Prognostic Influence of Galectin-1 in Gastric Adenocarcinoma
Abstract
:1. Introduction
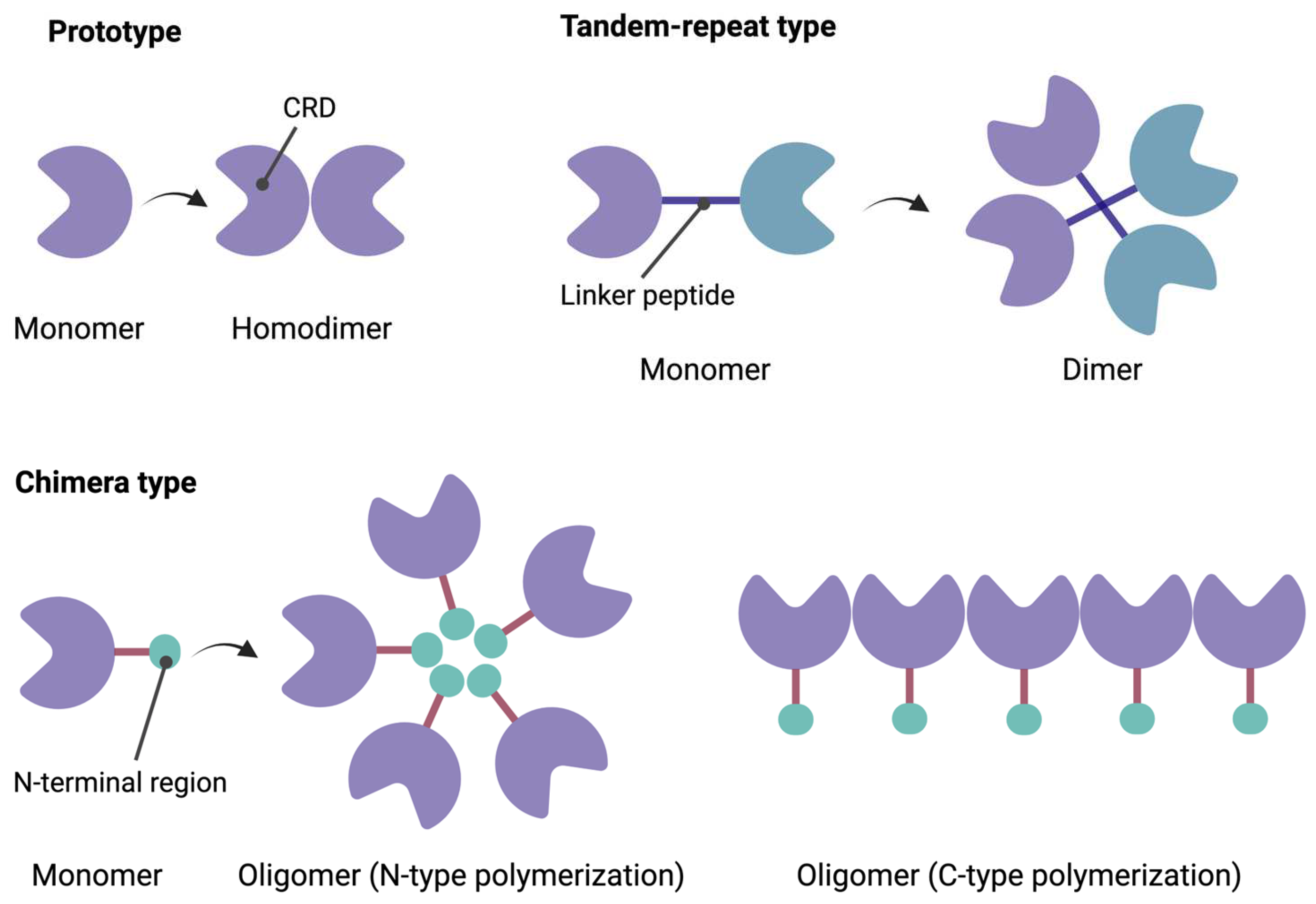
1.1. Structural Characteristics of Galectins
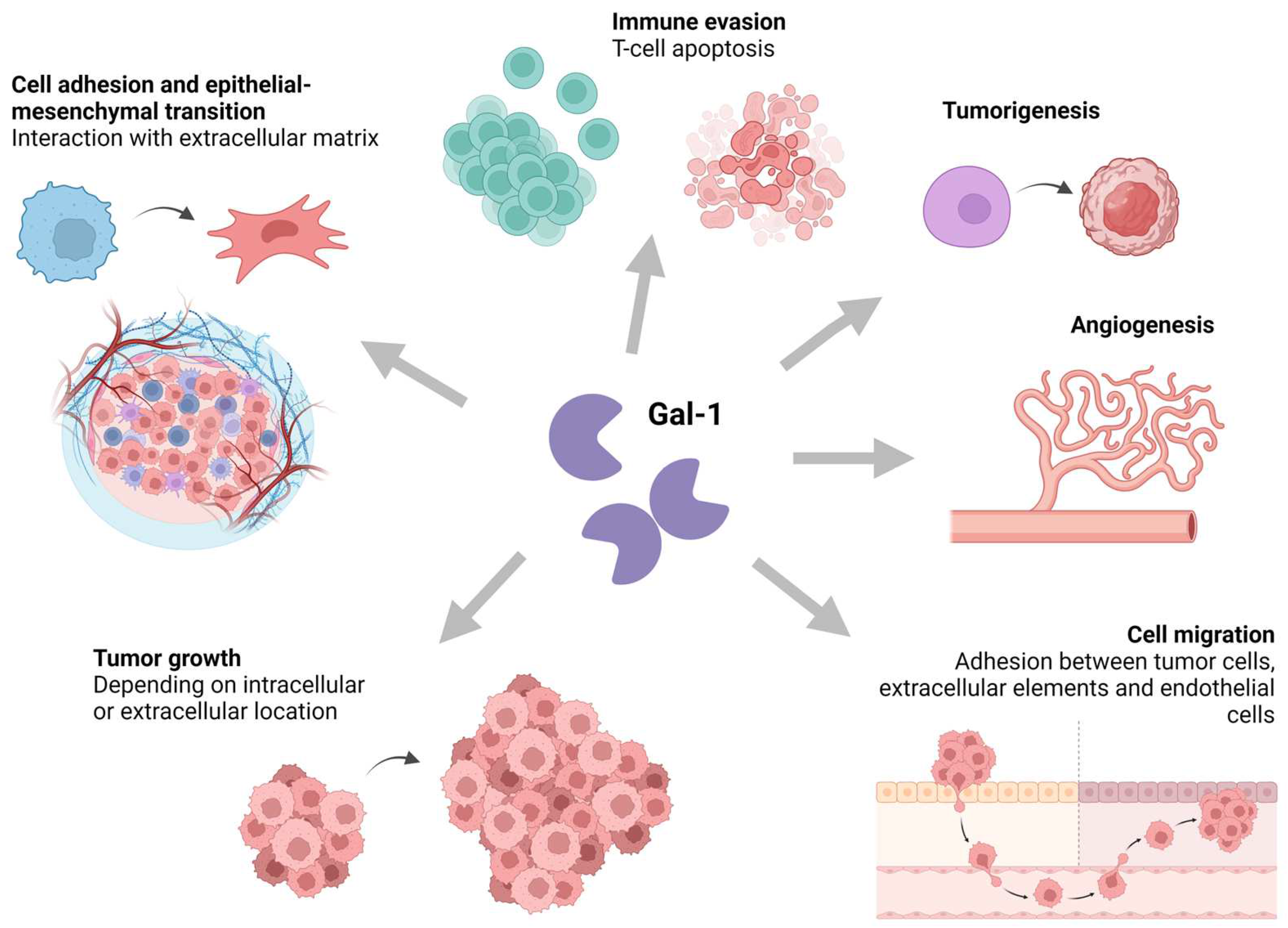
1.2. Functional Roles of Galectin-1
2. Materials and Methods
2.1. Immunohistochemical Study
2.2. Statistical Analysis
2.3. Ethical Approval
3. Results
3.1. Clinicopathological Characteristics of the Study Cohort
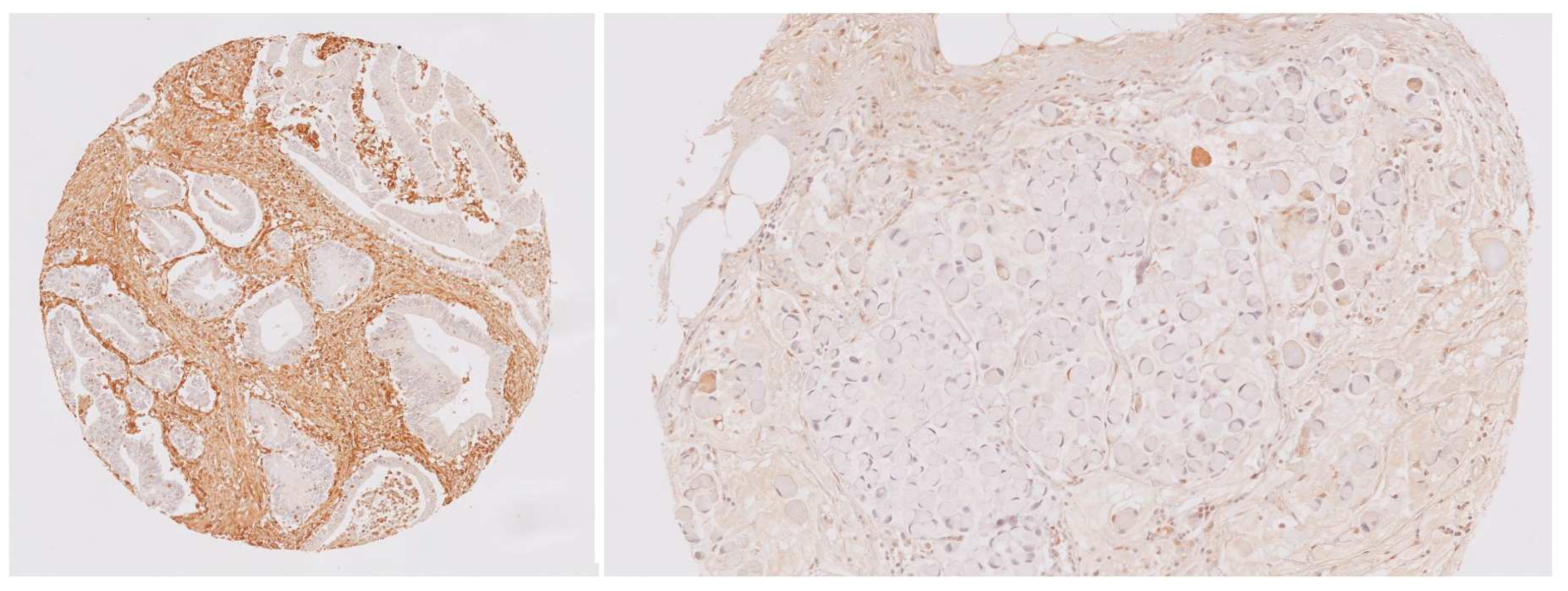
3.2. Gal-1 Immunohistochemical Expression
3.3. Association between Gal-1 Expression and Clinicopathological Findings
3.4. Association between Cancer-Related Death and Clinicopathological Factors
3.4.1. Univariable Analysis
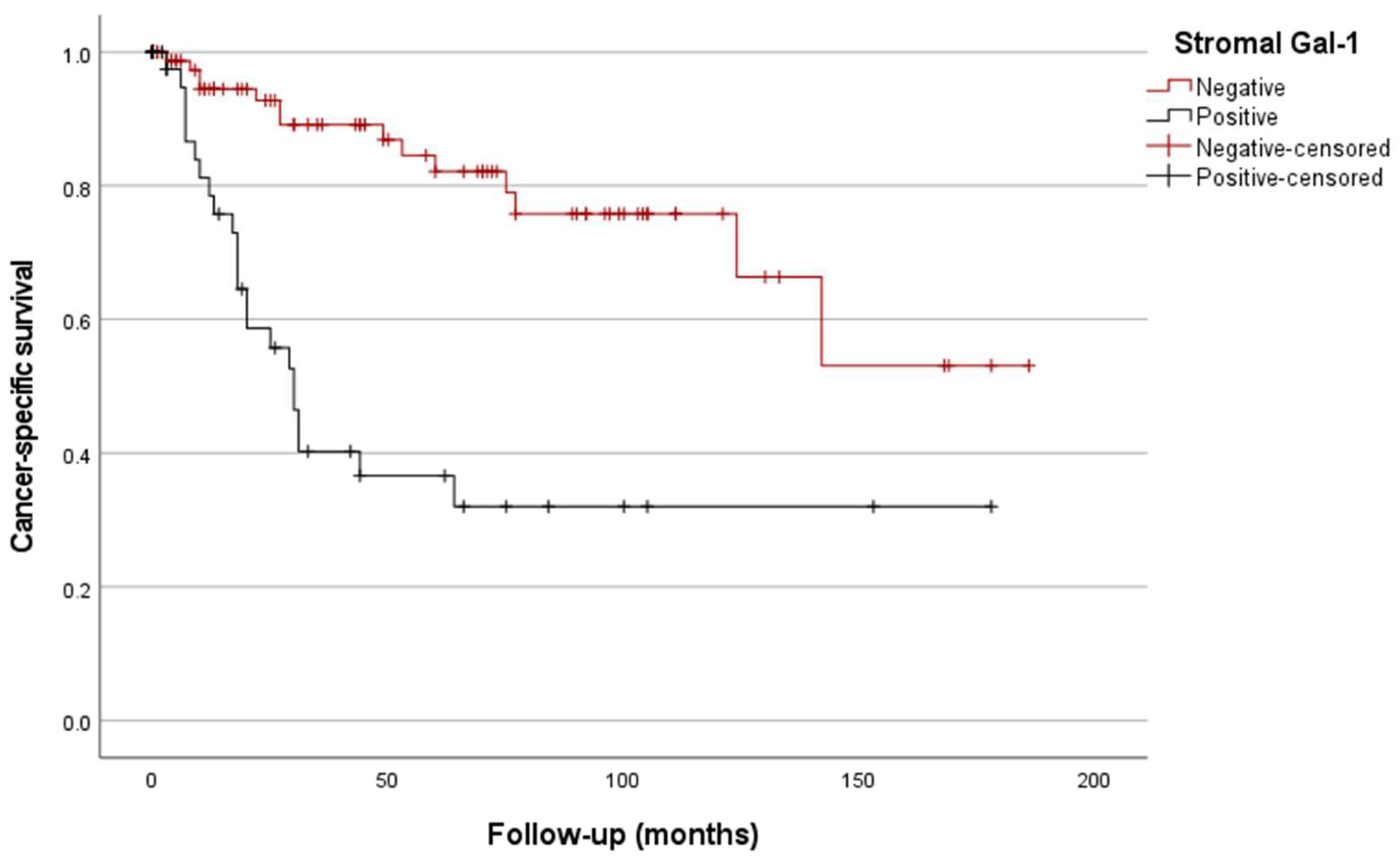
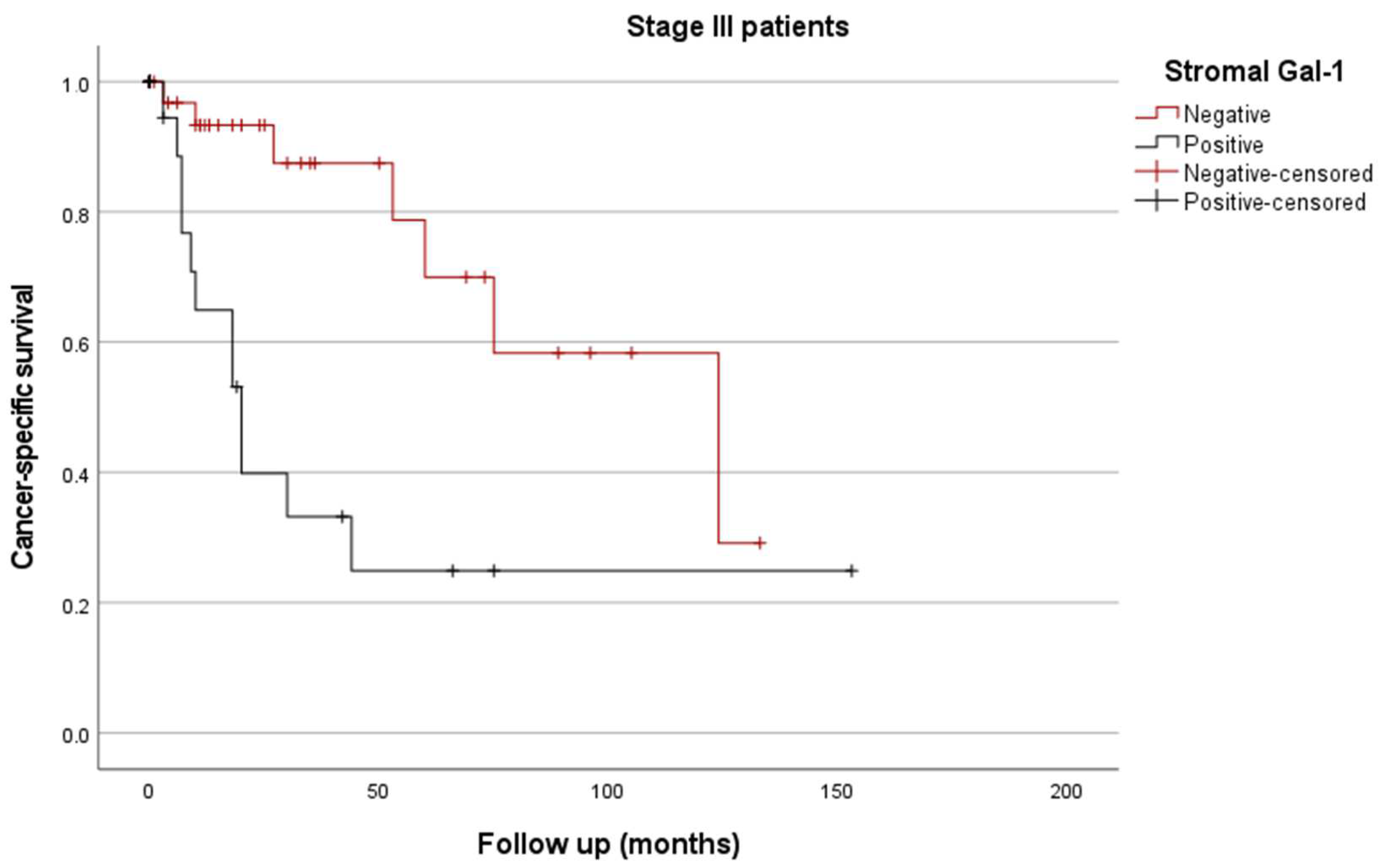
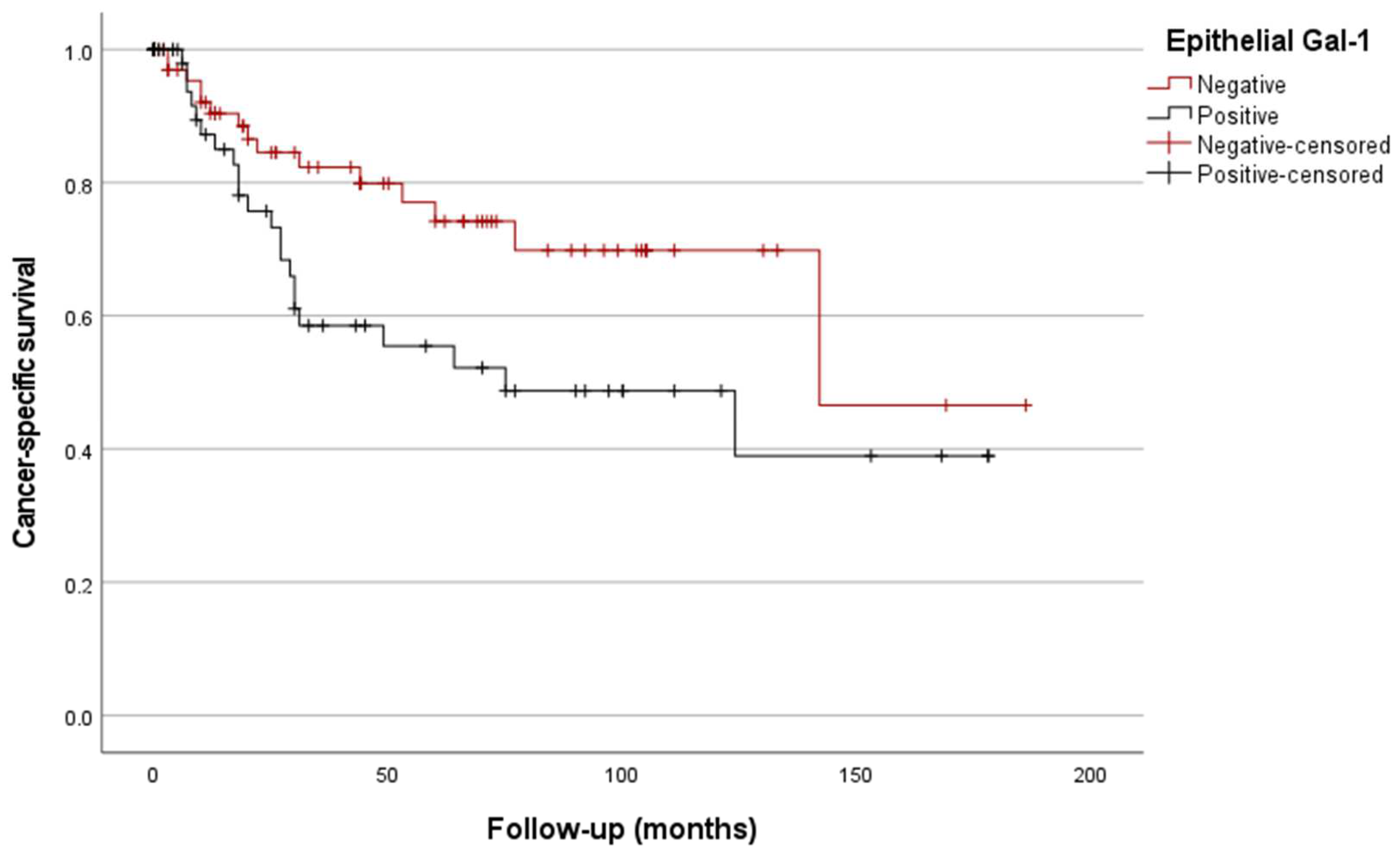
3.4.2. Kaplan/Meier Curves
3.4.3. Multivariable Analysis
4. Discussion
5. Strengths and Limitations of the Study
5.1. Strengths
5.2. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Xie, F.; Chen, B.; Yu, P.; Yu, J.; To, K.F.; Kang, W. Updated Epidemiology of Gastric Cancer in Asia: Decreased Incidence but Still a Big Challenge. Cancers 2023, 15, 2639. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Laversanne, M.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Piñeros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Antoni, S.; et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 2015, 137, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.; Carneiro, F.; Hurban, R.; Theise, N. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- Mantziari, S.; St Amour, P.; Abboretti, F.; Teixeira-Farinha, H.; Gaspar Figueiredo, S.; Gronnier, C.; Schizas, D.; Demartines, N.; Schäfer, M. A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma. Cancers 2023, 15, 1628. [Google Scholar] [CrossRef]
- Ang, Y.L.E.; Yong, W.P.; Tan, P. Translating gastric cancer genomics into targeted therapies. Crit. Rev. Oncol. Hematol. 2016, 100, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.T.; Tan, P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019, 110, 3405–3414. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Al Fatease, A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef] [PubMed]
- Baccili Cury Megid, T.; Farooq, A.R.; Wang, X.; Elimova, E. Gastric Cancer: Molecular Mechanisms, Novel Targets, and Immunotherapies: From Bench to Clinical Therapeutics. Cancers 2023, 15, 5075. [Google Scholar] [CrossRef]
- Selim, J.H.; Shaheen, S.; Sheu, W.C.; Hsueh, C.T. Targeted and novel therapy in advanced gastric cancer. Exp. Hematol. Oncol. 2019, 8, 25. [Google Scholar] [CrossRef]
- Li, H.; Shen, M.; Wang, S. Current therapies and progress in the treatment of advanced gastric cancer. Front. Oncol. 2024, 14, 1327055. [Google Scholar] [CrossRef]
- Mathias-Machado, M.C.; de Jesus, V.H.F.; Jácome, A.; Donadio, M.D.; Aruquipa, M.P.S.; Fogacci, J.; Cunha, R.G.; da Silva, L.M.; Peixoto, R.D. Claudin 18.2 as a New Biomarker in Gastric Cancer-What Should We Know? Cancers 2024, 16, 679. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk-Brzezinska, A.J.; Bujacz, A. H-type lectins–Structural characteristics and their applications in diagnostics, analytics and drug delivery. Int. J. Biol. Macromol. 2020, 152, 735–747. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Itabashi, T. Galectins and Their Ligand Glycoconjugates in the Central Nervous System Under Physiological and Pathological Conditions. Front. Neuroanat. 2021, 15, 767330. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key regulators of galectin-glycan interactions. Proteomics 2016, 16, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Modenutti, C.P.; Capurro, J.I.B.; Di Lella, S.; Martí, M.A. The Structural Biology of Galectin-Ligand Recognition: Current Advances in Modeling Tools, Protein Engineering, and Inhibitor Design. Front. Chem. 2019, 7, 823. [Google Scholar] [CrossRef] [PubMed]
- Guardia, C.M.; Caramelo, J.J.; Trujillo, M.; Méndez-Huergo, S.P.; Radi, R.; Estrin, D.A.; Rabinovich, G.A. Structural basis of redox-dependent modulation of galectin-1 dynamics and function. Glycobiology 2014, 24, 428–441. [Google Scholar] [CrossRef]
- Corral, J.M.; del Puerto-Nevado, L.; Cedeño, M.; Río-Vilariño, A.; Mahillo-Fernández, I.; Galeano, C.; Baños, N.; García-Foncillas, J.; Dómine, M.; Cebrián, A. Galectin-1, a novel promising target for outcome prediction and treatment in SCLC. Biomed. Pharmacother. 2022, 156, 113987. [Google Scholar] [CrossRef]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Neuzillet, C.; Albert, S.; Raymond, E.; Faivre, S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat. Rev. 2014, 40, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.S.; Soukya, P.S.L.; Ghouse, M.; Komal, D.; Alvala, R.; Alvala, M. Human Galectin-1 and Its Inhibitors: Privileged Target for Cancer and HIV. Mini Rev. Med. Chem. 2019, 19, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bosch, N.; Fernández-Barrena, M.G.; Moreno, M.; Ortiz-Zapater, E.; Munné-Collado, J.; Iglesias, M.; André, S.; Gabius, H.J.; Hwang, R.F.; Coise Poirier, F.; et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation. Cancer Res. 2014, 74, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Id Boufker, H.; Stamatopoulos, B.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Modulated expression of adhesion molecules and galectin-1: Role during mesenchymal stromal cell immunoregulatory functions. Exp. Hematol. 2010, 38, 922–932. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Li, C.Y.; Huang, Y.H.; Chang, T.S.; Lin, C.Y.; Chuang, C.H.; Wang, C.Y.; Anuraga, G.; Chang, T.H.; Shih, T.C.; et al. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene 2022, 41, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Porębska, N.; Poźniak, M.; Matynia, A.; Żukowska, D.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Galectins as modulators of receptor tyrosine kinases signaling in health and disease. Cytokine Growth Factor Rev. 2021, 60, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, K.; Richter, M.; Lavrik, I.N. Decoding the sweet regulation of apoptosis: The role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019, 26, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Rudjord-Levann, A.M.; Ye, Z.; Hafkenscheid, L.; Horn, S.; Wiegertjes, R.; Nielsen, M.A.I.; Song, M.; Mathiesen, C.B.K.; Stoop, J.; Stowell, S.; et al. Galectin-1 induces a tumor-associated macrophage phenotype and upregulates indoleamine 2,3-dioxygenase-1. iScience 2023, 26, 106984. [Google Scholar] [CrossRef]
- Chen, L.; Yao, Y.; Sun, L.; Tang, J. Galectin-1 promotes tumor progression via NF-κB signaling pathway in epithelial ovarian cancer. J. Cancer 2017, 8, 3733–3741. [Google Scholar] [CrossRef]
- Yu, X.; Qian, J.; Ding, L.; Yin, S.; Zhou, L.; Zheng, S. Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities. Int. J. Mol. Sci. 2023, 24, 6501. [Google Scholar] [CrossRef]
- Díaz Del Arco, C.; Ortega Medina, L.; Estrada Muñoz, L.; Molina Roldán, E.; Cerón Nieto, M.Á.; García Gómez de las Heras, S.; Fernández Aceñero, M.J. Are Borrmann’s Types of Advanced Gastric Cancer Distinct Clinicopathological and Molecular Entities? A Western Study. Cancers 2021, 13, 3081. [Google Scholar] [CrossRef] [PubMed]
- Horcic, M.; Koelzer, V.H.; Karamitopoulou, E.; Terracciano, L.; Puppa, G.; Zlobec, I.; Lugli, A. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum. Pathol. 2013, 44, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Shimazaki, H.; Aida, S.; Hase, K.; Matsukuma, S.; Kanai, T.; Kurihara, H.; Ozawa, K.; et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004, 127, 385–394. [Google Scholar] [CrossRef]
- Huang, S.F.; Chien, T.H.; Fang, W.L.; Wang, F.; Tsai, C.Y.; Te Hsu, J.; Yeh, C.N.; Chen, T.C.; Wu, R.C.; Chiu, C.T.; et al. The 8th edition American Joint Committee on gastric cancer pathological staging classification performs well in a population with high proportion of locally advanced disease. Eur. J. Surg. Oncol. 2018, 44, 1634–1639. [Google Scholar] [CrossRef]
- Porciúncula-González, C.; Cagnoni, A.J.; Fontana, C.; Mariño, K.V.; Saenz-Méndez, P.; Giacomini, C.; Irazoqui, G. Structural insights in galectin-1-glycan recognition: Relevance of the glycosidic linkage and the N-acetylation pattern of sugar moieties. Bioorg. Med. Chem. 2021, 44, 116309. [Google Scholar] [CrossRef] [PubMed]
- Pasmatzi, E.; Papadionysiou, C.; Monastirli, A.; Badavanis, G.; Tsambaos, D. Galectin 1 in dermatology: Current knowledge and perspectives. Acta Dermatovenerologica Alpina Pannonica Adriat. 2019, 28, 27–31. [Google Scholar] [CrossRef]
- Bacigalupo, M.L.; Carabias, P.; Troncoso, M.F. Contribution of galectin-1, a glycan-binding protein, to gastrointestinal tumor progression. World J. Gastroenterol. 2017, 23, 5266. [Google Scholar] [CrossRef]
- Bacigalupo, M.L.; Piazza, V.G.; Cicconi, N.S.; Carabias, P.; Bartke, A.; Fang, Y.; Sotelo, A.I.; Rabinovich, G.A.; Troncoso, M.F.; Miquet, J.G. Growth hormone upregulates the pro-tumorigenic galectin 1 in mouse liver. Endocr. Connect. 2019, 8, 1108–1117. [Google Scholar] [CrossRef]
- Dillon, M.; Lopez, A.; Lin, E.; Sales, D.; Perets, R.; Jain, P. Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers 2021, 13, 5059. [Google Scholar] [CrossRef]
- Wu, R.; Wu, T.; Wang, K.; Luo, S.; Chen, Z.; Fan, M.; Xue, D.; Lu, H.; Zhuang, Q.; Xu, X. Prognostic significance of galectin-1 expression in patients with cancer: A meta-analysis. Cancer Cell Int. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liu, Q.; Wu, J.; Wang, Y.; Dai, J.; Chen, D.; Zhou, Y.; Lian, Y. Galectin-1 Promotes Vasculogenic Mimicry in Gastric Cancer by Upregulating EMT Signaling. J. Cancer 2019, 10, 6286–6297. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, N.; Zhang, J.; Xie, J.; Liu, F.; Xu, D.; Yu, X.; Tian, Y. Advanced research on vasculogenic mimicry in cancer. J. Cell. Mol. Med. 2015, 19, 315–326. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, L.; Liang, N.; Xie, J.; Zhang, J.; Deng, G.; Luo, H.; Zhang, J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J. Cell. Mol. Med. 2016, 20, 1761. [Google Scholar] [CrossRef] [PubMed]
- Racordon, D.; Valdivia, A.; Mingo, G.; Erices, R.; Aravena, R.; Santoro, F.; Bravo, M.L.; Ramirez, C.; Gonzalez, P.; Sandoval, A.; et al. Structural and functional identification of vasculogenic mimicry in vitro. Sci. Rep. 2017, 7, 6985. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, E.; Oyanadel, C.; de la Peña, A.; Hernández, R.; Pérez-Molina, F.; Metz, C.; González, A.; Soza, A. Galectins in epithelial-mesenchymal transition: Roles and mechanisms contributing to tissue repair, fibrosis and cancer metastasis. Biol. Res. 2024, 57, 14. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Tao, H.Q.; Hu, Z.M.; Ma, Y.Y.; Xu, J.; Wang, H.J.; Xia, Y.J.; Li, L.; Fei, B.Y.; Li, Y.Q.; et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1. Cancer Sci. 2014, 105, 1402–1410. [Google Scholar] [CrossRef]
- Chong, Y.; Tang, D.; Gao, J.; Jiang, X.; Xu, C.; Xiong, Q.; Huang, Y.; Wang, J.; Zhou, H.; Shi, Y.; et al. Galectin-1 induces invasion and the epithelial-mesenchymal transition in human gastric cancer cells via non-canonical activation of the hedgehog signaling pathway. Oncotarget 2016, 7, 83611. [Google Scholar] [CrossRef]
- Wu, H.; Chen, P.; Liao, R.; Li, Y.W.; Yi, Y.; Wang, J.X.; Sun, T.W.; Zhou, J.; Shi, Y.H.; Yang, X.R.; et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J. Gastroenterol. Hepatol. 2012, 27, 1312–1319. [Google Scholar] [CrossRef]
- Barrow, H.; Rhodes, J.M.; Yu, L.G. The role of galectins in colorectal cancer progression. Int. J. cancer 2011, 129, 1–8. [Google Scholar] [CrossRef]
- Huang, C.S.; Tang, S.J.; Chung, L.Y.; Yu, C.P.; Ho, J.Y.; Cha, T.L.; Hsieh, C.C.; Wang, H.H.; Sun, G.H.; Sun, K.H. Galectin-1 upregulates CXCR4 to promote tumor progression and poor outcome in kidney cancer. J. Am. Soc. Nephrol. 2014, 25, 1486–1495. [Google Scholar] [CrossRef]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef]
- Wu, Z.; Boonmars, T.; Nagano, I.; Boonjaraspinyo, S.; Pinlaor, S.; Pairojkul, C.; Chamgramol, Y.; Takahashi, Y. Alteration of galectin-1 during tumorigenesis of Opisthorchis viverrini infection-induced cholangiocarcinoma and its correlation with clinicopathology. Tumour Biol. 2012, 33, 1169–1178. [Google Scholar] [CrossRef]
- Le, Q.T.; Shi, G.; Cao, H.; Nelson, D.W.; Wang, Y.; Chen, E.Y.; Zhao, S.; Kong, C.; Richardson, D.; O’Byrne, K.J.; et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. 2005, 23, 8932–8941. [Google Scholar] [CrossRef]
- Schulkens, I.A.; Heusschen, R.; Van Den Boogaart, V.; Van Suylen, R.J.; Dingemans, A.M.C.; Griffioen, A.W.; Thijssen, V.L. Galectin Expression Profiling Identifies Galectin-1 and Galectin-9Δ5 as Prognostic Factors in Stage I/II Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e107988. [Google Scholar] [CrossRef]
- Wu, T.F.; Li, C.F.; Chien, L.H.; Shen, K.H.; Huang, H.Y.; Su, C.C.; Liao, A.C. Galectin-1 dysregulation independently predicts disease specific survival in bladder urothelial carcinoma. J. Urol. 2015, 193, 1002–1008. [Google Scholar] [CrossRef]
- He, J.; Baum, L.G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Investig. 2006, 86, 578–590. [Google Scholar] [CrossRef]
- Croci, D.O.; Salatino, M.; Rubinstein, N.; Cerliani, J.P.; Cavallin, L.E.; Leung, H.J.; Ouyang, J.; Ilarregui, J.M.; Toscano, M.A.; Domaica, C.I.; et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 2012, 209, 1985–2000. [Google Scholar] [CrossRef]
- You, X.; Wang, Y.; Wu, J.; Lliu, Q.; Cchen, D.; Tang, D.; Wang, D. Prognostic significance of galectin-1 and vasculogenic mimicry in patients with gastric cancer. Onco. Targets. Ther. 2018, 11, 3237. [Google Scholar] [CrossRef]
- Kamper, P.; Ludvigsen, M.; Bendix, K.; Hamilton-Dutoit, S.; Rabinovich, G.A.; Møller, M.B.; Nyengaard, J.R.; Honoré, B.; D’Amore, F. Proteomic analysis identifies galectin-1 as a predictive biomarker for relapsed/refractory disease in classical Hodgkin lymphoma. Blood 2011, 117, 6638–6649. [Google Scholar] [CrossRef]
- Rorive, S.; Belot, N.; Decaestecker, C.; Lefrank, F.; Gordower, L.; Micik, S.; Maurage, C.-A.; Kaltner, H.; Ruchoux, M.M.; Danguy, A.; et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma–PubMed. Glia 2001, 33, 241–255. [Google Scholar] [CrossRef]
- You, X.; Wu, J.; Zhao, X.; Jiang, X.; Tao, W.; Chen, Z.; Huang, C.; Zheng, T.; Shen, X. Fibroblastic galectin-1-fostered invasion and metastasis are mediated by TGF-β1-induced epithelial-mesenchymal transition in gastric cancer. Aging 2021, 13, 18464. [Google Scholar] [CrossRef]
- Bektas, S.; Bahadir, B.; Ucan, B.H.; Ozdamar, S.O. CD24 and galectin-1 expressions in gastric adenocarcinoma and clinicopathologic significance. Pathol. Oncol. Res. 2010, 16, 569–577. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.J.; Zhang, Y.; Zhang, G.Q.; Zha, T.Z.; Feng, Y.Z.; Zhang, K. Clinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancer. World J. Gastroenterol. 2013, 19, 2073. [Google Scholar] [CrossRef]
- Chen, J.; Tang, D.; Wang, S.; Li, Q.G.; Zhang, J.R.; Li, P.; Lu, Q.; Niu, G.; Gao, J.; Ye, N.Y.; et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour Biol. 2014, 35, 2513–2519. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, C.; Guan, Z.; Su, X.; Xu, Z.; Cao, J.; Teng, L. Galectin-1 mediates TGF-β-induced transformation from normal fibroblasts into carcinoma-associated fibroblasts and promotes tumor progression in gastric cancer. Am. J. Transl. Res. 2016, 8, 1641. [Google Scholar]
- Chong, Y.; Tang, D.; Xiong, Q.; Jiang, X.; Xu, C.; Huang, Y.; Wang, J.; Zhou, H.; Shi, Y.; Wu, X.; et al. Galectin-1 from cancer-associated fibroblasts induces epithelial-mesenchymal transition through β1 integrin-mediated upregulation of Gli1 in gastric cancer. J. Exp. Clin. Cancer Res. 2016, 35, 175. [Google Scholar] [CrossRef]
- Schulz, H.; Schmoeckel, E.; Kuhn, C.; Hofmann, S.; Mayr, D.; Mahner, S.; Jeschke, U. Galectins-1, -3, and -7 Are Prognostic Markers for Survival of Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 1230. [Google Scholar] [CrossRef]
- Huang, M.Y.; He, J.P.; Zhang, W.Q.; Liu, J.L. Pooling analysis reveals that galectin-1 is a reliable prognostic biomarker in various cancers. J. Cell. Physiol. 2019, 234, 13788–13798. [Google Scholar] [CrossRef]
- Long, B.; Yu, Z.; Zhou, H.; Ma, Z.; Ren, Y.; Zhan, H.; Li, L.; Cao, H.; Jiao, Z. Clinical characteristics and prognostic significance of galectins for patients with gastric cancer: A meta-analysis. Int. J. Surg. 2018, 56, 242–249. [Google Scholar] [CrossRef]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Dimitroff, C.J. Galectin-1 research in T cell immunity: Past, present and future. Clin. Immunol. 2012, 142, 107–116. [Google Scholar] [CrossRef]
- Rabinovich, G.A. Galectin-1 as a potential cancer target. Br. J. Cancer 2005, 92, 1188–1192. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Krüger, C.; von Wasielewski, R.; Hoffmann, M.; Huster, K.M.; Busch, D.H.; Manns, M.P.; Korangy, F.; Greten, T.F. Necrotic tumor cell death in vivo impairs tumor-specific immune responses. J. Immunol. 2007, 178, 1573–1580. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Motran, C.C.; Molinder, K.M.; Liu, S.D.; Poirier, F.; Miceli, M.C. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur. J. Immunol. 2008, 38, 3015. [Google Scholar] [CrossRef]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; Von Eyben, R.; et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef]
- You, Y.; Tan, J.X.; Dai, H.S.; Chen, H.W.; Xu, X.J.; Yang, A.G.; Zhang, Y.J.; Bai, L.H.; Bie, P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget 2016, 7, 57099–57116. [Google Scholar] [CrossRef]
- He, J.; Baum, L.G. Presentation of galectin-1 by extracellular matrix triggers T cell death. J. Biol. Chem. 2004, 279, 4705–4712. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.C.; Zhao, J.; Wu, M.H.; Shih, T.C. Immunosuppressive Roles of Galectin-1 in the Tumor Microenvironment. Biomolecules 2021, 11, 1398. [Google Scholar] [CrossRef]
- Orozco, C.A.; Martinez-Bosch, N.; Guerrero, P.E.; Vinaixa, J.; Dalotto-Moreno, T.; Iglesias, M.; Moreno, M.; Djurec, M.; Poirier, F.; Gabius, H.J.; et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc. Natl. Acad. Sci. USA 2018, 115, E3769–E3778. [Google Scholar] [CrossRef]
- Soldati, R.; Berger, E.; Zenclussen, A.C.; Jorch, G.; Lode, H.N.; Salatino, M.; Rabinovich, G.A.; Fest, S. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int. J. Cancer 2012, 131, 1131–1141. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Giribaldi, M.L.; Blidner, A.G.; Cutine, A.M.; Gatto, S.G.; Morales, R.M.; Salatino, M.; Abba, M.C.; Croci, D.O.; Mariño, K.V.; et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2102950118. [Google Scholar] [CrossRef]
- Alhabbab, R.; Blair, P.; Smyth, L.A.; Ratnasothy, K.; Peng, Q.; Moreau, A.; Lechler, R.; Elgueta, R.; Lombardi, G. Galectin-1 is required for the regulatory function of B cells. Sci. Rep. 2018, 8, 2725. [Google Scholar] [CrossRef]
- Leung, Z.; Ko, F.C.F.; Tey, S.K.; Kwong, E.M.L.; Mao, X.; Liu, B.H.M.; Ma, A.P.Y.; Fung, Y.M.E.; Che, C.M.; Wong, D.K.H.; et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. 2019, 38, 423. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, A.; Li, S.; Tao, X.; Sheng, B.; Chetry, M.; Zhu, X. Predictive role of galectin-1 and integrin α5β1 in cisplatin-based neoadjuvant chemotherapy of bulky squamous cervical cancer. Biosci. Rep. 2017, 37, 37–38. [Google Scholar] [CrossRef]
- Su, Y.C.; Davuluri, G.V.N.; Chen, C.H.; Shiau, D.C.; Chen, C.C.; Chen, C.L.; Lin, Y.S.; Chang, C.P. Galectin-1-Induced Autophagy Facilitates Cisplatin Resistance of Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0148408. [Google Scholar] [CrossRef]
- Paz, H.; Joo, E.J.; Chou, C.H.; Fei, F.; Mayo, K.H.; Abdel-Azim, H.; Ghazarian, H.; Groffen, J.; Heisterkamp, N. Treatment of B-cell precursor acute lymphoblastic leukemia with the Galectin-1 inhibitor PTX008. J. Exp. Clin. Cancer Res. 2018, 37, 67. [Google Scholar] [CrossRef]
- Shih, T.C.; Liu, R.; Fung, G.; Bhardwaj, G.; Ghosh, P.M.; Lam, K.S. A Novel Galectin-1 Inhibitor Discovered through One-Bead Two-Compound Library Potentiates the Antitumor Effects of Paclitaxel in vivo. Mol. Cancer Ther. 2017, 16, 1212–1223. [Google Scholar] [CrossRef]
- Goud, N.S.; Bhattacharya, A. Human Galectin-1 in Multiple Cancers: A Privileged Molecular Target in Oncology. Mini Rev. Med. Chem. 2021, 21, 2169–2186. [Google Scholar] [CrossRef]
- Greer, P.F.C.; Rich, A.; Coates, D.E. Effects of galectin-1 inhibitor OTX008 on oral squamous cell carcinoma cells in vitro and the role of AP-1 and the MAPK/ERK pathway. Arch. Oral Biol. 2022, 134, 105335. [Google Scholar] [CrossRef]
- Gheysen, L.; Soumoy, L.; Trelcat, A.; Verset, L.; Journe, F.; Saussez, S. New Treatment Strategy Targeting Galectin-1 against Thyroid Cancer. Cells 2021, 10, 1112. [Google Scholar] [CrossRef]
| Features | Patients, n (Valid %), n = 149 | |
|---|---|---|
| Age (years) [median (range)] | 76 (33–91) | |
| Male gender | 82 (55) | |
| Symptoms | Symptomatic | 106 (89.3) |
| Local symptoms | 76 (65) | |
| Systemic symptoms | 65 (55.1) | |
| Size, mm [median (range)] | 50 (10–120) | |
| Depth, mm [median (range)] | 10 (1–29) | |
| Macroscopic type | Polypoid | 31 (21.8) |
| Flat | 11 (7.7) | |
| Ulcerative | 45 (31.7) | |
| Fungoid | 55 (38.7) | |
| Location | Cardias | 2 (1.5) |
| Fundus | 12 (9.2) | |
| Body | 43 (33.1) | |
| Antrum | 73 (56.2) | |
| Laurén subtype | Intestinal | 87 (59.2) |
| Diffuse | 43 (29.3) | |
| Mixed | 17 (11.6) | |
| High grade | 78 (53.1) | |
| Necrosis | 40 (27) | |
| Signet ring cells | 63 (42.9) | |
| Lymphovascular invasion | 64 (43.2) | |
| Perineural infiltration | 67 (45.3) | |
| Advancing front (infiltrative) | 91 (61.9) | |
| Budding | 24 (26.1) | |
| Desmoplasia | 72 (49.7) | |
| Intratumoral lymphocytic infiltration | Mild/moderate | 25 (18.1) |
| Intense | 105 (76.1) | |
| Peritumoral lymphocytic infiltration | 40 (27.4) | |
| Lymph node metastases | 96 (67.6) | |
| Number of metastatic lymph nodes [median (range)] | 3 (1–47) | |
| pT | T1 | 8 (5.5) |
| T2 | 29 (19.9) | |
| T3 | 90 (61.6) | |
| T4 | 19 (13) | |
| pN | N0 | 46 (32.4) |
| N1 | 26 (18.3) | |
| N2 | 37 (26.1) | |
| N3 | 33 (23.2) | |
| pTNM | I | 21 (15.2) |
| II | 51 (37) | |
| III | 66 (47.8) | |
| Gastrectomy | Subtotal | 105 (70.9) |
| Total | 43 (29.1) | |
| Lymphadenectomy | D1 | 7 (4.7) |
| D2 | 29 (19.5) | |
| NS a | 113 (75.8) | |
| Number of LN b resected | ≥16 | 87 (61.3) |
| <16 | 55 (38.7) | |
| Adjuvant therapy | 26 (23.2) | |
| Recurrence | 64 (44.1) | |
| Death due to tumor | 37 (26.6) | |
| Epithelial Expression (n = 95) | n | % | |
|---|---|---|---|
| Expression pattern | Cytoplasmic | 82 | 86.3 |
| Nuclear | 0 | ||
| Cytoplasmic and nuclear | 8 | 8.4 | |
| Cytoplasmic and membranous | 5 | 5.3 | |
| Staining intensity | Mild | 69 | 72.6 |
| Moderate | 26 | 27.4 | |
| Stromal Expression (n = 143) | n | % | |
| Expression pattern | Cytoplasmic | 143 | |
| Nuclear | 0 | 100 | |
| Staining intensity | Mild | 87 | 58.4 |
| Moderate | 56 | 41.6 | |
| Feature | Gal-1 Neg | Gal-1 + | p | |
|---|---|---|---|---|
| Necrosis | 19.8% | 42.6% | 0.004 | |
| Lymphovascular invasion | 37.6% | 55.3% | 0.043 | |
| pTNM stage (II–III) | 79.7% | 95.5% | 0.017 | |
| Recurrence | 38.14% | 56.2% | 0.039 | |
| Death due to tumor | 15.4% | 47.9% | <0.001 | |
| Peritumoral lymphocytic infiltration | 22.8% | 37.8% | 0.060 | |
| Lymph node metastases | 62.5% | 78.3% | 0.060 | |
| pN | pN0 | 37.5% | 21.7% | 0.066 |
| pN1 | 16.7% | 21.7% | ||
| pN2 | 28.12% | 21.7% | ||
| pN3 | 17.7% | 34.8% | ||
| Feature | Gal-1 Neg | Gal-1 + | p |
|---|---|---|---|
| Lymphovascular invasion | 34.9% | 54.8% | 0.016 |
| Peritumoral lymphocytic infiltration | 21.2% | 36.1% | 0.047 |
| Death due to tumor | 19.5% | 35.5% | 0.034 |
| Tumor grade (high) | 46.5% | 62.3% | 0.059 |
| pTNM stage (II–III) | 80.5% | 91.1% | 0.089 |
| Feature | OR (95% CI a) | p | |
|---|---|---|---|
| Necrosis | 2.5 (1.1–5.5) | 0.027 | |
| Laurén (diffuse) | 2.3 (1–5.2) | 0.039 | |
| pN | pN0 | 1 | 0.029 |
| pN1 | 1.7 (0.5–5.4) | ||
| pN2 | 0.9 (0.3–3) | ||
| pN3 | 3.8 (1.3–10.9) | ||
| Stromal Gal-1 + | 5.1 (2.3–11.3) | <0.001 | |
| Epithelial Gal-1 + | 2.3 (1.1–4.9) | 0.034 | |
| LNR b (median) | 0.25 vs. 0.1 | 0.011 | |
| Metastatic lymph nodes (median) | 4 vs. 2 | 0.043 | |
| Age at diagnosis (median) | 68 vs. 76 | 0.013 | |
| Signet ring cells | 2.15 (1–4.7) | 0.051 | |
| Covariates | HR (95% CI a) | p |
|---|---|---|
| Laurén (diffuse) | 2.65 (1.26–5.59) | 0.011 |
| LNR b | 7.71 (2.72–21.86) | <0.001 |
| Stromal Gal-1 + | 3.93 (1.84–8.4) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz del Arco, C.; Estrada Muñoz, L.; Cerón Nieto, M.d.l.Á.; Molina Roldán, E.; Fernández Aceñero, M.J.; García Gómez de las Heras, S. Prognostic Influence of Galectin-1 in Gastric Adenocarcinoma. Biomedicines 2024, 12, 1508. https://doi.org/10.3390/biomedicines12071508
Díaz del Arco C, Estrada Muñoz L, Cerón Nieto MdlÁ, Molina Roldán E, Fernández Aceñero MJ, García Gómez de las Heras S. Prognostic Influence of Galectin-1 in Gastric Adenocarcinoma. Biomedicines. 2024; 12(7):1508. https://doi.org/10.3390/biomedicines12071508
Chicago/Turabian StyleDíaz del Arco, Cristina, Lourdes Estrada Muñoz, María de los Ángeles Cerón Nieto, Elena Molina Roldán, María Jesús Fernández Aceñero, and Soledad García Gómez de las Heras. 2024. "Prognostic Influence of Galectin-1 in Gastric Adenocarcinoma" Biomedicines 12, no. 7: 1508. https://doi.org/10.3390/biomedicines12071508





