Comparative Efficacy of Various Exercise Therapies and Combined Treatments on Inflammatory Biomarkers and Morphological Measures of Skeletal Muscle among Older Adults with Knee Osteoarthritis: A Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol and Design
2.2. Search Strategy
2.3. PICOS Criteria for Study Selection
2.4. Outcome Measures
2.5. Data Extraction
2.6. Risks of Bias and Methodological Quality in Individual Study and across Studies
2.7. Data Synthesis and Analysis
2.8. Certainty of Evidence
3. Results
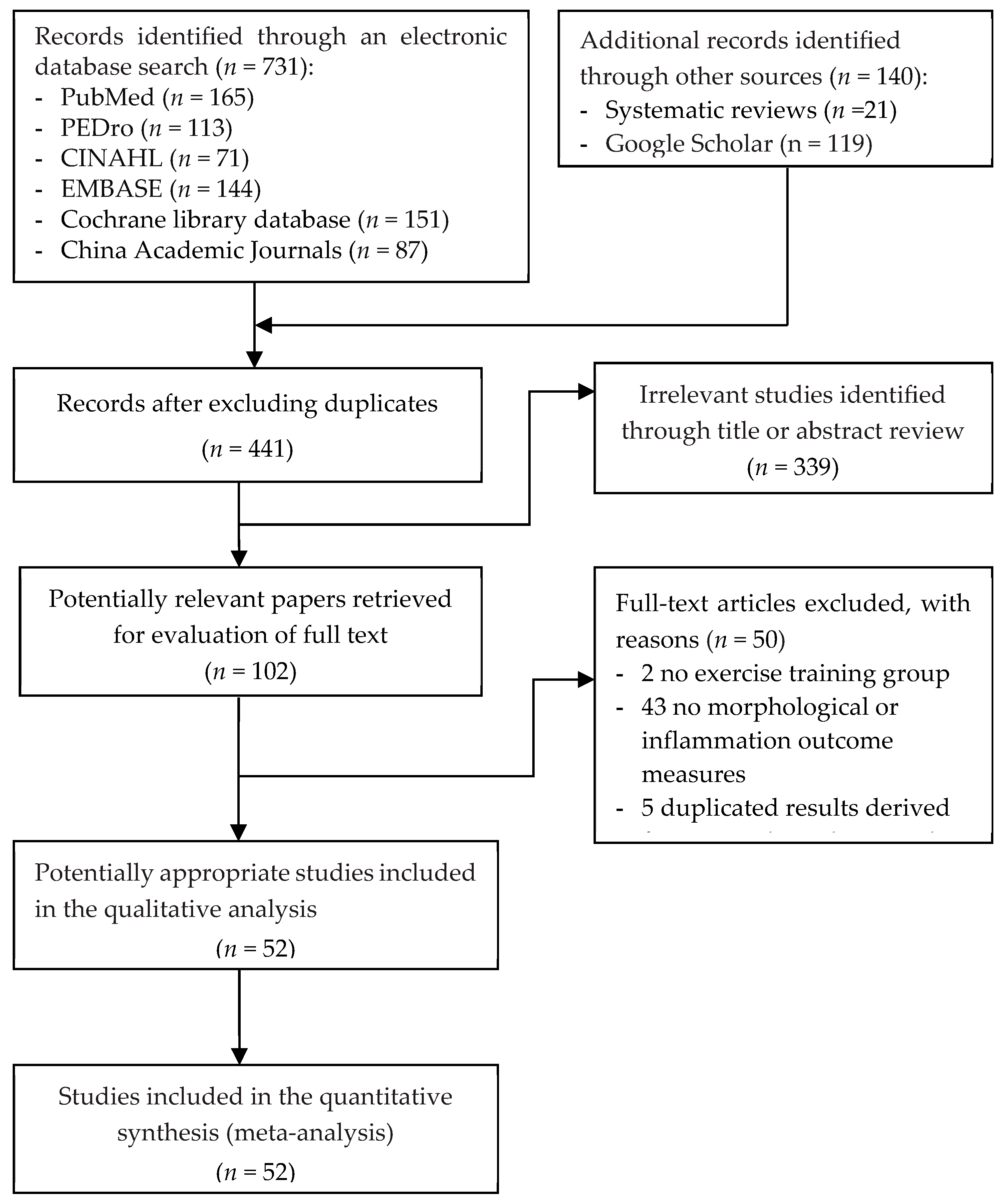
3.1. Selection of Studies
3.2. Characteristics of Analyzed Patients
3.3. Exercise Intervention Protocol
3.4. Risks of Bias in Individual Studies and across STUDIES
3.4.1. Selection Bias
3.4.2. Masks for Performance and Detection Biases
3.4.3. Attrition Bias
3.4.4. Research-Agenda Bias
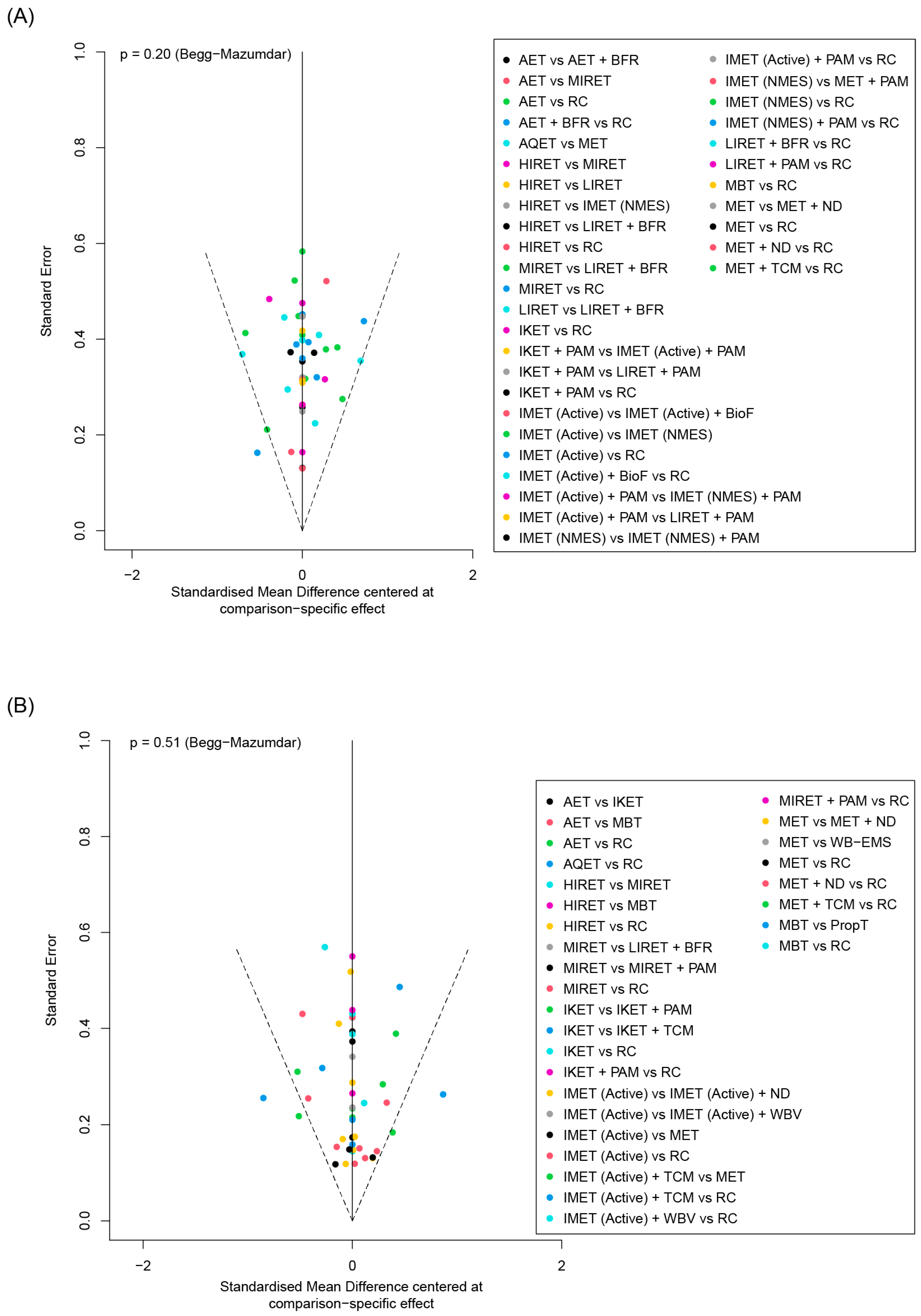
3.4.5. Publication Bias
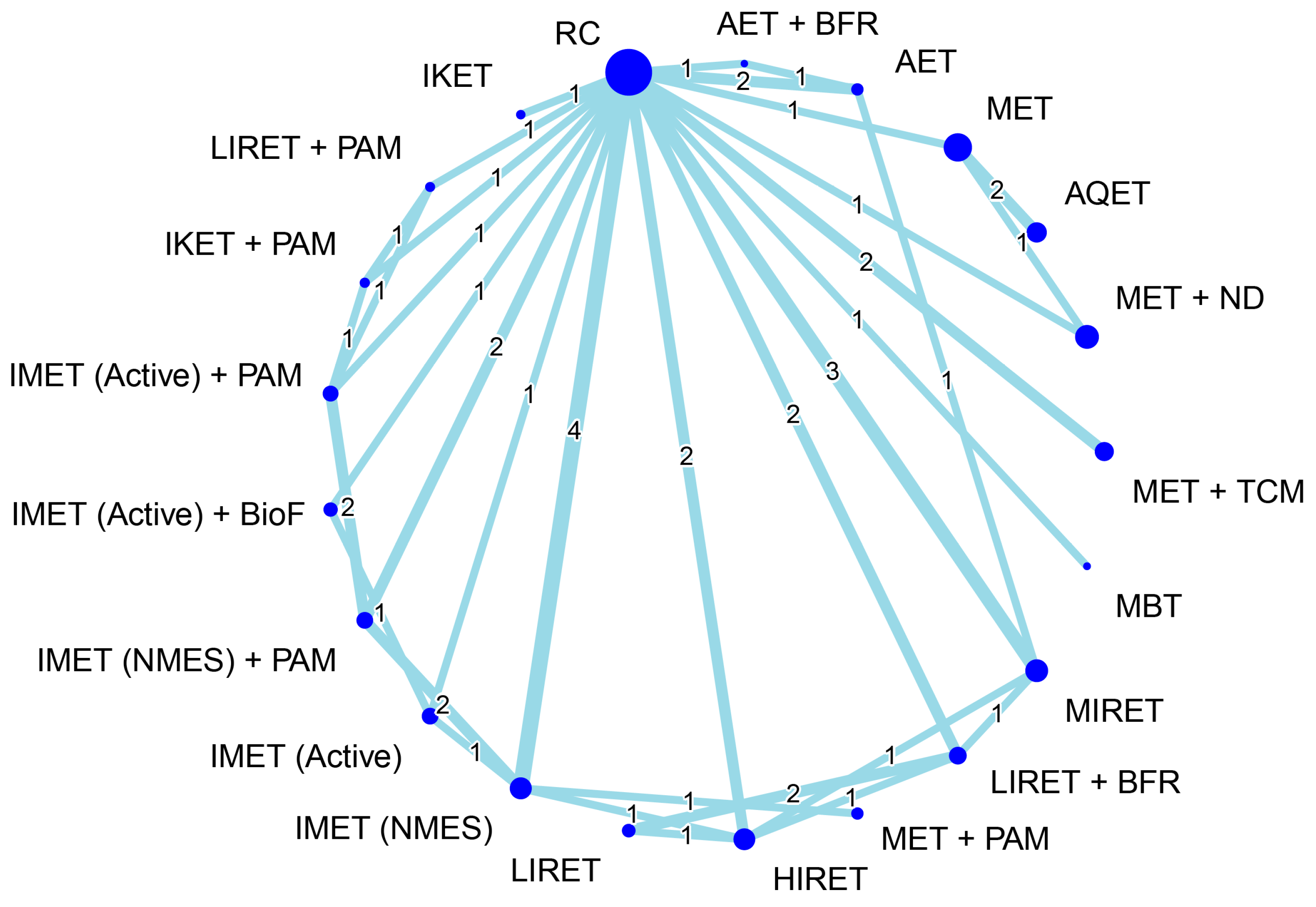
3.5. Effectiveness of Treatment for Muscle Hypertrophy
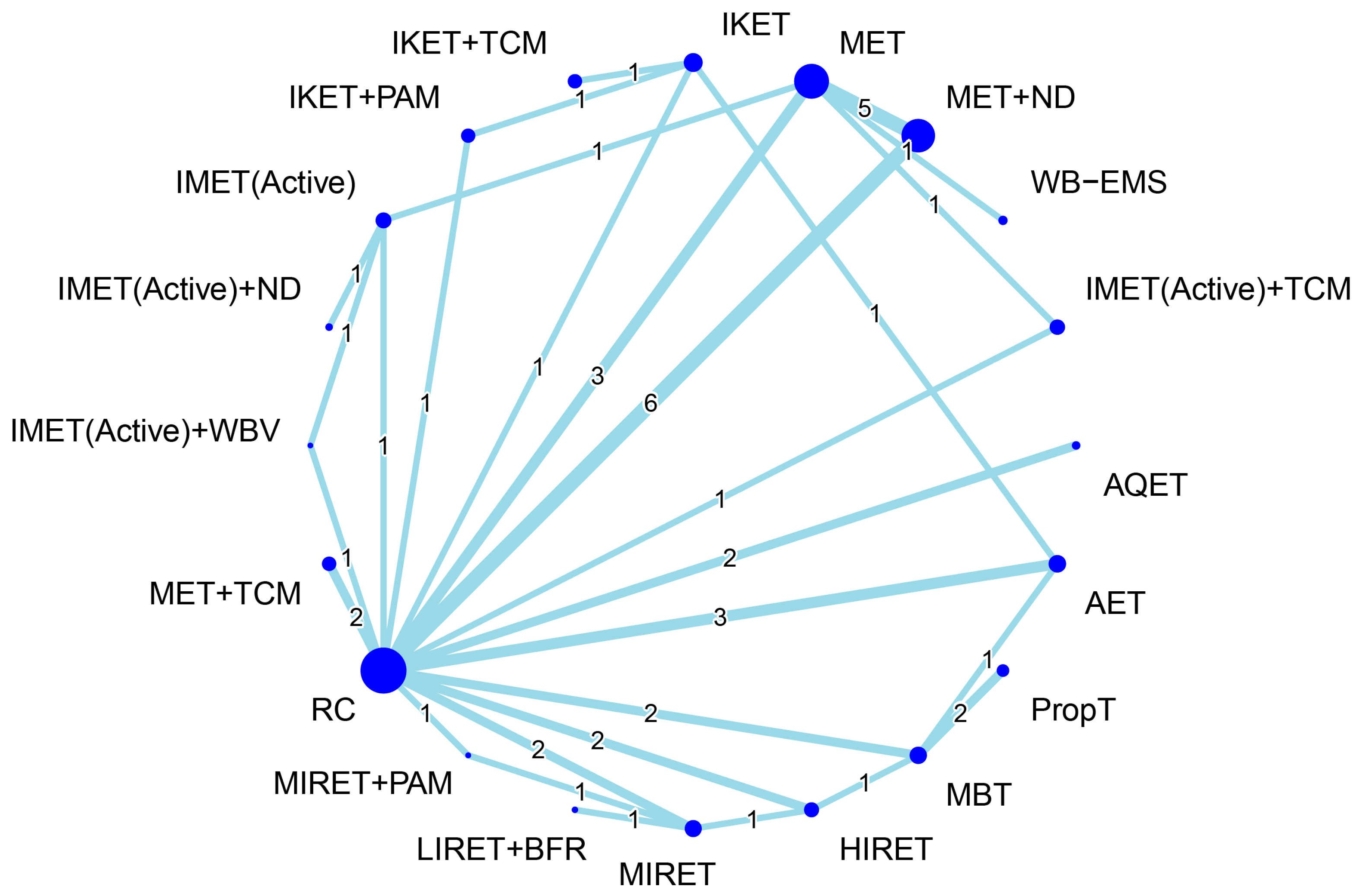
3.6. Effectiveness of Treatment for Serum Inflammation
3.7. Network Meta-Regression Analyses Results
3.8. Certainty of the Evidence
3.9. Compliance and Adverse Effects
4. Discussion
4.1. Summary of Main Findings
4.2. Comparisons of this NMA with Previous Studies
4.3. Moderator of Relative Efficiency among Treatment Regimens
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baos, S.; Prieto-Potin, I.; Román-Blas, J.A.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Front. Physiol. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- da Costa Teixeira, L.A.; Avelar, N.C.P.; Peixoto, M.F.D.; Parentoni, A.N.; Santos, J.M.D.; Pereira, F.S.M.; Danielewicz, A.L.; Leopoldino, A.A.O.; Costa, S.P.; Arrieiro, A.N.; et al. Inflammatory biomarkers at different stages of Sarcopenia in older women. Sci. Rep. 2023, 13, 10367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.Y.; Kim, C.Y. A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia. Nutrients 2023, 15, 2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, K.; Huang, S.; Li, W.; He, P. A review on associated factors and management measures for sarcopenia in type 2 diabetes mellitus. Medicine 2024, 103, e37666. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xiang, J.; Wang, B.; Duan, S.; Song, R.; Zhou, W.; Tan, S.; He, B. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1263650. [Google Scholar] [CrossRef]
- Dalle, S.; Koppo, K. Is inflammatory signaling involved in disease-related muscle wasting? Evidence from osteoarthritis, chronic obstructive pulmonary disease and type II diabetes. Exp. Gerontol. 2020, 137, 110964. [Google Scholar] [CrossRef]
- Moschou, D.; Krikelis, M.; Georgakopoulos, C.; Mole, E.; Chronopoulos, E.; Tournis, S.; Mavragani, C.; Makris, K.; Dontas, I.; Gazi, S. Sarcopenia in Rheumatoid arthritis. A narrative review. J. Frailty Sarcopenia Falls 2023, 8, 44–52. [Google Scholar] [CrossRef]
- Torii, M.; Itaya, T.; Minamino, H.; Katsushima, M.; Fujita, Y.; Tanaka, H.; Oshima, Y.; Watanabe, R.; Ito, H.; Arai, H.; et al. Management of sarcopenia in patients with rheumatoid arthritis. Mod. Rheumatol./Jpn. Rheum. Assoc. 2023, 33, 435–440. [Google Scholar] [CrossRef]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Yang, J.; Liu, P.; Wang, S.; Jiang, T.; Zhang, Y.; Liu, W. Causal relationship between sarcopenia and osteoarthritis: A bi-directional two-sample mendelian randomized study. Eur. J. Med. Res. 2023, 28, 327. [Google Scholar] [CrossRef]
- Omori, G.; Koga, Y.; Tanaka, M.; Nawata, A.; Watanabe, H.; Narumi, K.; Endoh, K. Quadriceps muscle strength and its relationship to radiographic knee osteoarthritis in Japanese elderly. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2013, 18, 536–542. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Ruhdorfer, A.; Juhl, C.; Eckstein, F.; Oiestad, B.E. Knee Extensor Strength and Risk of Structural, Symptomatic, and Functional Decline in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 649–658. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Loureiro, A.; Constantinou, M.; Diamond, L.E.; Beck, B.; Barrett, R. Individuals with mild-to-moderate hip osteoarthritis have lower limb muscle strength and volume deficits. BMC Musculoskelet. Disord. 2018, 19, 303. [Google Scholar] [CrossRef]
- Ruhdorfer, A.; Dannhauer, T.; Wirth, W.; Hitzl, W.; Kwoh, C.K.; Guermazi, A.; Hunter, D.J.; Benichou, O.; Eckstein, F. Thigh muscle cross-sectional areas and strength in advanced versus early painful osteoarthritis: An exploratory between-knee, within-person comparison in osteoarthritis initiative participants. Arthritis Care Res. 2013, 65, 1034–1042. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Magnusson, H.; Coster, M.; Karlsson, C.; Rosengren, B.E. Patients With Knee Osteoarthritis Have a Phenotype With Higher Bone Mass, Higher Fat Mass, and Lower Lean Body Mass. Clin. Orthop. Relat. Res. 2014, 473, 258–264. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, K.H.; Jung, G.U.; Shin, D.; Kim, K.; Park, S.M. Associations Between Fat Mass, Lean Mass, and Knee Osteoarthritis: The Fifth Korean National Health and Nutrition Examination Survey (KNHANES V). Calcif. Tissue Int. 2016, 99, 598–607. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ro, H.J.; Chung, S.G.; Kang, S.H.; Seo, K.M.; Kim, D.K. Low Skeletal Muscle Mass in the Lower Limbs Is Independently Associated to Knee Osteoarthritis. PLoS ONE 2016, 11, e0166385. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Kim, H.J.; Lee, B.; Kwon, M.; Jung, S.M.; Lee, S.W.; Park, Y.B.; Song, J.J. Decreased muscle mass is independently associated with knee pain in female patients with radiographically mild osteoarthritis: A nationwide cross-sectional study (KNHANES 2010-2011). Clin. Rheumatol. 2018, 37, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.H.; Kim, H.O.; Suh, Y.S.; Kim, M.G.; Yoo, W.H.; Kim, R.B.; Yang, H.S.; Lee, S.I.; Park, K.S. Relationship between decreased lower extremity muscle mass and knee pain severity in both the general population and patients with knee osteoarthritis: Findings from the KNHANES V 1-2. PLoS ONE 2017, 12, e0173036. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, C.; Mazières, B.; Verrouil, E.; Bernard, L.; Guillemin, F.; Rat, A.C. Body composition and clinical symptoms in patients with hip or knee osteoarthritis: Results from the KHOALA cohort. Semin. Arthritis Rheum. 2018, 47, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Chen, H.C.; Kuo, Y.C.; Tsauo, J.Y.; Huang, S.W.; Liou, T.H. Effects of Muscle Strength Training on Muscle Mass Gain and Hypertrophy in Older Adults With Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2020, 72, 1703–1718. [Google Scholar] [CrossRef]
- Li, S.; Ng, W.H.; Abujaber, S.; Shaharudin, S. Effects of resistance training on gait velocity and knee adduction moment in knee osteoarthritis patients: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16104. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 1, Cd004376. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, C.; Juhl, C.; Christensen, R.; Lund, H.; Zhang, W.; Henriksen, M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin. Arthritis Rheum. 2017, 47, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Chen, S.; Zhang, Y.; Zhang, Z.; Liu, C.; Lu, M.; Liu, F.; Li, S.; He, Z.; et al. The effects of resistance exercise in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 947–959. [Google Scholar] [CrossRef]
- Goh, S.L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 356–365. [Google Scholar] [CrossRef]
- Uthman, O.A.; van der Windt, D.A.; Jordan, J.L.; Dziedzic, K.S.; Healey, E.L.; Peat, G.M.; Foster, N.E. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. Br. J. Sports Med. 2014, 48, 1579. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Bulow, J.; Jensen, J.K.; Reitelseder, S.; Drummond, M.J.; Schjerling, P.; Scheike, T.; Serena, A.; Holm, L. Light-load resistance exercise increases muscle protein synthesis and hypertrophy signaling in elderly men. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E326–E338. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K.; Murton, A.J.; Greenhaff, P.L. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J. Appl. Physiol. 2011, 110, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.; Vechin, F.C.; Ugrinowitsch, C. A Review of Resistance Training-Induced Changes in Skeletal Muscle Protein Synthesis and Their Contribution to Hypertrophy. Sports Med. 2015, 45, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Partridge, T.A.; Yablonka-Reuveni, Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.B.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; AL, D.E.S.-P.; Roschel, H. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef]
- Mahmoud, W.S.; Elnaggar, R.K.; Ahmed, A.S. Influence of Isometric Exercise Training on Quadriceps Muscle Architecture and Strength in Obese Subjects with Knee Osteoarthritis. Int. J. Med. Res. Health Sci. 2017, 12, 688260. [Google Scholar]
- Valtonen, A.; Pöyhönen, T.; Sipilä, S.; Heinonen, A. Maintenance of aquatic training-induced benefits on mobility and lower-extremity muscles among persons with unilateral knee replacement. Arch. Phys. Med. Rehabil. 2011, 92, 1944–1950. [Google Scholar] [CrossRef]
- Lim, J.Y.; Tchai, E.; Jang, S.N. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: A randomized controlled trial. PM R. J. Inj. Funct. Rehabil. 2010, 2, 723–731. [Google Scholar] [CrossRef]
- Waller, B.; Munukka, M.; Rantalainen, T.; Lammentausta, E.; Nieminen, M.T.; Kiviranta, I.; Kautiainen, H.; Hakkinen, A.; Kujala, U.M.; Heinonen, A. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: A 4-month RCT with 12-month follow-up. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2017, 25, 1238–1246. [Google Scholar] [CrossRef]
- Aguiar, G.C.; Rocha, S.G.; Rezende, G.A.S.; Marcela Rêgo do Nascimento, M.R.; Scalzo, P.L. Effects of resistance training in individuals with knee osteoarthritis. Phys. Ther. Mov. 2016, 29, 589–596. [Google Scholar] [CrossRef]
- Santos, M.L.; Gomes, W.F.; Pereira, D.S.; Oliveira, D.M.; Dias, J.M.; Ferrioli, E.; Pereira, L.S. Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA). Arch. Gerontol. Geriatr. 2011, 52, 322–326. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Massey, G.J.; Maden-Wilkinson, T.M.; Morales-Artacho, A.J.; McKeown, A.; Appleby, C.L.; Folland, J.P. Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur. J. Appl. Physiol. 2017, 117, 631–640. [Google Scholar] [CrossRef]
- Marriott, K.; Chopp-Hurley, J.; Loukov, D.; Karampatos, S.; Kuntz, A.B.; Wiebenga, E.G.; Stratford, P.W.; Noseworthy, M.D.; Bowdish, D.M.E.; Maly, M.R. Muscle strength gains after strengthening exercise explained by reductions in serum inflammation in women with knee osteoarthritis. Clin. Biomech. 2021, 86, 105381. [Google Scholar] [CrossRef]
- Wang, S.Y.; Olson-Kellogg, B.; Shamliyan, T.A.; Choi, J.Y.; Ramakrishnan, R.; Kane, R.L. Physical therapy interventions for knee pain secondary to osteoarthritis: A systematic review. Ann. Intern. Med. 2012, 157, 632–644. [Google Scholar] [CrossRef]
- Escalante, Y.; García-Hermoso, A.; Saavedra, J.M. Effects of exercise on functional aerobic capacity in lower limb osteoarthritis: A systematic review. J. Sci. Med. Sport 2011, 14, 190–198. [Google Scholar] [CrossRef]
- Escalante, Y.; Saavedra, J.M.; Garcia-Hermoso, A.; Silva, A.J.; Barbosa, T.M. Physical exercise and reduction of pain in adults with lower limb osteoarthritis: A systematic review. J. Back. Musculoskelet. Rehabil. 2010, 23, 175–186. [Google Scholar] [CrossRef]
- Batterham, S.I.; Heywood, S.; Keating, J.L. Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet. Disord. 2011, 12, 123. [Google Scholar] [CrossRef]
- Lu, M.; Su, Y.; Zhang, Y.; Zhang, Z.; Wang, W.; He, Z.; Liu, F.; Li, Y.; Liu, C.; Wang, Y.; et al. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: Systematic review and meta-analysis. Z. Rheumatol. 2015, 74, 543–552. [Google Scholar] [CrossRef]
- Zampogna, B.; Papalia, R.; Papalia, G.F.; Campi, S.; Vasta, S.; Vorini, F.; Fossati, C.; Torre, G.; Denaro, V. The Role of Physical Activity as Conservative Treatment for Hip and Knee Osteoarthritis in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1167. [Google Scholar] [CrossRef]
- Kus, G.; Yeldan, I. Strengthening the quadriceps femoris muscle versus other knee training programs for the treatment of knee osteoarthritis. Rheumatol. Int. 2019, 39, 203–218. [Google Scholar] [CrossRef]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthrit. Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef]
- Heikkinen, R.; Waller, B.; Munukka, M.; Multanen, J.; Heinonen, A.; Karvanen, J. Impact or No Impact for Women With Mild Knee Osteoarthritis: A Bayesian Meta-Analysis of Two Randomized Controlled Trials With Contrasting Interventions. Arthrit. Care Res. 2022, 74, 1133–1141. [Google Scholar] [CrossRef]
- Song, J.A.; Oh, J.W. Effects of Aquatic Exercises for Patients with Osteoarthritis: Systematic Review with Meta-Analysis. Healthcare 2022, 10, 560. [Google Scholar] [CrossRef]
- Khalafi, M.; Akbari, A.; Symonds, M.E.; Pourvaghar, M.J.; Rosenkranz, S.K.; Tabari, E. Influence of different modes of exercise training on inflammatory markers in older adults with and without chronic diseases: A systematic review and meta-analysis. Cytokine 2023, 169, 156303. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation: Melbourne, Australia, 2023. Available online: www.covidence.org(accessed on 28 May 2024).
- McKeown, S.; Mir, Z.M. Considerations for conducting systematic reviews: Evaluating the performance of different methods for de-duplicating references. Syst. Rev. 2021, 10, 38. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Hong-Baik, I.; Úbeda-D’Ocasar, E.; Cimadevilla-Fernández-Pola, E.; Jiménez-Díaz-Benito, V.; Hervás-Pérez, J.P. The Effects of Non-Pharmacological Interventions in Fibromyalgia: A Systematic Review and Metanalysis of Predominants Outcomes. Biomedicines 2023, 11, 2367. [Google Scholar] [CrossRef]
- Furlan, A.D.; Malmivaara, A.; Chou, R.; Maher, C.G.; Deyo, R.A.; Schoene, M.; Bronfort, G.; van Tulder, M.W. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine 2015, 40, 1660–1673. [Google Scholar] [CrossRef]
- Knottnerus, J.A.; Tugwell, P. Research-agenda bias. J. Clin. Epidemiol. 2018, 98, vii–viii. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage Publications: Newbury Park, CA, USA, 1993. [Google Scholar]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Jackson, D.; Boddington, P.; White, I.R. The design-by-treatment interaction model: A unifying framework for modelling loop inconsistency in network meta-analysis. Res. Synth. Methods 2016, 7, 329–332. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Chapter 12: Network Meta-Analysis. In Doing Meta-Analysis with R: A Hands-On Guide; Harrer, M., Cuijpers, P., Furukawa, T.A., Ebert, D.D., Eds.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- Shim, S.R.; Kim, S.-J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019013. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Esteban-Sopeña, J.; Beltran-Alacreu, H.; Terradas-Monllor, M.; Avendaño-Coy, J.; García-Magro, N. Effectiveness of Virtual Reality on Postoperative Pain, Disability and Range of Movement after Knee Replacement: A Systematic Review and Meta-Analysis. Life 2024, 14, 289. [Google Scholar] [CrossRef]
- Beavers, K.M.; Beavers, D.P.; Newman, J.J.; Anderson, A.M.; Loeser, R.F., Jr.; Nicklas, B.J.; Lyles, M.F.; Miller, G.D.; Mihalko, S.L.; Messier, S.P. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2015, 23, 249–256. [Google Scholar] [CrossRef]
- Bruce-Brand, R.A.; Walls, R.J.; Ong, J.C.; Emerson, B.S.; O’Byrne, J.M.; Moyna, N.M. Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2012, 13, 118. [Google Scholar] [CrossRef]
- Chen, G.H.; Wang, J.; Xiao, C. The effect of hot compress of chinese medicine combined with manipulation pulling and pressing leg exercise on pain mediators, anti-inflammatory and pro-inflammatory factors of chronic knee osteoarthritis. World J. Integr. Tradit. West. Med. 2021, 16, 1068–1072. [Google Scholar] [CrossRef]
- Chen, L.L.; Kang, H.; Cai, J.; Wu, G.X. Clinical observation on manipulation combined with muscle strength training in treatment of knee osteoarthritis. J. Hubei Univ. Chin. Med. 2023, 25, 75–78. [Google Scholar] [CrossRef]
- Choi, Y.L.; Kim, B.K.; Hwang, Y.P.; Moon, O.K.; Choi, W.S. Effects of isometric exercise using biofeedback on maximum voluntary isometric contraction, pain, and muscle thickness in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 149–153. [Google Scholar] [CrossRef]
- Christensen, P.; Frederiksen, R.; Bliddal, H.; Riecke, B.F.; Bartels, E.M.; Henriksen, M.; Juul, S.R.T.; Gudbergsen, H.; Winther, K.; Astrup, A.; et al. Comparison of three weight maintenance programs on cardiovascular risk, bone and vitamins in sedentary older adults. Obesity 2013, 21, 1982–1990. [Google Scholar] [CrossRef]
- Cook, S.B.; LaRoche, D.P.; Villa, M.R.; Barile, H.; Manini, T.M. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp. Gerontol. 2017, 99, 138–145. [Google Scholar] [CrossRef]
- de Almeida, A.C.; Aily, J.B.; Pedroso, M.G.; Gonçalves, G.H.; de Carvalho Felinto, J.; Ferrari, R.J.; Pastre, C.M.; Mattiello, S.M. A periodized training attenuates thigh intermuscular fat and improves muscle quality in patients with knee osteoarthritis: Results from a randomized controlled trial. Clin. Rheumatol. 2020, 39, 1265–1275. [Google Scholar] [CrossRef]
- Devrimsel, G.; Metin, Y.; Serdaroglu Beyazal, M. Short-term effects of neuromuscular electrical stimulation and ultrasound therapies on muscle architecture and functional capacity in knee osteoarthritis: A randomized study. Clin. Rehabil. 2019, 33, 418–427. [Google Scholar] [CrossRef]
- Franz, A.; Ji, S.; Bittersohl, B.; Zilkens, C.; Behringer, M. Impact of a Six-Week Prehabilitation With Blood-Flow Restriction Training on Pre- and Postoperative Skeletal Muscle Mass and Strength in Patients Receiving Primary Total Knee Arthroplasty. Front. Physiol. 2022, 13, 881484. [Google Scholar] [CrossRef]
- Gur, H.; Cakin, N.; Akova, B.; Okay, E.; Kucukoglu, S. Concentric versus combined concentric-eccentric isokinetic training: Effects on functional capacity and symptoms in patients with osteoarthrosis of the knee. Arch. Phys. Med. Rehabil. 2002, 83, 308–316. [Google Scholar] [CrossRef]
- Ha, G.C.; Yoon, J.R.; Yoo, C.G.; Kang, S.J.; Ko, K.J. Effects of 12-week aquatic exercise on cardiorespiratory fitness, knee isokinetic function, and Western Ontario and McMaster University osteoarthritis index in patients with knee osteoarthritis women. J. Exerc. Rehabil. 2018, 14, 870–876. [Google Scholar] [CrossRef]
- Harper, S.A.; Roberts, L.M.; Layne, A.S.; Jaeger, B.C.; Gardner, A.K.; Sibille, K.T.; Wu, S.S.; Vincent, K.R.; Fillingim, R.B.; Manini, T.M.; et al. Blood-Flow Restriction Resistance Exercise for Older Adults with Knee Osteoarthritis: A Pilot Randomized Clinical Trial. J. Clin. Med. 2019, 8, 265. [Google Scholar] [CrossRef]
- Ji, L.Y.; Quan, X.M.; Chen, Q.L.; He, Y.Q.; Yi, C.Z. Effect of dialectical nursing combined with rehabilitation training on serum inflammatory cytokines in patients with bilateral knee osteoarthritis. J. Guangzhou Univ. Tradit. Chin. Med. 2016, 33, 477–481. [Google Scholar] [CrossRef]
- Jiang, Y.; Lai, Z.Q.; Fan, K.Y.; Wang, Y.C.; Liu, C.; Xu, Z.L.; Huang, R.; Lai, J.H.; Wang, X.Q.; Wang, L. Effects of 12-week Health Qigong Baduanjin on Lower Limb Body Compositions in Knee Osteoarthritis Patients. J. Liaoning Univ. Tradit. Chin. Med. 2020, 22, 90–93. [Google Scholar] [CrossRef]
- Kelmendi, B.; Kast, S.; von Stengel, S.; Kohl, M.; Roemer, F.; Uder, M.; Kemmler, W.; Kob, R. The Effect of Whole-Body Electromyostimulation on Inflam-matory Biomarkers and Adipokines in Overweight to Obese Adults with Knee Osteoarthritis: A Randomized Controlled Study. Ger. J. Sports Med. 2024, 75, 57–62. [Google Scholar]
- Kim, S.; Hsu, F.C.; Groban, L.; Williamson, J.; Messier, S. A pilot study of aquatic prehabilitation in adults with knee osteoarthritis undergoing total knee arthroplasty—Short term outcome. BMC Musculoskelet. Disord. 2021, 22, 388. [Google Scholar] [CrossRef]
- Kocaman, O.; Koyuncu, H.; Dinç, A.; Toros, H.; Karamehmetoğlu, S.S. The Comparison of the Effects of Electrical Stimulation and Exercise in the Treatment of Knee Osteoarthritis. Turk. J. Phys. Med. Rehabil. 2008, 54. [Google Scholar]
- Kuntz, A.B.; Chopp-Hurley, J.N.; Brenneman, E.C.; Karampatos, S.; Wiebenga, E.G.; Adachi, J.D.; Noseworthy, M.D.; Maly, M.R. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS ONE 2018, 13, e0195653. [Google Scholar] [CrossRef]
- Li, Y.T.; Ye, Y.Y.; Niu, X.M.; Qiu, Z.W.; Zhong, W.H. Effects of Yi Jin Jing exercise on the coordination activation ability of lower limb muscles of knee osteoarthritis. Chin. J. Tradit. Chin. Med. Pharm. 2022, 37, 2380–2385. [Google Scholar]
- Lin, Z.; Liu, T.; Hu, Z.; Que, W.; Qiu, H.; Chen, L. Effects of Different Running Intensity on Serum Levels of IL-6 and TNF-α in Patients with Early Knee Osteoarthritis. J. Coll. Physicians Surg. Pak. 2022, 32, 899–903. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Chen, X.; Hu, K.; Tu, Y.; Lin, M.; Huang, J.; Liu, W.; Wu, J.; Qiu, Z.; et al. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: A randomised multimodal magnetic resonance imaging study. Br. J. Anaesth. 2019, 123, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yang, J.Q.; Zhang, L.Z.; Li, Y. Effect of diclofenac sodium sustained release capsules combined with traditional Chinese medicine and rehabilitation exercise training on primary knee osteoarthritis. J. Baotou Med. Coll. 2022, 38, 1–4. [Google Scholar] [CrossRef]
- Ma, L.; Pan, H.Y. Effect of heat-sensitive moxibustion combined with constant velocity myocardial training on knee osteoarthritis. J. Chang. Univ. Tradit. Chin. Med. 2019, 35, 283–285. [Google Scholar] [CrossRef]
- Mahmoud, W.S.; Osailan, A.; Ahmed, A.S.; Elnaggar, R.K.; Radwan, N.L. Optimal parameters of blood flow restriction and resistance training on quadriceps strength and cross-sectional area and pain in knee osteoarthritis. Isokinet. Exerc. Sci. 2021, 29, 393–402. [Google Scholar] [CrossRef]
- Malas, F.Ü.; Özçakar, L.; Kaymak, B.; Ulaşlı, A.; Güner, S.; Kara, M.; Akıncı, A. Effects of different strength training on muscle architecture: Clinical and ultrasonographic evaluation in knee osteoarthritis. PM R. J. Inj. Funct. Rehabil. 2013, 5, 655–662. [Google Scholar] [CrossRef]
- McLeod, A.; Schiffer, L.; Castellanos, K.; DeMott, A.; Olender, S.; Fitzgibbon, M.; Hughes, S.; Fantuzzi, G.; Tussing-Humphreys, L. Impact of Physical Activity and Weight Loss on Fat Mass, Glucose Metabolism, and Inflammation in Older African Americans with Osteoarthritis. Nutrients 2020, 12, 3299. [Google Scholar] [CrossRef]
- Melo Mde, O.; Pompeo, K.D.; Brodt, G.A.; Baroni, B.M.; da Silva Junior, D.P.; Vaz, M.A. Effects of neuromuscular electrical stimulation and low-level laser therapy on the muscle architecture and functional capacity in elderly patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2015, 29, 570–580. [Google Scholar] [CrossRef]
- Messier, S.P.; Loeser, R.F.; Mitchell, M.N.; Valle, G.; Morgan, T.P.; Rejeski, W.J.; Ettinger, W.H. Exercise and weight loss in obese older adults with knee osteoarthritis: A preliminary study. J. Am. Geriatr. Soc. 2000, 48, 1062–1072. [Google Scholar] [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Beavers, D.P.; Nicklas, B.J.; DeVita, P.; Carr, J.J.; Hunter, D.J.; Lyles, M.; Guermazi, A.; Bennell, K.L.; et al. Effect of High-Intensity Strength Training on Knee Pain and Knee Joint Compressive Forces among Adults with Knee Osteoarthritis: The START Randomized Clinical Trial. JAMA 2021, 325, 646–657. [Google Scholar] [CrossRef]
- Miller, G.D.; Nicklas, B.J.; Loeser, R.F. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J. Am. Geriatr. Soc. 2008, 56, 644–651. [Google Scholar] [CrossRef]
- Mu, Z.Y.; Li, Y.J.; Wang, C.Y.; Zhu, M.H.; Li, Q.B. Therapeutic observation of laser acupuncture-moxibustion plus isokinetic muscle strength training for knee osteoarthritis. Shanghai J. Acupunct. Moxibust 2019, 38, 1409–1413. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Ambrosius, W.; Messier, S.P.; Miller, G.D.; Penninx, B.W.; Loeser, R.F.; Palla, S.; Bleecker, E.; Pahor, M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: A randomized controlled clinical trial. Am. J. Clin. Nutr. 2004, 79, 544–551. [Google Scholar] [CrossRef]
- Oldham, J.A.; Howe, T.E.; Petterson, T.; Smith, G.P.; Tallis, R.C. Electrotherapeutic rehabilitation of the quadriceps in elderly osteoarthritic patients: A double blind assessment of patterned neuromuscular stimulation. Clin. Rehabil. 1995, 9, 10–20. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Sedighipour, L.; Bossaghzade, Z.; Abdollahzadeh, M.H.; Nikray, R.; Mollayi, F. The efficacy of electromyographic biofeedback on pain, function, and maximal thickness of vastus medialis oblique muscle in patients with knee osteoarthritis: A randomized clinical trial. J. Pain. Res. 2018, 11, 2781–2789. [Google Scholar] [CrossRef]
- Samut, G.; Dinçer, F.; Özdemir, O. The effect of isokinetic and aerobic exercises on serum interleukin-6 and tumor necrosis factor alpha levels, pain, and functional activity in patients with knee osteoarthritis. Mod. Rheumatol./Jpn. Rheum. Assoc. 2015, 25, 919–924. [Google Scholar] [CrossRef]
- Segal, N.A.; Williams, G.N.; Davis, M.C.; Wallace, R.B.; Mikesky, A.E. Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM R. J. Inj. Funct. Rehabil. 2015, 7, 376–384. [Google Scholar] [CrossRef]
- Simao, A.P.; Avelar, N.C.; Tossige-Gomes, R.; Neves, C.D.; Mendonça, V.A.; Miranda, A.S.; Teixeira, M.M.; Teixeira, A.L.; Andrade, A.P.; Coimbra, C.C.; et al. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2012, 93, 1692–1700. [Google Scholar] [CrossRef]
- Sterzi, S.; Giordani, L.; Morrone, M.; Lena, E.; Magrone, G.; Scarpini, C.; Milighetti, S.; Pellicciari, L.; Bravi, M.; Panni, I.; et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: A randomized, double-blind, placebo-controlled study. Eur. J. Phys. Rehabil. Med. 2016, 52, 321–330. [Google Scholar]
- Tok, F.; Aydemir, K.; Peker, F.; Safaz, I.; Taşkaynatan, M.A.; Ozgül, A. The effects of electrical stimulation combined with continuous passive motion versus isometric exercise on symptoms, functional capacity, quality of life and balance in knee osteoarthritis: Randomized clinical trial. Rheumatol. Int. 2011, 31, 177–181. [Google Scholar] [CrossRef]
- Varzaityte, L.; Kubilius, R.; Rapoliene, L.; Bartuseviciute, R.; Balcius, A.; Ramanauskas, K.; Nedzelskiene, I. The effect of balneotherapy and peloid therapy on changes in the functional state of patients with knee joint osteoarthritis: A randomized, controlled, single-blind pilot study. Int. J. Biometeorol. 2020, 64, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Vassao, P.G.; de Souza, A.C.F.; da Silveira Campos, R.M.; Garcia, L.A.; Tucci, H.T.; Renno, A.C.M. Effects of photobiomodulation and a physical exercise program on the expression of inflammatory and cartilage degradation biomarkers and functional capacity in women with knee osteoarthritis: A randomized blinded study. Adv. Rheumatol. 2021, 61, 62. [Google Scholar] [CrossRef]
- Walls, R.J.; McHugh, G.; O’Gorman, D.J.; Moyna, N.M.; O’Byrne, J.M. Effects of preoperative neuromuscular electrical stimulation on quadriceps strength and functional recovery in total knee arthroplasty. A pilot study. BMC Musculoskelet. Disord. 2010, 11, 119. [Google Scholar] [CrossRef]
- Walrabenstein, W.; Wagenaar, C.A.; van de Put, M.; van der Leeden, M.; Gerritsen, M.; Twisk, J.W.R.; van der Esch, M.; van Middendorp, H.; Weijs, P.J.M.; Roorda, L.D.; et al. A multidisciplinary lifestyle program for metabolic syndrome-associated osteoarthritis: The “Plants for Joints” randomized controlled trial. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2023, 31, 1491–1500. [Google Scholar] [CrossRef]
- Wang, Q. Observation of clinical effectiveness of external use of Zhenggu Powder combined with functional exercise for the treatment of knee osteoarthritis. Beijing J. Tradit. Chin. Med. 2016, 35, 1014–1017. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.W.; Zhang, H.C.; Wang, Y.Z. Zhenggu Powder Combined with Functional Exercise in Knee Osteoarthritis and Its Effect on Knee Joint Function. World Chin. Med. 2017, 12, 1332–1339. [Google Scholar]
- Wang, X.T.; Zhou, C.B.; Yang, L.H.; Yan, L.Q.; Wang, K.; Wang, Q. Effects of personalized neuromuscular training on knee function, pain, stiffness and quality of life in patients with knee osteoarthritis. J. Hebei Med. Univ. 2021, 42, 395–400. [Google Scholar] [CrossRef]
- Wyatt, F.B.; Milam, S.; Manske, R.C.; Deere, R. The effects of aquatic and traditional exercise programs on persons with knee osteoarthritis. J. Strength. Cond. Res./Natl. Strength. Cond. Assoc. 2001, 15, 337–340. [Google Scholar]
- Yang, J.F.; Yang, Q.; Xu, Y.K. Clinical observation on the treatment of 42 cases of knee osteoarthritis with baduanjin combined with core muscle group training. Rheum. Arthritis 2023, 12, 6–10,60. [Google Scholar] [CrossRef]
- Yin, N.; Tang, F.W.; Fu, F.; Han, G.; Xing, J. Effects of low intensity pulsed focused ultrasound combined with isokinetic muscle strength training on knee proprioception, quality of life and inflammatory factors levels in patients with knee osteoarthritis. Progr Mod. Biomed. 2021, 21, 4275–4278. [Google Scholar] [CrossRef]
- von Stengel, S.; Fröhlich, M.; Ludwig, O.; Eifler, C.; Berger, J.; Kleinöder, H.; Micke, F.; Wegener, B.; Zinner, C.; Mooren, F.C.; et al. Revised contraindications for the use of non-medical WB-electromyostimulation. Evidence-based German consensus recommendations. Front. Sports Act. Living 2024, 6, 1371723. [Google Scholar] [CrossRef]
- Dos Santos, L.P.; Santo, R.; Ramis, T.R.; Portes, J.K.S.; Chakr, R.; Xavier, R.M. The effects of resistance training with blood flow restriction on muscle strength, muscle hypertrophy and functionality in patients with osteoarthritis and rheumatoid arthritis: A systematic review with meta-analysis. PLoS ONE 2021, 16, e0259574. [Google Scholar] [CrossRef]
- Grantham, B.; Korakakis, V.; O’Sullivan, K. Does blood flow restriction training enhance clinical outcomes in knee osteoarthritis: A systematic review and meta-analysis. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2021, 49, 37–49. [Google Scholar] [CrossRef]
- Wang, H.N.; Chen, Y.; Cheng, L.; Cai, Y.H.; Li, W.; Ni, G.X. Efficacy and Safety of Blood Flow Restriction Training in Patients With Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2022, 74, 89–98. [Google Scholar] [CrossRef]
- de Oliveira Melo, M.; Aragão, F.A.; Vaz, M.A. Neuromuscular electrical stimulation for muscle strengthening in elderly with knee osteoarthritis—A systematic review. Complement. Ther. Clin. Pract. 2013, 19, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bahşi, A.; Altındağ, Ö.; Akaltun, M.S.; Aydeniz, A.; Avcı, E.E.; Gür, A. Comparison of the Effects of Isokinetic, Isometric, and Isotonic Exercises on Knee Osteoarthritis Using Ultrasound. Cureus 2022, 14, e28324. [Google Scholar] [CrossRef] [PubMed]
- Benli Küçük, E.; Özyemişci Taşkıran, Ö.; Tokgöz, N.; Meray, J. Effects of isokinetic, isometric, and aerobic exercises on clinical variables and knee cartilage volume using magnetic resonance imaging in patients with osteoarthritis. Turk. J. Phys. Med. Rehabil. 2018, 64, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Rosa, U.H.; Velásquez Tlapanco, J.; Lara Maya, C.; Villarreal Ríos, E.; Martínez González, L.; Vargas Daza, E.R.; Galicia Rodríguez, L. Comparison of the effectiveness of isokinetic vs isometric therapeutic exercise in patients with osteoarthritis of knee. Reumatol. Clin. 2012, 8, 10–14. [Google Scholar] [CrossRef] [PubMed]




| Participant (P) |
| 1. Age: 50 years or older |
| 2. Having a diagnosis of knee osteoarthritis |
| Intervention (I) |
| 1. Monotherapy: any exercise therapy alone. The specified exercise therapies are defined as follows: |
| (1) Resistance-based exercise training: Any exercise that requires muscles to contract against an external resistance force throughout an entire joint movement. The extra force can be free weights (e.g., dumbbells or barbells), elastic bands or tubing, self-body weight, or any other object that causes counter actions of the muscles. |
| (2) Isometric exercise training: Any exercise that requires the muscles to contract against an external resistance force without joint movement. The muscle contraction can be actively initiated or passively activated by neuromuscular electrical stimulation. |
| (3) Isokinetic exercise training: An exercise that requires the muscles to contract at a constant speed of joint movements, irrespective of the amount of resistance applied. It is termed isokinetic contraction. |
| (4) Aerobic exercise training: an exercise that refers to cardiovascular conditioning such as walking, running, swimming, and bicycling. |
| (5) Aquatic or water-based exercise training: an exercise that refers to therapeutic motion regimens performed in a water environment |
| (6) Proprioceptive or sensory-motor training: An exercise aimed at improving balance and reducing fall risk in older adults. It is also termed kinesiotherapy, which challenges an individual’s ability of postural control to stabilize a joint during static or dynamic functional tasks. |
| (7) Mind–body therapy: An exercise approach that combines body movement, breathing control, and attention focus to improve physical and overall health such as yoga, tai chi, and qigong. It emphasizes the relationships among a person’s mental, physical, and spiritual experiences. |
| (8) Whole-body electromyostimulation: an application of electrical stimulations for main muscle groups during body movement tasks. |
| (9) Muti-component exercise regimen: an exercise regimen composed of two or more of the exercise types listed above. |
| 2. Combined treatment: an exercise therapy in combination with an adjunct noninvasive treatment. The adjunct treatment included the following: |
| (1) Physical assistant agents (such as biofeedback, blood-flow restriction, whole-body vibration) or modalities (such as therapeutic ultrasound diathermy, interferential current, and transcutaneous electrical nerve stimulation) |
| (2) Traditional Chinese medicine (such as herbal medicine and electric acupoint therapy) |
| (3) Nutrition or diet intervention (such as protein supplementation, weight loss, or an energy-restricted program) |
| Comparison (C) |
| The comparator included the following: |
| 1. Different exercise type |
| 2. Lower training intensity |
| 3. Regular care without any exercise training |
| Outcome (O) |
| 1. Inflammation biomarker |
| (1) C-reactive protein |
| (2) Pro-inflammatory cytokine (a) Interleukins (IL), such as IL-1, IL-6, IL-8, and IL-1β (b) Tumor necrosis factor (TNF) family, such as TNF-α, TNF-α receptor, and tumor necrosis-like weak inducer of apoptosis |
| 2. Measures of muscular morphology |
| Cross-sectional area, muscle volume, muscle thickness |
| Study design (S) |
| 1. Randomized parallel (two-arm or multiple-arm) controlled trial |
| 2. Randomized crossover trial |
| Study Year | Country (Area) | Study Arm | Age (Years) a | BMI (kg/m2) a | Sex | N | K-L Grade | Disease Duration (Month) | Exercise Intervention | Outcome Measures b | Follow- up Time (Week) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (Session /Week) | Duration (Week) | Adherence (%) | Muscle Morphology | Inflammatory Biomarker | |||||||||||||||
| Female | Male | CSA | MT | TVol | Interleukin | TNF | CRP | ||||||||||||
| Beavers | USA | MET + ND | 65.5 | 33.5 | 108 | 43 | 151 | 2, 3 | NR | 3 | 72 | 70 | v | v | v | 0, 24, | |||
| 2015 [80] | MET | 65.5 | 33.5 | 108 | 42 | 150 | 66 | 72 | |||||||||||
| RC | 65.8 | 33.7 | 105 | 44 | 149 | 61 | |||||||||||||
| Bruce-Brand | Ireland | HI-RET | 63.4 | 33.9 | 4 | 6 | 10 | 3, 4 | NR | 3 | 6 | 83.3 | v | 0, 6 | |||||
| 2012 [81] | IMET (NMES) | 63.9 | 33.7 | 4 | 6 | 10 | 91.3 | ||||||||||||
| RC | 65.2 | 31.7 | 3 | 3 | 6 | ||||||||||||||
| Chen | China | IMET (Active) + TCM | 65.8 | NR | 27 | 19 | 46 | 1–3 | 58.3 | 7 | 4 | NR | v | v | 0, 4 | ||||
| 2021 [82] | MET | 66.3 | 26 | 20 | 46 | 60.4 | |||||||||||||
| Chen | China | IMET (Active) + TCM | 61.2 | NR | 23 | 27 | 50 | 1–3 | 33.7 | 7 | 5 | NR | v | v | 0, 5 | ||||
| 2023 [83] | RC | 60.9 | 35 | 25 | 60 | 33.7 | |||||||||||||
| Choi | Korea | IMET (Active) + BioF | 72.8 c | 24.3 c | 20 | 0 | 20 | 2.5 d | NR | 3 | 8 | NR | v | 0, 8 | |||||
| 2015 [84] | RC | 10 | 0 | 10 | |||||||||||||||
| Christensen | USA | MET + ND | 67.4 | 36.5 | 52 | 12 | 64 | ≥1 | 114 | 4 | 52 | 13.8 | v | 0, 52 | |||||
| 2013 [85] | RC | 66.4 | 37.8 | 103 | 25 | 128 | 96.0 | 61.5 | |||||||||||
| Cook | USA | MI-RET | 76.7 | 26.8 | 7 | 5 | 12 | NR | NR | 2 | 12 | 95 | v | 0, 6, | |||||
| 2017 [86] | LI-RET + BFR | 76.5 | 26.8 | 7 | 5 | 12 | 96 | 12 | |||||||||||
| LI-RET | 74.8 | 26.2 | 7 | 5 | 12 | 100 | |||||||||||||
| de Almeida | Brazil | AET | 55.6 | 26 | 15 | 5 | 20 | 2, 3 | 27.1 | 3 | 14 | 92 c | v | 0, 14 | |||||
| 2020 [87] | MI-RET | 55.2 | 26 | 16 | 5 | 21 | 26.6 | ||||||||||||
| RC | 53.8 | 27 | 16 | 4 | 20 | 24.2 | |||||||||||||
| Devrimsel | Turkey | IMET (NMES) | 61.2 | 30 | 23 | 7 | 30 | 2, 3 | 69.1 | 5 | 3 | 100 | v | 0, 3 | |||||
| 2019 [88] | MET + PAM | 62.8 | 28.9 | 24 | 6 | 30 | 83.5 | 96.7 | |||||||||||
| Ferraz | Brazil | HI-RET | 59.9 | 30.3 | 16 | 0 | 16 | 2, 3 | 52.8 | 2 | 12 | 90 | v | 0, 12 | |||||
| 2018 [39] | LI-RET + BFR | 60.3 | 30.2 | 16 | 0 | 16 | 52.8 | 91 | |||||||||||
| LI-RET | 60.7 | 29.9 | 16 | 0 | 16 | 56.4 | 85 | ||||||||||||
| Franz | Germany | AET + BFR | 61.5 | 26.8 | 3 | 7 | 10 | 3, 4 | NR | 2 | 6 | 100 | v | 0, 3, | |||||
| 2022 [89] | AET + PLA | 64.2 | 27.7 | 3 | 7 | 10 | 100 | 6 | |||||||||||
| RC | 66.3 | 29.4 | 4 | 6 | 10 | ||||||||||||||
| Gur | Turkey | IKET | 55.5 | 31.3 | NR | 17 | 2, 3 | NR | 3 | 8 | 100 | v | 0, 8 | ||||||
| 2002 [90] | RC | 57.0 | 32.3 | 6 | 100 | ||||||||||||||
| Ha | China | AQET | 60.9 | 25.2 | 9 | 0 | 9 | NR | NR | 3 | 4 | NR | v | 0, 4 | |||||
| 2018 [91] | RC | 61.3 | 24.6 | 8 | 0 | 8 | |||||||||||||
| Harper | USA | LI-RET + BFR | 67.2 | 31.7 | 10 | 6 | 16 | ≥2 | NR | 3 | 12 | 81.4 | v | 0, 12 | |||||
| 2019 [92] | MI-RET | 69.1 | 29.8 | 15 | 4 | 19 | 83 | ||||||||||||
| Ji | China | MET + TCM | 65.2 | NR | 12 | 18 | 30 | ≥2 | 37.4 | 7 | 4 | v | v | 0, 4 | |||||
| 2016 [93] | RC | 64.3 | 10 | 20 | 30 | 38.9 | |||||||||||||
| Jiang | China | MBT | 64.2 | 25.8 | 11 | 0 | 11 | 1, 2 | 47.0 | 5 | 12 | NR | v | 0, 12 | |||||
| 2020 [94] | RC | 62.9 | 22.9 | 12 | 0 | 12 | 42.6 | ||||||||||||
| Kelmendi | Germany | WB-EMS | 58.3 | 31.1 | 22 | 14 | 36 | 2, 3 | NR | 1.5 | 29 | 88 | v | v | 0, 29 | ||||
| 2024 [95] | MET | 57.9 | 29.5 | 24 | 12 | 36 | >90 | ||||||||||||
| Kim | USA | AQET | 67.4 | 32.9 | 10 | 10 | 20 | 3, 4 | NR | 3 | 12 | 96.6 | v | v | v | 0, 12 | |||
| 2021 [96] | RC | 66.9 | 31.9 | 9 | 14 | 23 | |||||||||||||
| Kocaman | Turkey | IMET (NMES) + PAM | 61.6 | 29.4 | 15 | 4 | 19 | 1–3 | NR | 5 | 4 | NR | v | 0, 4 | |||||
| 2008 [97] | IMET (Active) + PAM | 60.4 | 31.2 | 14 | 5 | 19 | |||||||||||||
| Kuntz | Canada | HI-RET | 63.7 | 28.9 | 11 | 0 | 11 | NR | NR | 3 | 12 | 90 | v | v | v | 0, 12 | |||
| 2018 [98] | MBT | 65.5 | 30.1 | 10 | 0 | 10 | 100 | ||||||||||||
| RC | 71.1 | 32.3 | 10 | 0 | 10 | 90 | |||||||||||||
| Li | China | MBT | 63.7 | 23.9 | 22 | 8 | 30 | 2, 3 | NR | 3 | 12 | NR | v | v | 0, 12 | ||||
| 2022 [99] | PropT | 62.7 | 24.2 | 23 | 5 | 28 | |||||||||||||
| Lin | China | AET | 65 | NR | 45 | 52 | 97 | 1, 2 | 15.7 | 5 | 24 | NR | v | v | 0, 24 | ||||
| 2022 [100] | RC | 65.4 | 16 | 18 | 34 | 16 | |||||||||||||
| Liu | China | AET | 55 | 23.4 | 23 | 4 | 27 | 2, 3 | 9.0 | 5 | 12 | 93 | v | 0, 12 | |||||
| 2019 [101] | MBT | 54.5 | 23.0 | 46 | 11 | 57 | 6.5 | 93.5 | |||||||||||
| RC | 55 | 23.4 | 14 | 10 | 24 | 6.5 | 97 | ||||||||||||
| Lu | China | MET + TCM | 64.9 | NR | 30 | 34 | 64 | 2, 3 | 24.7 | 1~5 | 16 | NR | v | 0, 16 | |||||
| 2022 [102] | RC | 64.9 | 28 | 36 | 64 | 24.1 | |||||||||||||
| Ma | China | IKET + TCM | 56.3 | NR | 42 | 50 | 92 | 2, 3 | 8.7 | 2 | 8 | NR | v | v | v | 0, 8 | |||
| 2019 [103] | IKET | 57.1 | 41 | 52 | 93 | 8.9 | |||||||||||||
| Mahmoud | KSA | IMET (Active) | 54.6 | 35.0 | 0 | 32 | 32 | 2, 3 | 42.0 | 3 | 12 | NR | v | 0, 12 | |||||
| 2017 [40] | RC | 53.2 | 34.8 | 0 | 12 | 12 | 45.6 | ||||||||||||
| Mahmoud | KSA | LI-RET + BFR | 60.2 | 30.8 | 0 | 17 | 17 | 2, 3 | 59.9 | 3 | 8 | 85 | v | 0, 8 | |||||
| 2021 [104] | RC | 59.1 | 29.7 | 0 | 18 | 18 | 60.1 | 90 | |||||||||||
| Malas | Turkey | IKET + PAM | 56.2 | 31.8 | 51 c | 10 c | 20 | 1–3 | 49.2 | 5 | 5 | NR | v | 0, 5 | |||||
| 2013 [105] | IMET (Active) + PAM | 61.2 | 33.2 | 22 | 58.8 | ||||||||||||||
| LI-RET + PAM | 59.1 | 30.0 | 19 | 54.0 | |||||||||||||||
| RC | 58.9 | 33.2 | 61 | 54.2 | |||||||||||||||
| McLeod | USA | MET + ND | 66.5 | 33.7 | 64 | 8 | 72 | NR | NR | 3 | 8 | 64 | v | v | v | 0, 8 | |||
| 2020 [106] | MET | 67.2 | 33.9 | 73 | 10 | 83 | 70 | ||||||||||||
| Melo Mde | Brazil | IMET (NMES) + PAM | 69.6 | 29.0 | 14 | 0 | 14 | 2, 3 | ≥6 | 2 | 8 | NR | v | v | 0, 8 | ||||
| 2015 [107] | IMET (NMES) | 69.3 | 33.0 | 15 | 0 | 15 | |||||||||||||
| RC | 67.7 | 30.0 | 15 | 0 | 15 | ||||||||||||||
| Messier | USA | MET + ND | 67 | 35.0 | 10 | 3 | 13 | ≥2 | NR | 3 | 24 | 94.7 | v | 0, 24 | |||||
| 2000 [108] | MET | 69 | 38.0 | 7 | 4 | 11 | 82.6 | ||||||||||||
| Messier | USA | HI-RET | 67 | 31.0 | 52 | 75 | 127 | ≥2 | NR | 3 | 72 | 66 | v | 0, 72 | |||||
| 2021 [109] | MI-RET | 64 | 31.0 | 51 | 75 | 126 | 69 | ||||||||||||
| RC | 64 | 32.0 | 48 | 76 | 124 | 80 | |||||||||||||
| Miller | USA | MET + ND | 69.8 | 34.9 | 20 | 11 | 31 | NR | NR | 3 | 24 | 77.5 | v | v | v | 0, 24 | |||
| 2008 [110] | RC | 69.5 | 34.4 | 20 | 16 | 36 | |||||||||||||
| Mu | China | IKET + PAM | 55 | 22.5 | 23 | 32 | 55 | 2, 3 | NR | 5 | 4 | NR | v | 0, 4 | |||||
| 2019 [111] | IKET | 53 | 23.1 | 24 | 30 | 54 | |||||||||||||
| Nicklas | USA | MET + ND | 68 | 33.9 | 47 | 17 | 64 | ≥1 | NR | 3 | 72 | 64 | v | v | 0, 24, | ||||
| 2004 [112] | MET | 69 | 34.6 | 59 | 8 | 67 | 60 | 72 | |||||||||||
| RC | 68.5 | 34.4 | 97 | 44 | 141 | 72.5 | |||||||||||||
| Oldham | UK | IMET (NMES) | 69 c | NR | 17 c | 13 c | 22 | 1–3 | NR | 7 | 18 | 90 c | v | 0, 6, | |||||
| 1995 [113] | RC | 8 | 12, 18 | ||||||||||||||||
| Raeissadat | Iran | IMET (Active) + BioF | 60.2 | 27.6 | 19 | 2 | 21 | 1, 2 | 42.0 | 1~2 | 8 | 91.3 | v | 0, 8 | |||||
| 2018 [114] | IMET (Active) | 61.9 | 28.5 | 16 | 4 | 20 | 32.4 | 86.9 | |||||||||||
| Samut | USA | AET | 57.6 | 33.9 | 14 | 0 | 14 | 2, 3 | 60.0 c | 3 | 6 | NR | v | v | v | 0, 6 | |||
| 2015 [115] | IKET | 62.5 | 30.5 | 15 | 0 | 15 | |||||||||||||
| RC | 60.9 | 30.4 | 13 | 0 | 13 | ||||||||||||||
| Segal | USA | LI-RET + BFR | 56.1 | 28.7 | 19 | 0 | 19 | 1–3 | ≥1 | 3 | 4 | 97.2 | v | 0, 4 | |||||
| 2015 [116] | LI-RET | 54.6 | 32.5 | 21 | 0 | 21 | 100 | ||||||||||||
| Simao | Turkey | IMET (Active) | 69 | 27.4 | 9 | 1 | 10 | ≥2 | NR | 3 | 12 | 98.6 | v | 0, 12 | |||||
| 2012 [117] | IMET (Active) + WBV | 75 | 29.8 | 8 | 2 | 10 | 99.7 | ||||||||||||
| RC | 71 | 26.7 | 10 | 1 | 11 | ||||||||||||||
| Sterzi | Italy | IMET (Active) + ND | 71.3 | 34.8 | 14 | 9 | 23 | ≥2 | 81.6 | 3 | 8 | 90 | v | 0, 12 | |||||
| 2016 [118] | IMET (Active) | 71 | 34.3 | 19 | 8 | 27 | 86.4 | 90 | |||||||||||
| Tok | Turkey | IMET (NMES) + PAM | 61.8 | NR | 16 | 4 | 20 | 2, 3 | NR | 5 | 3 | 100 | v | 0, 3 | |||||
| 2011 [119] | IMET (Active) + PAM | 66.6 | 14 | 6 | 20 | 100 | |||||||||||||
| Varzaityte | Lithuania | AQET | 63.1 | 29.3 | 52 | 10 | 62 | 1–3 | NR | 3 | 4 | NR | v | 0, 4, | |||||
| 2020 [120] | MET | 67.9 | 29.8 | 28 | 2 | 30 | 8 | ||||||||||||
| Vassao | Brazil | MI-RET + PAM | 61.6 | 30.5 | 13 | 0 | 13 | 2, 3 | ≥6 | 2 | 8 | NR | v | v | 0, 8 | ||||
| 2021 [121] | MI-RET | 62.3 | 30.1 | 13 | 0 | 13 | |||||||||||||
| RC | 66.5 | 27.2 | 10 | 0 | 10 | ||||||||||||||
| Walls | Ireland | IMET (NMES) | 64.4 | 30.7 | 6 | 3 | 9 | 3, 4 | NR | 3 | 8 | 99 | v | 0, 8 | |||||
| 2010 [122] | IMET (Active) | 63.2 | 32.8 | 4 | 1 | 5 | 99.4 | ||||||||||||
| Walrabenstein | Netherlands | MET + ND | 63.3 | 33.2 | 28 | 4 | 32 | ≥1 | NR | 2 | 16 | NR | v | 0, 8, | |||||
| 2023 [123] | RC | 63.4 | 33.4 | 26 | 6 | 32 | 16 | ||||||||||||
| Wang | China | MET + TCM | 61.4 | NR | 18 | 12 | 30 | 1, 2 | 39.2 c | 6 | 4 | NR | v | 0, 4 | |||||
| 2016 [124] | RC | 61.1 | 18 | 12 | 30 | ||||||||||||||
| Wang | China | MET + TCM | 64.1 | NR | 24 | 21 | 45 | 1, 2 | 33.3 | 6 | 4 | NR | v | 0, 4 | |||||
| 2017 [125] | RC | 63.6 | 24 | 21 | 45 | 32.7 | |||||||||||||
| Wang | China | IMET (Active) | 57.8 | NR | 35 | 40 | 75 | 2, 3 | 7.8 | 3 | 6 | NR | v | v | 0, 6 | ||||
| 2021 [126] | MET | 58.1 | 39 | 36 | 75 | 7.5 | |||||||||||||
| Wyatt | USA | AQET | 40–70 c | NR | NR | 23 | 2, 3 | NR | 3 | 6 | NR | v | 0, 6 | ||||||
| 2001 [127] | MET | 23 | |||||||||||||||||
| Yang | China | MBT | 64.5 | NR | 33 | 9 | 42 | 1–3 | 87 | 7 | 8 | NR | v | v | 0, 8 | ||||
| 2023 [128] | PropT | 64.5 | 34 | 8 | 42 | 86.2 | |||||||||||||
| Yin | China | IKET + PAM | 54.3 | NR | 23 | 12 | 35 | 1, 2 | 27.8 | 3 | 8 | NR | v | v | 0, 8 | ||||
| 2021 [129] | RC | 54.8 | 21 | 14 | 35 | 27.4 | |||||||||||||
| Treatment Arm | Abbreviation | |
|---|---|---|
| Primary Exercise Training | Adjunct Treatment | |
| Monotherapy | ||
| Aerobic exercise training | AET | |
| Aquatic exercise therapy | AQET | |
| High-intensity resistance exercise training | HIRET | |
| Moderate-intensity resistance exercise training | MIRET | |
| Low-intensity resistance exercise training | LIRET | |
| Isokinetic exercise training | IKET | |
| Isometric exercise training, self-activated muscle contraction | IMET (active) | |
| Isometric exercise training, activated by neuromuscular electrical stimulation | IMET (NMES) | |
| Multicomponent exercise training | MET | |
| Mind–body therapy | MBT | |
| Proprioceptive training | PropT | |
| Whole-body electromyostimulation | WB-EMS | |
| Combined treatment | ||
| Aerobic exercise training | Blood-flow restriction | AET + BFR |
| Medium-intensity resistance exercise training | Physical agent modality | MIRET + PAM |
| Low-intensity resistance exercise training | Blood-flow restriction | LIRET + BFR |
| Physical agent modality | LIRET + PAM | |
| Isokinetic exercise training | Physical agent modality | IKET + PAM |
| Traditional Chinese medicine | IKET + TCM | |
| Isometric exercise training, | Biofeedback | IMET (Active) + BioF |
| self-activated muscle contraction | Nutrition and diet interventions | IMET (Active) + ND |
| Physical agent modality | IMET (Active) + PAM | |
| Traditional Chinese medicine | IMET (Active) + TCM | |
| Whole-body vibration | IMET (Active) + WBV | |
| Isometric exercise training, activated by neuromuscular electrical stimulation | Physical agent modality | IMET (NMES) + PAM |
| Multicomponent exercise training | Nutrition and diet interventions | MET + ND |
| Physical agent modality | MET + PAM | |
| Traditional Chinese medicine | MET + TCM | |
| Regular care | RC | |
| Moderator | Effects on Muscle Hypertrophy a | Effects on Inflammation Reduction a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | B | SE | Median | 95% CrI | N | B | SE | Median | 95% CrI | |||
| Participant factor | ||||||||||||
| Age | 26 | −0.733 | 0.0026 | −0.737 | −1.446, | −0.029 | 30 | −0.374 | 0.0029 | −0.374 | −1.169, | 0.408 |
| BMI | 21 | 0.134 | 0.0028 | 0.124 | −0.614, | 0.957 | 21 | −0.239 | 0.0021 | −0.242 | −0.813, | 0.349 |
| Sex distribution b | 24 | −0.010 | 0.0028 | −0.101 | −0.882, | 0.697 | 30 | 0.036 | 0.0021 | 0.038 | −0.559, | 0.621 |
| Area of population c | 26 | 0.102 | 0.0025 | 0.109 | −0.621, | 0.778 | 30 | −0.381 | 0.0021 | −0.383 | −0.989, | 0.215 |
| Disease duration | 11 | −0.094 | 0.0082 | −0.119 | −2.353, | 2.354 | 13 | 0.165 | 0.0078 | 0.185 | −1.997, | 2.312 |
| KL III-IV proportion d | 19 | −0.539 | 0.0037 | −0.547 | −1.558, | 0.509 | 14 | −0.935 | 0.0175 | −0.521 | −7.256, | 2.999 |
| Study design factor | ||||||||||||
| ROB e | 26 | 0.296 | 0.0031 | 0.312 | −0.599 | 1.104 | 30 | −0.073 | 0.002 | −0.068 | −0.647, | 0.481 |
| Follow-up duration | 26 | −0.466 | 0.0015 | −0.463 | −0.890, | −0.040 | 30 | 0.350 | 0.0018 | 0.348 | −0.144, | 0.889 |
| Intervention factor | ||||||||||||
| Treatment composition f | 26 | 0.054 | 0.0047 | 0.033 | −1.293, | 1.326 | 30 | 0.368 | 0.0018 | 0.372 | −0.153, | 0.879 |
| Treatment duration | 26 | −0.451 | 0.0014 | −0.453 | −0.846, | −0.056 | 30 | 0.342 | 0.0019 | 0.334 | −0.149, | 0.918 |
| Treatment (Common Comparator: RC) | Muscle Volume | Serum Level of Inflammation | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Participants (Studies) | Treatment Effect, SMD (95%CI) a | Certainty of Evidence (GRADE) b | Number of Participants (Studies) | Treatment Effect, SMD (95%CI) a | Certainty of Evidence (GRADE) b | |||
| Direct Estimate | Network Estimate | Direct Estimate | Network Estimate | |||||
| Monotherapy | ||||||||
| HIRET | 99 (3) | 0.23 (−0.53; 1.00) | 0.63 (0.01; 1.24) | ⨁⨁⨁⊝ c | 100 (2) | −0.10 (−0.76; 0.57) | −0.15 (−0.77; 0.47) | ⨁⨁⨁⊝ e |
| MIRET | 109 (3) | 0.62 (0.01; 1.23) | 0.70 (0.13; 1.27) | ⨁⨁⊝⊝ cd | 131 (3) | −0.29 (−0.92; 0.35) | −0.26 (−0.88; 0.36) | ⨁⨁⊝⊝ de |
| LIRET | 37 (2) | 0.19 (−0.75; 1.13) | ⨁⨁⨁⊝ e | |||||
| AET | 30 (2) | 0.28 (−0.53; 1.09) | 0.26 (−0.51; 1.02) | ⨁⨁⊝⊝ ce | 139 (3) | −0.92 (−1.45; −0.38) | −0.84 (−1.35; −0.32) | ⨁⨁⊝⊝ cd |
| MET | 170 (3) | −0.31 (−1.22; 0.60) | −0.31 (−1.22; 0.60) | ⨁⨁⊝⊝ ce | 571 (8) | 0.13 (−0.32; 0.57) | −0.15 (−0.50; 0.21) | ⨁⊝⊝⊝ cde |
| IMET (Active) | 57 (3) | 1.15 (0.02; 2.27) | 0.57 (−0.22; 1.36) | ⨁⨁⊝⊝ ce | 113 (3) | −0.60 (−1.70; 0.50) | 0.36 (−0.33; 1.05) | ⨁⨁⊝⊝ ce |
| IMET (NMES) | 101 (6) | 0.67 (0.07; 1.27) | 0.75 (0.21; 1.29) | ⨁⨁⨁⊝ c | ||||
| IKET | 17 (1) | 0.14 (−1.14; 1.42) | 0.14 (−1.14; 1.42) | ⨁⊝⊝⊝ cef | 162 (3) | −0.77 (−1.82; 0.28) | −0.40 (−1.15; 0.34) | ⨁⨁⨁⊝ e |
| AQET | 85 (2) | −0.06 (−1.22; 1.10) | ⨁⨁⊝⊝ ef | 29 (2) | −0.39 (−1.13; 0.36) | −0.39 (−1.13; 0.36) | ⨁⊝⊝⊝ cef | |
| MBT | 11 (1) | 0.10 (−1.10; 1.29) | 0.10 (−1.10; 1.29) | ⨁⊝⊝⊝ cef | 135 (4) | −0.52 (−1.25; 0.21) | −0.60 (−1.25; 0.06) | ⨁⊝⊝⊝ cef |
| PropT | 69 (2) | 0.23 (−0.68; 1.14) | ⨁⨁⊝⊝ ce | |||||
| WB-EMS | 36 (1) | −0.16 (−1.09; 0.77) | ⨁⨁⊝⊝ ef | |||||
| Combined therapy | ||||||||
| MIRET + PAM | 13 (1) | −1.04 (−2.16; 0.08) | −0.76 (−1.76; 0.24) | ⨁⨁⊝⊝ ef | ||||
| LIRET + PAM | 19 (1) | 0.00 (−1.01; 1.02) | 0.12 (−0.85; 1.09) | ⨁⨁⊝⊝ ef | ||||
| LIRET + BFR | 64 (4) | 1.72 (0.87; 2.58) | 1.28 (0.60; 1.97) | ⨁⨁⨁⨁ | 16 (1) | −0.55 (−1.72; 0.61) | ⨁⨁⊝⊝ ef | |
| AET + BFR | 10 (1) | 0.50 (−0.74; 1.75) | 0.51 (−0.63; 1.66) | ⨁⨁⊝⊝ ef | ||||
| MET + ND | 119 (1) | 0.09 (−0.82; 1.00) | 0.09 (−0.82; 1.00) | ⨁⨁⊝⊝ ce | 529 (8) | −0.12 (−0.45; 0.21) | −0.19 (−0.50; 0.12) | ⨁⨁⊝⊝ ce |
| MET + PAM | 30 (1) | . | 0.18 (−0.97; 1.33) | ⨁⨁⨁⊝ e | ||||
| MET + TCM | 75 (2) | 0.57 (−0.14; 1.27) | 0.57 (−0.14; 1.27) | ⨁⨁⨁⊝e | 92 (2) | −1.19 (−1.81; −0.58) | −1.19 (−1.81; −0.58) | ⨁⨁⨁⨁ |
| IMET (Active) + BioF | 41 (2) | 0.70 (−0.47; 1.88) | 0.85 (−0.05; 1.75) | ⨁⨁⨁⊝ e | ||||
| IMET (Active) + PAM | 39 (2) | 0.17 (−0.83; 1.17) | 0.44 (−0.31; 1.18) | ⨁⨁⊝⊝ ce | ||||
| IMET (Active) + ND | 23 (1) | 0.81 (−0.34; 1.96) | ⨁⨁⊝⊝ ef | |||||
| IMET (Active) + TCM | 106 (2) | −1.61 (−2.45; −0.78) | −1.23 (−1.85; −0.62) | ⨁⨁⨁⨁ | ||||
| IMET (Active) + WBV | 12 (1) | −1.14 (−2.25; −0.03) | −0.65 (−1.67; 0.37) | ⨁⊝⊝⊝ cef | ||||
| IMET (NMES) + PAM | 57 (4) | 1.15 (0.33; 1.97) | 0.88 (0.22; 1.54) | ⨁⨁⨁⊝ c | ||||
| IKET + PAM | 20 (1) | 0.09 (−0.92; 1.10) | 0.21 (−0.76; 1.17) | ⨁⨁⊝⊝ ef | 90 (2) | −1.35 (−2.25; −0.46) | −1.89 (−2.62; −1.16) | ⨁⨁⨁⨁ |
| IKET + TCM | 92 (1) | −1.55 (−2.63; −0.46) | ⨁⨁⨁⨁ | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-L.; Chen, H.-C.; Huang, M.-H.; Huang, S.-W.; Liao, C.-D. Comparative Efficacy of Various Exercise Therapies and Combined Treatments on Inflammatory Biomarkers and Morphological Measures of Skeletal Muscle among Older Adults with Knee Osteoarthritis: A Network Meta-Analysis. Biomedicines 2024, 12, 1524. https://doi.org/10.3390/biomedicines12071524
Lin C-L, Chen H-C, Huang M-H, Huang S-W, Liao C-D. Comparative Efficacy of Various Exercise Therapies and Combined Treatments on Inflammatory Biomarkers and Morphological Measures of Skeletal Muscle among Older Adults with Knee Osteoarthritis: A Network Meta-Analysis. Biomedicines. 2024; 12(7):1524. https://doi.org/10.3390/biomedicines12071524
Chicago/Turabian StyleLin, Che-Li, Hung-Chou Chen, Mao-Hua Huang, Shih-Wei Huang, and Chun-De Liao. 2024. "Comparative Efficacy of Various Exercise Therapies and Combined Treatments on Inflammatory Biomarkers and Morphological Measures of Skeletal Muscle among Older Adults with Knee Osteoarthritis: A Network Meta-Analysis" Biomedicines 12, no. 7: 1524. https://doi.org/10.3390/biomedicines12071524
APA StyleLin, C.-L., Chen, H.-C., Huang, M.-H., Huang, S.-W., & Liao, C.-D. (2024). Comparative Efficacy of Various Exercise Therapies and Combined Treatments on Inflammatory Biomarkers and Morphological Measures of Skeletal Muscle among Older Adults with Knee Osteoarthritis: A Network Meta-Analysis. Biomedicines, 12(7), 1524. https://doi.org/10.3390/biomedicines12071524







