Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study
Abstract
1. Background
2. Materials and Methods
2.1. Study Design
2.2. Data Sources for Exposures and Outcomes
2.3. Selection of Tool Variables
2.4. Statistical Analysis
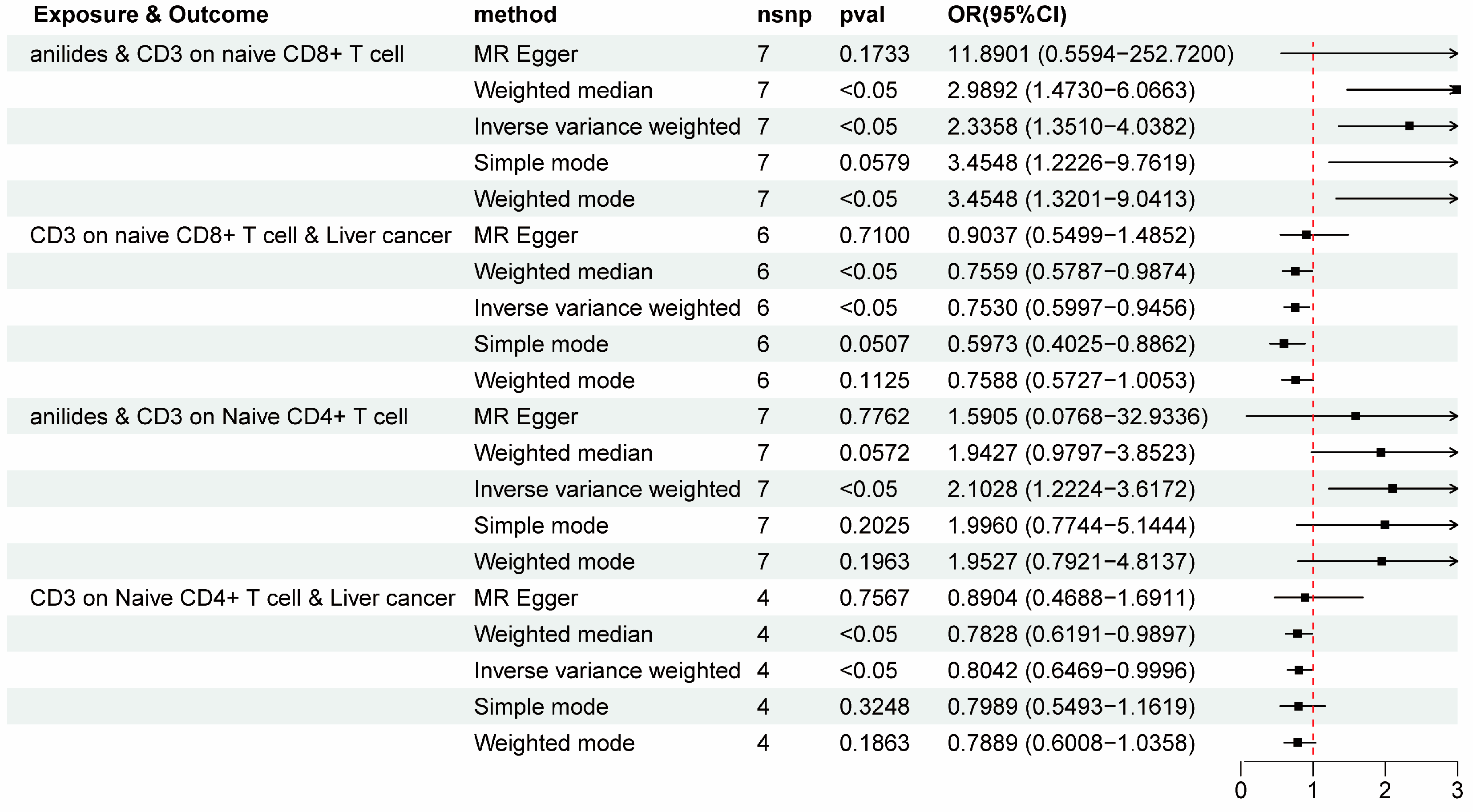
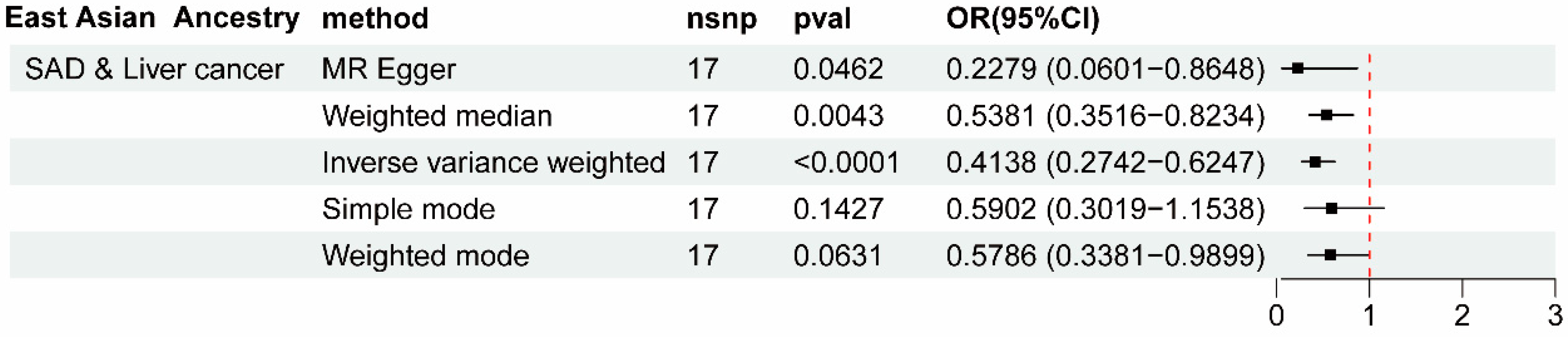
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef]
- Llovet, J.M.; Willoughby, C.E.; Singal, A.G.; Greten, T.F.; Heikenwälder, M.; El-Serag, H.B.; Finn, R.S.; Friedman, S.L. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 487–503. [Google Scholar] [CrossRef]
- Hemminki, K.; Sundquist, K.; Sundquist, J.; Forsti, A.; Liska, V.; Hemminki, A.; Li, X. Personal comorbidities and their subsequent risks for liver, gallbladder and bile duct cancers. Int. J. Cancer 2023, 152, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Z.; Zhu, M.; Huang, Y.; Jin, Z.; Xiong, Z. Low-density lipoprotein cholesterol and risk of hepatocellular carcinoma: A Mendelian randomization and mediation analysis. Lipids Health Dis. 2023, 22, 110. [Google Scholar] [CrossRef]
- Lu, L.; Wan, B.; Li, L.; Sun, M. Hypothyroidism has a protective causal association with hepatocellular carcinoma: A two-sample Mendelian randomization study. Front. Endocrinol. 2022, 13, 987401. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, J.; Lin, X.; Yang, Y.; Liu, B.; Lan, T.; Xiao, S.; Deng, A.; Yin, Z.; Xu, Y.; et al. Peripheral immune characteristics of hepatitis B virus-related hepatocellular carcinoma. Front. Immunol. 2023, 14, 1079495. [Google Scholar] [CrossRef]
- Gach, K.; Wyrębska, A.; Fichna, J.; Janecka, A. The role of morphine in regulation of cancer cell growth. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 221–230. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Arra, C. The role of morphine in animal models of human cancer: Does morphine promote or inhibit the tumor growth? Biomed Res. Int. 2013, 2013, 258141. [Google Scholar] [CrossRef]
- Rayyan, Y.; Williams, J.; Rigas, B. The role of NSAIDs in the prevention of colon cancer. Cancer Investig. 2002, 20, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Petrick, J.L.; Chen, J.; Hagberg, K.W.; Sahasrabuddhe, V.V.; Graubard, B.I.; Jick, S.; McGlynn, K.A. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016, 43, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Verdoodt, F.; Kjaer, S.K.; Friis, S. Influence of aspirin and non-aspirin NSAID use on ovarian and endometrial cancer: Summary of epidemiologic evidence of cancer risk and prognosis. Maturitas 2017, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keall, R.; Keall, P.; Kiani, C.; Luckett, T.; McNeill, R.; Lovell, M. A systematic review of assessment approaches to predict opioid misuse in people with cancer. Support Care Cancer 2022, 30, 5645–5658. [Google Scholar] [CrossRef] [PubMed]
- Porteous, T.; Bond, C.; Hannaford, P.; Sinclair, H. How and why are non-prescription analgesics used in Scotland? Fam. Pract. 2005, 22, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Fach, M.; Bischof, G.; Schmidt, C.; Rumpf, H.J. Prevalence of dependence on prescription drugs and associated mental disorders in a representative sample of general hospital patients. Gen. Hosp. Psychiatry 2007, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Wang, J.; Charboneau, R.; Loh, H.H.; Barke, R.A. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J. Immunol. 2005, 175, 6361–6367. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.; Sharma, B.; Salhan, D.; Husain, M.; Malhotra, A.; Buch, S.; Singhal, P.C. Vitamin D receptor activation and downregulation of renin-angiotensin system attenuate morphine-induced T cell apoptosis. Am. J. Physiol. Cell. Physiol. 2012, 303, C607–C615. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, S.; Hu, X. New insights into T-cell exhaustion in liver cancer: From mechanism to therapy. J. Cancer Res. Clin. Oncol. 2023, 149, 12543–12560. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Lapierre, P.; Lamarre, A. Regulatory T Cells in Autoimmune and Viral Chronic Hepatitis. J. Immunol. Res. 2015, 2015, 479703. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Yang, Q.; Shi, W.; Kahlert, U.D.; Li, Z.; Lin, S.; Song, Q.; Fan, W.; Wang, L.; Zhu, Y.; et al. Mendelian Randomization Analyses of Chronic Immune-Mediated Diseases, Circulating Inflammatory Biomarkers, and Cytokines in Relation to Liver Cancer. Cancers 2023, 15, 2930. [Google Scholar] [CrossRef]
- Ming, R.; Wu, H.; Liu, H.; Zhan, F.; Qiu, X.; Ji, M. Causal effects and metabolites mediators between immune cell and risk of breast cancer: A Mendelian randomization study. Front. Genet. 2024, 15, 1380249. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, X.; Li, J.; Su, M.; Li, X. Causal effects and immune cell mediators between prescription analgesic use and risk of infectious diseases: A Mendelian randomization study. Front. Immunol. 2023, 14, 1319127. [Google Scholar] [CrossRef] [PubMed]
- Boef, A.G.; Dekkers, O.M.; le Cessie, S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015, 44, 496–511. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Orru, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Li, Q.; Li, Y.; Wu, J. A causal relationship between childhood obesity and risk of osteoarthritis: Results from a two-sample Mendelian randomization analysis. Ann. Med. 2022, 54, 1636–1645. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.; Wang, W. Exploring the causality and pathogenesis of systemic lupus erythematosus in breast cancer based on Mendelian randomization and transcriptome data analyses. Front. Immunol. 2022, 13, 1029884. [Google Scholar] [CrossRef] [PubMed]
- Genomes Project, C.; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.; George, D.S.; Philip, C.H.; Stephen, B.J.G.E. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, H.; Qu, B.; Ma, X.; Lu, L. Protective effect of higher free thyroxine levels within the reference range on biliary tract cancer risk: A multivariable mendelian randomization and mediation analysis. Front. Endocrinol. 2024, 15, 1379607. [Google Scholar] [CrossRef]
- Rigual, M.D.M.; Sanchez Sanchez, P.; Djouder, N. Is liver regeneration key in hepatocellular carcinoma development? Trends Cancer 2023, 9, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shu, L.; Zhang, Z.; Li, J.; Zong, J.; Cheong, L.Y.; Ye, D.; Lam, K.S.L.; Song, E.; Wang, C.; et al. Adipocyte Fatty Acid Binding Protein Promotes the Onset and Progression of Liver Fibrosis via Mediating the Crosstalk between Liver Sinusoidal Endothelial Cells and Hepatic Stellate Cells. Adv. Sci. 2021, 8, e2003721. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Wang, J.; El-Khoueiry, A.B. Liver Cancer Immunity. Hepatology 2021, 73 (Suppl. S1), 86–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, G.; Ma, Q.; Wang, X.; Wang, S.; Niu, Y. Causal relationship between anti-inflammatory drugs and cancer: A pan-cancer study with Mendelian randomization. Front. Genet. 2024, 15, 1392745. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Ahn, S.H.; Yoon, J.H.; Kim, B.K. Clinical Indication of Aspirin Associated with Reduced Risk of Liver Cancer in Chronic Hepatitis B: A Nationwide Cohort Study. Am. J. Gastroenterol. 2022, 117, 758–768. [Google Scholar] [CrossRef]
- Simon, T.G.; Ma, Y.; Ludvigsson, J.F.; Chong, D.Q.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; Chung, R.T.; Zhang, X.; et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018, 4, 1683–1690. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Lin, M.; He, J.; Zhang, X.; Sun, X.; Dong, W.; Zhang, R.; Xu, Y.; Lv, L. Targeting fibrinogen-like protein 1 enhances immunotherapy in hepatocellular carcinoma. J. Clin. Investig. 2023, 133, e164528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Feng, J.Y.; Zhao, L.N.; Zhao, M.; Wei, X.F.; Geng, Y.; Yuan, H.F.; Hou, C.Y.; Zhang, H.H.; Wang, G.W.; et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-kappaB p65-activated SLC7A11 transcription. Acta Pharmacol. Sin. 2023, 44, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Mi, N.; Wang, L.; Huang, C.; Fu, W.; Bai, M.; Gao, L.; Ma, H.; Zhang, C.; Lu, Y.; et al. Regular use of paracetamol and risk of liver cancer: A prospective cohort study. BMC Cancer 2024, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Bergman, K.; Muller, L.; Teigen, S.W. Series: Current issues in mutagenesis and carcinogenesis, No. 65. The genotoxicity and carcinogenicity of paracetamol: A regulatory (re)view. Mutat Res. 1996, 349, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.G.; Feldbrugge, L.; Tapper, E.B.; Popov, Y.; Ghaziani, T.; Afdhal, N.; Robson, S.C.; Mukamal, K.J. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment. Pharmacol. Ther. 2016, 43, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Birkova, A.; Hubkova, B.; Cizmarova, B.; Bolerazska, B. Current View on the Mechanisms of Alcohol-Mediated Toxicity. Int. J. Mol. Sci. 2021, 22, 9686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Lee, Y.P.; Wu, M.L.; Chi, Y.C.; Liu, C.M.; Lai, C.L.; Yin, S.J. Inhibition of human alcohol and aldehyde dehydrogenases by aspirin and salicylate: Assessment of the effects on first-pass metabolism of ethanol. Biochem. Pharmacol. 2015, 95, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Alcover, A.; Alarcon, B.; Di Bartolo, V. Cell Biology of T Cell Receptor Expression and Regulation. Annu. Rev. Immunol. 2018, 36, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Niu, Z.; Zhang, Z.; Zhang, J.; Wang, G.; Wang, Y.; Yang, J. Back on the scene: Advances and challenges in CD3-related drugs in tumor therapy. Drug Discov. Today 2022, 27, 2199–2208. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, W.; Wang, S.; Ding, H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients with Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 729705. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Y.; Wang, H.; Liang, W.; Xie, L.; Li, N.; Fang, Y.; Wang, Y.; Liu, J.; Chi, H.; et al. ITPRIPL1 binds CD3epsilon to impede T cell activation and enable tumor immune evasion. Cell 2024, 187, 2305–2323. [Google Scholar] [CrossRef] [PubMed]




| Exposure | Outcome | Heterogeneity Test | Pleiotropy Test | |||||
|---|---|---|---|---|---|---|---|---|
| Q (MR Egger) | Q_pval (MR Egger) | Q (IVW) | Q_pval (IVW) | Egger Intercept | SE | Global Test p | ||
| SAD | Liver cancer | 25.8421 | 0.2585 | 27.9063 | 0.2193 | 0.0941 | 0.0710 | 0.229 |
| Anilides | Liver cancer | 3.1766 | 0.6728 | 4.2535 | 0.6425 | 0.20484 | 0.1974 | 0.082 |
| SAD | LFC | 26.7920 | 0.2192 | 37.8024 | 0.0267 | 0.1335 | 0.0444 | <0.001 |
| NSAIDs | ALD | 1.4543 | 0.8347 | 5.34232 | 0.3755 | 0.1353 | 0.0686 | 0.443 |
| Anilides | ALD | 4.5954 | 0.4672 | 13.9467 | 0.0302 | 0.2812 | 0.0920 | 0.051 |
| Mediators | Anilides and Liver Cancer | ||||||
|---|---|---|---|---|---|---|---|
| Anilides and Mediator | Mediator and Liver Cancer | Direct Effect | Mediation Effect | ||||
| β | p | OR | p | Effect | Proportion | ||
| CD3 on naive CD8+ T cell | 0.8483 | 0.0024 | 0.7530 | 0.0146 | −1.5403 | −0.2406 | 12.49% |
| CD3 on naive CD4+ T cell | 0.7433 | 0.0072 | 0.8042 | 0.0496 | −1.5981 | −0.162 | 9.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Mao, S.; Wang, J.; Li, G.; Sun, B. Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study. Biomedicines 2024, 12, 1537. https://doi.org/10.3390/biomedicines12071537
Tao X, Mao S, Wang J, Li G, Sun B. Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study. Biomedicines. 2024; 12(7):1537. https://doi.org/10.3390/biomedicines12071537
Chicago/Turabian StyleTao, Xuewen, Shuai Mao, Jincheng Wang, Guoqiang Li, and Beicheng Sun. 2024. "Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study" Biomedicines 12, no. 7: 1537. https://doi.org/10.3390/biomedicines12071537
APA StyleTao, X., Mao, S., Wang, J., Li, G., & Sun, B. (2024). Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study. Biomedicines, 12(7), 1537. https://doi.org/10.3390/biomedicines12071537




