Lipoprotein(a) and Lung Function Are Associated in Older Adults: Longitudinal and Cross-Sectional Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Exclusion Criteria
2.3. Lipoprotein(a) Measurement
2.4. Measurement of Pulmonary Function
2.5. Covariables
2.6. Statistical Analysis
2.7. Mendelian Randomization Analyses
3. Results
3.1. Descriptive Characteristics of Baseline Data and Lung Function Measurements at Follow-Up
3.2. Descriptive Characteristics Comparing Baseline and Follow-Up Data
3.3. Descriptive Characteristics According to Lp(a) Quintile 1 vs. Lp(a) Quintiles 2-5
3.4. Multiple Linear Regression Models Assessing the Association between Baseline Lp(a) and Follow-Up Lung Function Measurements
3.5. Adjusted Mean of FEV1 and FVC at Follow-Up According to Individual Lp(a) Quintile
3.6. Delta FEV1 and Delta FVC According to Individual Lp(a) Quintile
3.7. Cross-Sectional Analyses: Association between Re-Assessed Lp(a) and Lung Function Measurements at Follow-Up
3.8. Two-Sample MR Analyses Do Not Suggest a Causal Relationship between Lp(a) and FEV1/FVC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, K. A New Serum Type System in Man—The Lp System. Acta Pathol. Microbiol. Scand. 1963, 59, 369–382. [Google Scholar] [CrossRef]
- Gaubatz, J.W.; Heideman, C.; Gotto, A.M.; Morrisett, J.D.; Dahlen, G.H. Human plasma lipoprotein [a]. Structural properties. J. Biol. Chem. 1983, 258, 4582–4589. [Google Scholar] [CrossRef] [PubMed]
- Jawi, M.M.; Frohlich, J.; Chan, S.Y. Lipoprotein(a) the Insurgent: A New Insight into the Structure, Function, Metabolism, Pathogenicity, and Medications Affecting Lipoprotein(a) Molecule. J. Lipids 2020, 2020, 3491764. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Utermann, G. Lipoprotein(a): Resurrected by genetics. J. Intern. Med. 2013, 273, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Sabouret, P.; Angoulvant, D.; Ray, K.K. Lipoprotein(a), the rediscovered risk factor, or how to get “back to the future”. Arch. Cardiovasc. Dis. 2020, 113, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Kronenberg, F.; Stroes, E.S.; Mach, F. Lipoprotein(a): The revenant. Eur. Heart J. 2017, 38, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein(a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef] [PubMed]
- Utermann, G.; Weber, W. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 1983, 154, 357–361. [Google Scholar] [CrossRef]
- Kronenberg, F. Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovasc. Drugs Ther. 2016, 30, 87–100. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J. Am. Coll. Cardiol. 2013, 61, 1146–1156. [Google Scholar] [CrossRef]
- König, M.; Joshi, S.; Leistner, D.M.; Landmesser, U.; Sinning, D.; Steinhagen-Thiessen, E.; Demuth, I. Cohort profile: Role of lipoproteins in cardiovascular disease—The LipidCardio study. BMJ Open 2019, 9, e030097. [Google Scholar] [CrossRef]
- Tipping, R.W.; Ford, C.E.; Simpson, L.M.; Walldius, G.; Jungner, I.; Folsom, A.R.; Chambless, L.; Panagiotakos, D.; Pitsavos, C.; Chrysohoou, C.; et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA J. Am. Med. Assoc. 2009, 302, 412. [Google Scholar] [CrossRef]
- Freeman, D.J.; Griffin, B.A.; Murray, E.; Lindsay, G.M.; Gaffney, D.; Packard, C.J.; Shepherd, J. Smoking and plasma lipoproteins in man: Effects on low density lipoprotein cholesterol levels and high density lipoprotein subfraction distribution. Eur. J. Clin. Investig. 1993, 23, 630–640. [Google Scholar] [CrossRef]
- Jang, A.Y.; Han, S.H.; Sohn, I.S.; Oh, P.C.; Koh, K.K. Lipoprotein(a) and Cardiovascular Diseases—Revisited. Circ. J. 2020, 84, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Yamagami, K.; Sakata, K.; Usui, S.; Kawashiri, M.A.; Takamura, M. Healthy lifestyle, lipoprotein (a) levels and the risk of coronary artery disease. Eur. J. Clin. Investig. 2024, 54, e14093. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Mach, F. Potential of Lipoprotein(a)-Lowering Strategies in Treating Coronary Artery Disease. Drugs 2020, 80, 229–239. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J.; Sanchis-Gomar, F. Antisense lipoprotein[a] therapy: State-of-the-art and future perspectives. Eur. J. Intern. Med. 2020, 76, 8–13. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.-C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Nordestgaard, B.G. Antisense Oligonucleotides Targeting Lipoprotein(a). Curr. Atheroscler. Rep. 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A. Lipoprotein(a)-antisense therapy. Clin. Res. Cardiol. Suppl. 2019, 14, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Lipoprotein(a): From Causality to Treatment. Curr. Atheroscler. Rep. 2024, 26, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Ojeda, F.M.; Rzayeva, N.; Zeller, T.; Sinning, C.R.; Pfeiffer, N.; Beutel, M.; Blettner, M.; Lackner, K.J.; Blankenberg, S.; et al. FEV1 and FVC predict all-cause mortality independent of cardiac function—Results from the population-based Gutenberg Health Study. Int. J. Cardiol. 2017, 234, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Liu, M.A.; Barrett-Connor, E.; Wong, N.D. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: The Rancho Bernardo study. Respir. Med. 2014, 108, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Burroughs Pena, M.S.; Dunning, A.; Schulte, P.J.; Durheim, M.T.; Kussin, P.; Checkley, W.; Velazquez, E.J. Pulmonary function and adverse cardiovascular outcomes: Can cardiac function explain the link? Respir. Med. 2016, 121, 4–12. [Google Scholar] [CrossRef]
- Polak, M.; Dorynska, A.; Szafraniec, K.; Pajak, A. Cardiovascular risk assessment, cardiovascular disease risk factors, and lung function parameters. Kardiol. Pol. 2018, 76, 1055–1063. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Xiao, L.; Guo, Y.; Ma, J.; Zhou, M.; Shi, T.; Tan, A.; Yuan, J.; Chen, W. Association of lung function with cardiovascular risk: A cohort study. Respir. Res. 2018, 19, 214. [Google Scholar] [CrossRef]
- Buchmann, N.; Kassner, U.; Norman, K.; Goldeck, D.; Eckardt, R.; Pawelec, G.; Steinhagen-Thiessen, E.; Demuth, I. Higher Lipoprotein (a) Levels Are Associated with Better Pulmonary Function in Community-Dwelling Older People—Data from the Berlin Aging Study II. PLoS ONE 2015, 10, e0139040. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.K.; Kwon, M.J.; Song, J.U. Decline in lung function is associated with elevated lipoprotein (a) in individuals without clinically apparent disease: A cross-sectional study. Respirology 2019, 24, 68–75. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Bockenhoff, A.; Demuth, I.; Duzel, S.; Eckardt, R.; Li, S.C.; Lindenberger, U.; Pawelec, G.; Siedler, T.; Wagner, G.G.; et al. Cohort profile: The Berlin Aging Study II (BASE-II). Int. J. Epidemiol. 2014, 43, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Demuth, I.; Banszerus, V.; Drewelies, J.; Duzel, S.; Seeland, U.; Spira, D.; Tse, E.; Braun, J.; Steinhagen-Thiessen, E.; Bertram, L.; et al. Cohort profile: Follow-up of a Berlin Aging Study II (BASE-II) subsample as part of the GendAge study. BMJ Open 2021, 11, e045576. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Pompei, P.; Ales, K.L.; Mackenzie CCharlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice, C. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Zuber, V.; Grinberg, N.F.; Gill, D.; Manipur, I.; Slob, E.A.W.; Patel, A.; Wallace, C.; Burgess, S. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 2022, 109, 767–782. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, N.; Tanigawa, Y.; Amar, D.; Mars, N.; Benner, C.; Aguirre, M.; Venkataraman, G.R.; Wainberg, M.; Ollila, H.M.; Kiiskinen, T.; et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021, 53, 185–194. [Google Scholar] [CrossRef]
- Shrine, N.; Izquierdo, A.G.; Chen, J.; Packer, R.; Hall, R.J.; Guyatt, A.L.; Batini, C.; Thompson, R.J.; Pavuluri, C.; Malik, V.; et al. Multi-ancestry genome-wide association analyses improve resolution of genes and pathways influencing lung function and chronic obstructive pulmonary disease risk. Nat. Genet. 2023, 55, 410–422. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N.; Scholz, M.; Lill, C.M.; Burkhardt, R.; Eckardt, R.; Norman, K.; Loeffler, M.; Bertram, L.; Thiery, J.; Steinhagen-Thiessen, E.; et al. Association between lipoprotein(a) level and type 2 diabetes: No evidence for a causal role of lipoprotein(a) and insulin. Acta Diabetol. 2017, 54, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Jae, S.Y.; Heffernan, K.S.; Lee, M.K.; Fernhall, B.; Park, W.H. Relation of cardiorespiratory fitness to inflammatory markers, fibrinolytic factors, and lipoprotein(a) in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2008, 102, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Natarajan, P.; Klarin, D.; Won, H.H.; Peloso, G.M.; Stitziel, N.O.; Nomura, A.; Zekavat, S.M.; Bick, A.G.; et al. Phenotypic Characterization of Genetically Lowered Human Lipoprotein(a) Levels. J. Am. Coll. Cardiol. 2016, 68, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Basili, S.; Ferroni, P.; Vieri, M.; Cardelli, P.; Ceci, F.; Paradiso, M.; Labbadia, G.; Gazzaniga, P.P.; Cordova, C.; Alessandri, C. Lipoprotein(a) serum levels in patients affected by chronic obstructive pulmonary disease. Atherosclerosis 1999, 147, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Hoover-Plow, J.; Huang, M. Lipoprotein(a) metabolism: Potential sites for therapeutic targets. Metabolism 2013, 62, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Nair, K.S. Age effect on fibrinogen and albumin synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E1023–E1030. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan Afr. Med. J. 2009, 3, 17. [Google Scholar]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein (a): Impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Wild, S.H.; Byrne, C.D. Lipoprotein (a), metabolic syndrome and coronary calcium score in a large occupational cohort. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Lin, L.; Qi, H.Y.; Du, R.; Hu, C.Y.; Ma, L.N.; Peng, K.; Li, M.; Xu, Y.; Xu, M.; et al. Association between Lipoprotein (a) Levels and Metabolic Syndrome in a Middle-aged and Elderly Chinese Cohort. Biomed. Environ. Sci. 2019, 32, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Kamstrup, P.R.; Rifai, N.; Nordestgaard, B.G.; Buring, J.E.; Ridker, P.M. Lipoprotein(a) and risk of type 2 diabetes. Clin. Chem. 2010, 56, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Haycock, P.C.; Gurdasani, D.; Pomilla, C.; Boekholdt, S.M.; Tsimikas, S.; Khaw, K.T.; Wareham, N.J.; Sandhu, M.S.; Forouhi, N.G. The association between circulating lipoprotein(a) and type 2 diabetes: Is it causal? Diabetes 2014, 63, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N.; Ittermann, T.; Demuth, I.; Markus, M.R.P.; Volzke, H.; Dorr, M.; Friedrich, N.; Lerch, M.M.; Santos, R.D.; Schipf, S.; et al. Lipoprotein(a) and Metabolic Syndrome. Dtsch. Arztebl. Int. 2022, 119, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Baffi, C.W.; Wood, L.; Winnica, D.; Strollo, P.J., Jr.; Gladwin, M.T.; Que, L.G.; Holguin, F. Metabolic Syndrome and the Lung. Chest 2016, 149, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.L.; Krishnan, J.A.; Glick, S.; Smith, L.J. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet. Med. 2010, 27, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N.; Norman, K.; Steinhagen-Thiessen, E.; Demuth, I.; Eckardt, R. Lung function in elderly subjects with metabolic syndrome and type II diabetes: Data from the Berlin Aging Study II. Z. Gerontol. Geriatr. 2015. [Google Scholar] [CrossRef]
- Onat, A.; Coban, N.; Can, G.; Yuksel, M.; Karagoz, A.; Yuksel, H.; Ademoglu, E.; Erginel-Unaltuna, N. Low "quotient" Lp(a) concentration mediates autoimmune activation and independently predicts cardiometabolic risk. Exp. Clin. Endocrinol. Diabetes 2015, 123, 11–18. [Google Scholar] [CrossRef]
- Baritussio, A.; Enzi, G.; Inelmen, E.M.; Schiavon, M.; de Biasi, F.; Allegra, L.; Ursini, F.; Baldo, G. Altered surfactant synthesis and function in rats with diet-induced hyperlipidemia. Metabolism 1980, 29, 503–510. [Google Scholar] [CrossRef]
- Barochia, A.V.; Kaler, M.; Cuento, R.A.; Gordon, E.M.; Weir, N.A.; Sampson, M.; Fontana, J.R.; MacDonald, S.; Moss, J.; Manganiello, V.; et al. Serum Apolipoprotein A-I and Large HDL Particles are Positively Correlated with FEV in Atopic Asthma. Am. J. Respir. Crit. Care Med. 2015, 19, 990–1000. [Google Scholar] [CrossRef]
- Fessler, M.B. Revisiting “good” and “bad” cholesterol the battle over flow through arteries now shifts to flow through airways. Am. J. Respir. Crit. Care Med. 2015, 191, 969–970. [Google Scholar] [CrossRef]
- Fessler, M.B. A new frontier in immunometabolism cholesterol in lung health and disease. Ann. Am. Thorac. Soc. 2017, 14, S399–S405. [Google Scholar] [CrossRef]
- Gowdy, K.M.; Fessler, M.B. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm. Pharmacol. Ther. 2013, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hass, M.A.; Longmore, W.J. Regulation of lung surfactant cholesterol metabolism by serum lipoproteins. Lipids 1980, 15, 401–406. [Google Scholar] [CrossRef]
- McCrae, K.C.; Weltman, B.; Alyward, S.; Shaw, R.A.; Sowa, M.G.; Unruh, H.W.; Rand, T.G.; Thliveris, J.A.; Scott, J.E. The effect of elevated dietary cholesterol on pulmonary surfactant function in adolescent mice. Pediatr. Pulmonol. 2008, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Nahak, P.; Nag, K.; Hillier, A.; Devraj, R.; Thompson, D.W.; Manna, K.; Makino, K.; Ohshima, H.; Nakahara, H.; Shibata, O.; et al. Effect of serum, cholesterol and low density lipoprotein on the functionality and structure of lung surfactant films. J. Oleo Sci. 2014, 63, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.D.; Andersen, J.M.; Dietschy, J.M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J. Lipid Res. 1981, 22, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Summer, R.S. Surfactant lipids at the host-environment interface metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 624–635. [Google Scholar] [CrossRef]
- Pian, M.S.; Dobbs, L.G. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: Mediation by heterotrimeric G proteins. Am. J. Physiol. 1997, 273, L634–L639. [Google Scholar] [CrossRef] [PubMed]
- Simony, S.B.; Mortensen, M.B.; Langsted, A.; Afzal, S.; Kamstrup, P.R.; Nordestgaard, B.G. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: The Copenhagen General Population Study. Atherosclerosis 2022, 355, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Sandkamp, M.; Kokott, R.; Schulte, H.; Assmann, G. Relationship of lipoprotein(a) to variables of coagulation and fibrinolysis in a healthy population. Clin. Chem. 1991, 37, 1950–1954. [Google Scholar] [CrossRef]
- Slunga, L.; Asplund, K.; Johnson, O.; Dahlen, G.H. Lipoprotein (a) in a randomly selected 25-64 year old population: The Northern Sweden Monica Study. J. Clin. Epidemiol. 1993, 46, 617–624. [Google Scholar] [CrossRef]
- Ushioda, M.; Makita, K.; Takamatsu, K.; Horiguchi, F.; Aoki, D. Serum lipoprotein(a) dynamics before/after menopause and long-term effects of hormone replacement therapy on lipoprotein(a) levels in middle-aged and older Japanese women. Horm. Metab. Res. 2006, 38, 581–586. [Google Scholar] [CrossRef]
- Amaral, A.F.; Strachan, D.P.; Gomez Real, F.; Burney, P.G.; Jarvis, D.L. Lower lung function associates with cessation of menstruation: UK Biobank data. Eur. Respir. J. 2016, 48, 1288–1297. [Google Scholar] [CrossRef]
- Real, F.G.; Svanes, C.; Omenaas, E.R.; Anto, J.M.; Plana, E.; Jarvis, D.; Janson, C.; Neukirch, F.; Zemp, E.; Dratva, J.; et al. Lung function, respiratory symptoms, and the menopausal transition. J. Allergy Clin. Immunol. 2008, 121, 72–80.e73. [Google Scholar] [CrossRef]
- Hong, Y.S.; Park, H.Y.; Chang, Y.; Jang, E.H.; Zhao, D.; Kim, S.; Guallar, E.; Kim, H.; Cho, J.; Ryu, S. Stages of menopause and abnormal lung function: A cross-sectional study of middle-aged women. Menopause 2021, 28, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Triebner, K.; Accordini, S.; Calciano, L.; Johannessen, A.; Benediktsdottir, B.; Bifulco, E.; Demoly, P.; Dharmage, S.C.; Franklin, K.A.; Garcia-Aymerich, J.; et al. Exogenous female sex steroids may reduce lung ageing after menopause: A 20-year follow-up study of a general population sample (ECRHS). Maturitas 2019, 120, 29–34. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Sex and gender in respiratory physiology. Eur. Respir. Rev. 2021, 30. [Google Scholar] [CrossRef]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Song, A.; Dai, M.; Xu, M.; Sun, W.; Xu, B.; Sun, J.; Wang, T.; Xu, Y.; Lu, J.; et al. Serum lipoprotein (a) concentrations are inversely associated with T2D, prediabetes, and insulin resistance in a middle-aged and elderly Chinese population. J. Lipid Res. 2015, 56, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Nordestgaard, B.G. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: A Mendelian randomisation study. Lancet Diabetes Endocrinol. 2013, 1, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Neele, D.M.; de Wit, E.C.; Princen, H.M. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia 1999, 42, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Janzen, B.; Karunanayake, C.; Rennie, D.; Pickett, W.; Lawson, J.; Kirychuk, S.; Hagel, L.; Senthilselvan, A.; Koehncke, N.; Dosman, J.; et al. Gender Differences in the Association of Individual and Contextual Exposures with Lung Function in a Rural Canadian Population. Lung 2017, 195. [Google Scholar] [CrossRef]
- Talaminos Barroso, A.; Marquez Martin, E.; Roa Romero, L.M.; Ortega Ruiz, F. Factors Affecting Lung Function: A Review of the Literature. Arch. Bronconeumol. (Engl. Ed.) 2018, 54, 327–332. [Google Scholar] [CrossRef]




| Baseline | All (n = 679) | Men (n = 326) | Women (n = 353) | p-Value * |
|---|---|---|---|---|
| Age [years] | 68 (65–71) | 68 (65–71) | 68 (65–70) | 0.108 |
| BMI [kg/m2] | 26.2 (23.8–28.7) | 26.9 (24.8–29.0) | 25.1 (22.9–28.0) | <0.001 |
| Current smoker [n; %] | 61 (9) | 30 (9) | 31 (9) | 0.894 |
| Pack-years [years] | 0.5 (0.0–15.0) | 7 (0.0–22.2) | 0 (0.0–6.1) | <0.001 |
| Regular alcohol intake [n; %] | 611 (90) | 297 (91) | 314 (89) | 0.289 |
| Self-reported physical inactivity [n; %] | 55 (8) | 33 (10) | 22 (6) | 0.066 |
| Morbidity index [pts.] | 1 (0–2) | 1 (0–2) | 0 (0–1) | 0.052 |
| T2D [n; %] | 74 (11) | 52 (16) | 22 (6) | <0.001 |
| MetS [n; %] | 230 (34) | 141 (44) | 89 (26) | <0.001 |
| Lipoprotein(a) [mg/dL] | 10.6 (5.0–28.1) | 9.0 (4.0–21.0) | 12.0 (5.0–42.0) | 0.002 |
| Follow-up | ||||
| FEV1 [ml] | 2337 ± 615 | 2737 ± 563 | 1967 ± 387 | <0.001 |
| FVC [ml] | 3115 ± 798 | 3686 ± 671 | 2588 ± 480 | <0.001 |
| FEV1/FVC [%] | 76 (72–79) | 75 (71–79) | 76 (73–80) | <0.001 |
| Men (n = 326) | Women (n = 353) | All (n = 679) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value * | Baseline | Follow-Up | p-Value * | Baseline | Follow-Up | p-Value * | |
| Age [years] | 68 (65–71) | 76 (72–78) | <0.001 | 68 (65–70) | 76 (73–78) | <0.001 | 68 (65–71) | 76 (73–78) | <0.001 |
| BMI [kg/m2] | 26.9 (24.8–29.0) | 26.8 (24.7–29.1) | 0.136 | 25.1 (22.9–28.0) | 25.5 (23.0–28.7) | <0.001 | 26.2 (23.8–28.7) | 26.3 (23.9–29.0) | <0.001 |
| Current smoker [n; %] | 30 (9) | 16 (5) | 0.001 | 31 (9) | 20 (6) | 0.003 | 61 (9) | 36 (5) | <0.001 |
| Pack-years [years] | 7 (0.0–22.2) | 6.3 (0.0–22-5) | 0.853 | 0 (0.0–6.1) | 0.0 (0.0–5–0) | 0.270 | 0.5 (0.0–15.0) | 0.0 (0.0–15.0) | 0.423 |
| Regular alcohol intake [n; %] | 297 (91) | 275 (84) | <0.001 | 314 (89) | 288 (82) | <0.001 | 611 (90) | 563 (83) | <0.001 |
| Self-reported physical inactivity [n; %] | 33 (10) | 41 (13) | 0.401 | 22 (6) | 40 (11) | 0.028 | 55 (8) | 81 (12) | 0.024 |
| Morbidity index [pts.] | 1 (0–2) | 1 (0–2) | <0.001 | 0 (0–1) | 1 (0–2) | <0.001 | 1 (0–1) | 1 (0–2) | <0.001 |
| T2D [n; %] | 52 (16) | 70 (21) | <0.001 | 22 (6) | 42 (12) | <0.001 | 74 (11) | 112 (16) | <0.001 |
| GGT [U/L] | 25 (19–37) | 27 (20–37) | 0.033 | 17 (14–25) | 19 (15–28) | <0.001 | 21 (15–31) | 23 (16–33) | <0.001 |
| ALT [U/L] | 22 (17–28) | 21 (17–27) | 0.559 | 18 (14–22) | 18 (14–23) | 0.104 | 19 (15–25) | 20 (15–24) | 0.496 |
| AP [U/L] | 58 (51–70) | N/A | N/A | 63 (53–74) | N/A | N/A | 60 (52–72) | N/A | NA |
| Lp(a) [mg/dL] | 9.0 (4.0–21.0) | 8.2 (3.4–19.0) | 0.354 | 12.0 (5.8–42.0) | 11.4 (5.5–43.1) | 0.244 | 10.9 (5.0–28.3) | 9.4 (5.3–28.2) | 0.821 |
| Lp(a) quintile 1 [n; %] | 65 (20) | 82 (25) | 0.010 | 70 (20) | 74 (21) | 0.345 | 128 (19) | 156 (23) | 0.001 |
| Men (n = 173) | Women (n = 183) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value * | Baseline | Follow-Up | p-Value * | |
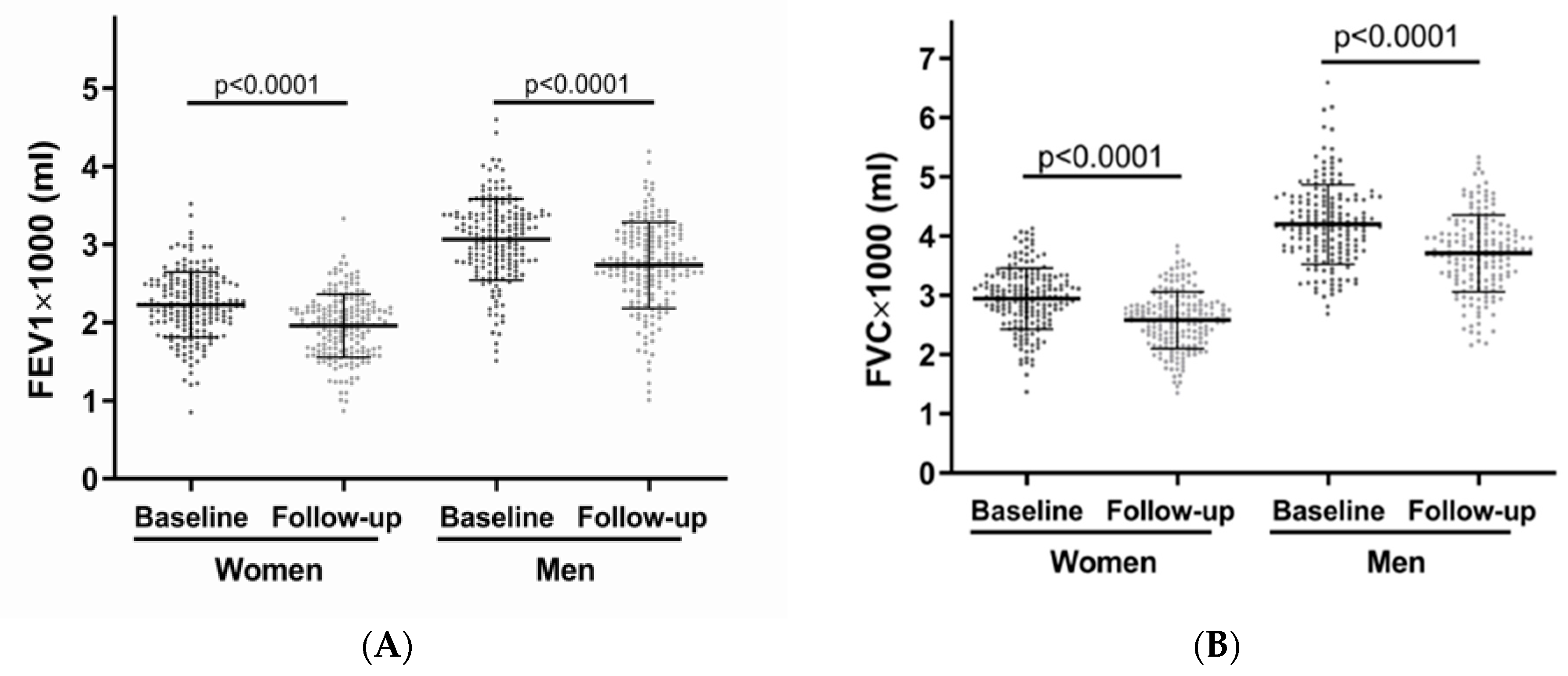
| FEV1 [mL] | 3064 ± 521 | 2733 ± 511 | <0.001 | 2228 ± 418 | 1958 ± 404 | <0.001 |
| FVC [mL] | 4195 ± 671 | 3707 ± 647 | <0.001 | 2944 ± 516 | 2580 ± 479 | <0.001 |
| FEV1/FVC [%] | 74 (69–78) | 74 (70–78) | 0.183 | 76 (72–80) | 77 (73–80) | 0.369 |
| Men (n = 326) | Women (n = 353) | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Lp(a) Quintile 1 | Lp(a) Quintiles 2-5 | p-Value * | Lp(a) Quintile 1 | Lp(a) Quintiles 2-5 | p-Value * | Lp(a) Quintile 1 | Lp(a) Quintiles 2-5 | p-Value * |
| Age [years] | 67 (65–71) | 69 (66–71) | 0.288 | 68 (65–70) | 68 (65–70) | 0.205 | 68 (65–71) | 68 (66–71) | 0.066 |
| BMI [kg/m2] | 27.2 (24.5–29.5) | 26.9 (24.9–29.0) | 0.546 | 24.4 (23.0–28.1) | 25.4 (22.8–27.9) | 0.695 | 26.0 (23.8–28.7) | 26.2 (23.8–28.7) | 0.887 |
| Current smoker [n; %] | 9 (14) | 21 (8) | 0.136 | 7 (10) | 24 (9) | 0.906 | 14 (11) | 47 (9) | 0.383 |
| Pack-years [years] | 9.0 (0.0–33.8) | 6.3 (0.0–20.0) | 0.177 | 0.0 (0.0–7.5) | 0.0 (0.0–6.0) | 0.957 | 1.0 (0.0–19.3) | 0.0 (0.0–14.0) | 0.563 |
| Regular alcohol intake [n; %] | 57 (88) | 240 (92) | 0.460 | 65 (93) | 249 (88) | 0.244 | 115 (91) | 496 (90) | 0.856 |
| Self-reported physical inactivity [n; %] | 9 (14) | 24 (9) | 0.271 | 5 (7) | 17 (6) | 0.783 | 14 (11) | 41 (8) | 0.200 |
| Morbidity index [pts.] | 1 (0–2) | 1 (0–2) | 0.751 | 1 (0–1) | 0 (0–1) | 0.463 | 1 (0–2) | 1 (0–1) | 0.380 |
| T2D [n; %] | 15 (23) | 37 (14) | 0.082 | 8 (12) | 14 (5) | 0.041 | 23 (17) | 51 (10) | 0.010 |
| MetS [n; %] | 28 (43) | 113 (44) | 0.897 | 22 (32) | 67 (24) | 0.196 | 50 (37) | 180 (34) | 0.441 |
| Follow-up | |||||||||
| FEV1 [mL] | 2577 ± 570 | 2776 ± 555 | 0.010 | 1936 ± 380 | 1975 ± 390 | 0.461 | 2293 ± 576 | 2347 ± 624 | 0.372 |
| FVC [mL] | 3520 ± 630 | 3728 ± 676 | 0.026 | 2541 ± 461 | 2600 ± 484 | 0.359 | 3084 ± 731 | 3123 ± 813 | 0.623 |
| FEV1/FVC [%] | 74 (70–78) | 75 (71–79) | 0.140 | 77 (72–80) | 76 (73–79) | 0.729 | 76 (71–79) | 76 (72–79) | 0.335 |
| Lp(a) Quintile 1 * | Men | Women | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 | FVC | FEV1/FVC | FEV1 | FVC | FEV1/FVC | |||||||||||||
| Beta | SE | p | Beta | SE | p | Beta | SE | p | Beta | SE | p | Beta | SE | p | Beta | SE | p | |
| Model 1 | −225 | 74 | 0.003 | −237 | 89 | 0.008 | −17 | 9 | 0.078 | −55 | 50 | 0.272 | −78 | 62 | 0.205 | −1 | 8 | 0.926 |
| Model 2 | −216 | 77 | 0.005 | −231 | 92 | 0.013 | −15 | 10 | 0.135 | −69 | 50 | 0.166 | −99 | 61 | 0.105 | −1 | 8 | 0.923 |
| Model 3 | −218 | 81 | 0.008 | −234 | 97 | 0.017 | −15 | 10 | 0.156 | −60 | 52 | 0.251 | −87 | 64 | 0.175 | −1 | 8 | 0.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.K.; Ohlei, O.; Keller, T.; Regitz-Zagrosek, V.; Toepfer, S.; Steinhagen-Thiessen, E.; Bertram, L.; Buchmann, N.; Demuth, I. Lipoprotein(a) and Lung Function Are Associated in Older Adults: Longitudinal and Cross-Sectional Analyses. Biomedicines 2024, 12, 1502. https://doi.org/10.3390/biomedicines12071502
Song CK, Ohlei O, Keller T, Regitz-Zagrosek V, Toepfer S, Steinhagen-Thiessen E, Bertram L, Buchmann N, Demuth I. Lipoprotein(a) and Lung Function Are Associated in Older Adults: Longitudinal and Cross-Sectional Analyses. Biomedicines. 2024; 12(7):1502. https://doi.org/10.3390/biomedicines12071502
Chicago/Turabian StyleSong, Chae Kyung, Olena Ohlei, Theresa Keller, Vera Regitz-Zagrosek, Sarah Toepfer, Elisabeth Steinhagen-Thiessen, Lars Bertram, Nikolaus Buchmann, and Ilja Demuth. 2024. "Lipoprotein(a) and Lung Function Are Associated in Older Adults: Longitudinal and Cross-Sectional Analyses" Biomedicines 12, no. 7: 1502. https://doi.org/10.3390/biomedicines12071502





