute | Animal | Serum | CF | CF was not able to detect antibodies from infected chickens, pheasants, sparrow hawks, magpies, or ravens CF detected antibodies from infected pigeons with maximal detection between the 3rd and 5th infection week CF detected antibodies from one red-tailed hawk and one marsh hawk in the 2nd and the 3rd infection week | Lundgren, Thorpe, and Haskell (1966)/USA [51] |
| - | Human | Serum | CF | 9.5% of infected individuals showed maximal titer detection 64% of serum samples from infected individuals showed cross-reaction with typhus antigens | Shepard et al., (1976)/USA [52] |
| Acute and convalescent-phase | Human | Serum | Micro-IF MA HA CF | Reactivity - Micro-IF: 30% MA: 26% HA: 30% CF: 13% | Philip et. al. (1977)/USA [64] |
| Convalescent-phase | Animal | Kidney and skin | Direct IFA | Direct IFA detected 7 (7/10) samples from infected individuals Samples from individuals negative for RMSF did not show immunofluorescence | Walker and Cain (1978)/USA [56] |
| - | Human and animal | Lung, heart, epididymis, and testis | Direct IFA | The formalin-fixed method showed a slightly reduced intensity of staining, with improved morphology Normal tissues showed no staining | Hall and Bagley (1978)/USA [55] |
| - | Human | Serum | Weil–Felix Micro-IF CF | Weil–Felix: only 6% of similarity with micro-IF. Agreement between these two tests was higher when considering paired serums CF: 86% false-negative | Hechemy (1979)/USA [66] |
| Acute | Human | Skin | Direct IFA | Direct IFA was able to detect the presence of R. rickettsii in only 1 (1/2) positive sample | Fleisher, Lennette, and Honig (1979)/USA [57] |
| Acute | Human | Serum and skin | Direct IFA CF HA Weil–Felix | Direct IFA - Sensitivity: 70% Specificity: 100% CF - Sensitivity: 0% Specificity: 0% HA - Sensitivity: 19% Specificity: 99% Weil–Felix Proteus Ox-2 - Sensitivity: 18% Specificity: 96% Weil–Felix Proteus Ox-19 - Sensitivity: 65% Specificity: 78% | Walker et. al. (1980)/USA [67] |
| Acute and convalescent-phase | Human | Serum | ELISA and Indirect IFA | ELISA - Sensitivity: 100% Specificity: 100% IFA - Sensitivity: 100% Specificity: 83% | Clements et. al. (1983)/USA [68] |
| - | Human | Serum | Latex-R. rickettsii test and micro-IF | New York State laboratory - Sensitivity: 84.45% Specificity: 99.98% Collaborating laboratories - Sensitivity: 79.20% Specificity: 95.81% | Hechemy et. al. (1983)/USA [69] |
| - | Animal | Skin | Direct IFA | Direct IFA was able to detect the presence of R. rickettsii in 18 (18/23) samples from erythematous macules Using normal inguinal skin, direct IFA was unable to detect the presence of R. rickettsii | Davidson et. al, (1989)/USA [58] |
| - | Human | Serum | ELISA | Sensitivity: 100% Specificity: 100% | Radulovic et. al. (1993)/USA [35] |
| - | Human | Serum Skin biopsy, and autopsy samples | Direct IFA and immunoperoxidase test | Direct IFA and immunoperoxidase tests detected the presence of R. rickettsii in 9 (9/10) positive samples | White, Patrick, and Miller (1994)/USA [70] |
| - | Human and animal | Skin | Direct IFA | Sensitivity was improved when using a higher concentration of fetal bovine serum | Melles, Colombo, and Lemos (1999)/Brazil [59] |
| - | Human | Serum whole blood liver, myocardium, spleen, kidney, lung, adrenal gland, pancreas, cerebral cortex, cerebellum, skin, stomach, colon, bone marrow, lymph node, small intestine, trachea, skeletal muscle, thymus, thyroid, coronary artery, aorta, hippocampus, medulla, pons, pineal gland, choroid plexus, ovary, tongue, and appendix | IHC | IHC was able to detect the presence of R. rickettsii in 12 (12/16) samples | Paddock et. al. (1999)/USA [63] |
| Acute and convalesce- cent-phase | Human | Serum | Indirect IFA | Anti-R. rickettsii IgM and/or IgG were detected in at least one collected serum sample from 10 (10/13) infected individuals | McQuiston et. al. (2014)/USA [37] |
| Acute and convalesce- cent-phase | Human | Serum | Indirect IFA | Only 11.1% of Georgia donors and 6% of Pacific Northwest donors had IgG titers ≥ 64 | Straily et. al. (2020)/USA [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.A.; Prado, V.B.d.; Silva, R.R.; Rocha, M.v.P.; de Oliveira, R.A.R.; Falcão, T.d.J.R.; Serpa, C.C.; Rocha, M.A.; Pereira, S.P.; Silva, L.S.; et al. A Mini-Review of Diagnostic Methods for the Antigen and Antibody Detection of Rocky Mountain and Brazilian Spotted Fever. Biomedicines 2024, 12, 1501. https://doi.org/10.3390/biomedicines12071501
Silva KA, Prado VBd, Silva RR, Rocha MvP, de Oliveira RAR, Falcão TdJR, Serpa CC, Rocha MA, Pereira SP, Silva LS, et al. A Mini-Review of Diagnostic Methods for the Antigen and Antibody Detection of Rocky Mountain and Brazilian Spotted Fever. Biomedicines. 2024; 12(7):1501. https://doi.org/10.3390/biomedicines12071501
Chicago/Turabian StyleSilva, Kamila Alves, Vanesa Borges do Prado, Rafael Rodrigues Silva, Marcelo van Petten Rocha, Rafael Almeida Ribeiro de Oliveira, Tarumim de Jesus Rodrigues Falcão, Clara Cristina Serpa, Marina Andrade Rocha, Sabrina Paula Pereira, Líria Souza Silva, and et al. 2024. "A Mini-Review of Diagnostic Methods for the Antigen and Antibody Detection of Rocky Mountain and Brazilian Spotted Fever" Biomedicines 12, no. 7: 1501. https://doi.org/10.3390/biomedicines12071501
APA StyleSilva, K. A., Prado, V. B. d., Silva, R. R., Rocha, M. v. P., de Oliveira, R. A. R., Falcão, T. d. J. R., Serpa, C. C., Rocha, M. A., Pereira, S. P., Silva, L. S., Machado, J. M., Machado-de-Ávila, R. A., Fujiwara, R. T., Chávez-Fumagalli, M. A., Coelho, E. A. F., Giunchetti, R. C., Campos-da-Paz, M., Gonçalves, A. A. M., & Galdino, A. S. (2024). A Mini-Review of Diagnostic Methods for the Antigen and Antibody Detection of Rocky Mountain and Brazilian Spotted Fever. Biomedicines, 12(7), 1501. https://doi.org/10.3390/biomedicines12071501











