Role of ncRNAs in the Pathogenesis of Sjögren’s Syndrome
Abstract
1. Introduction
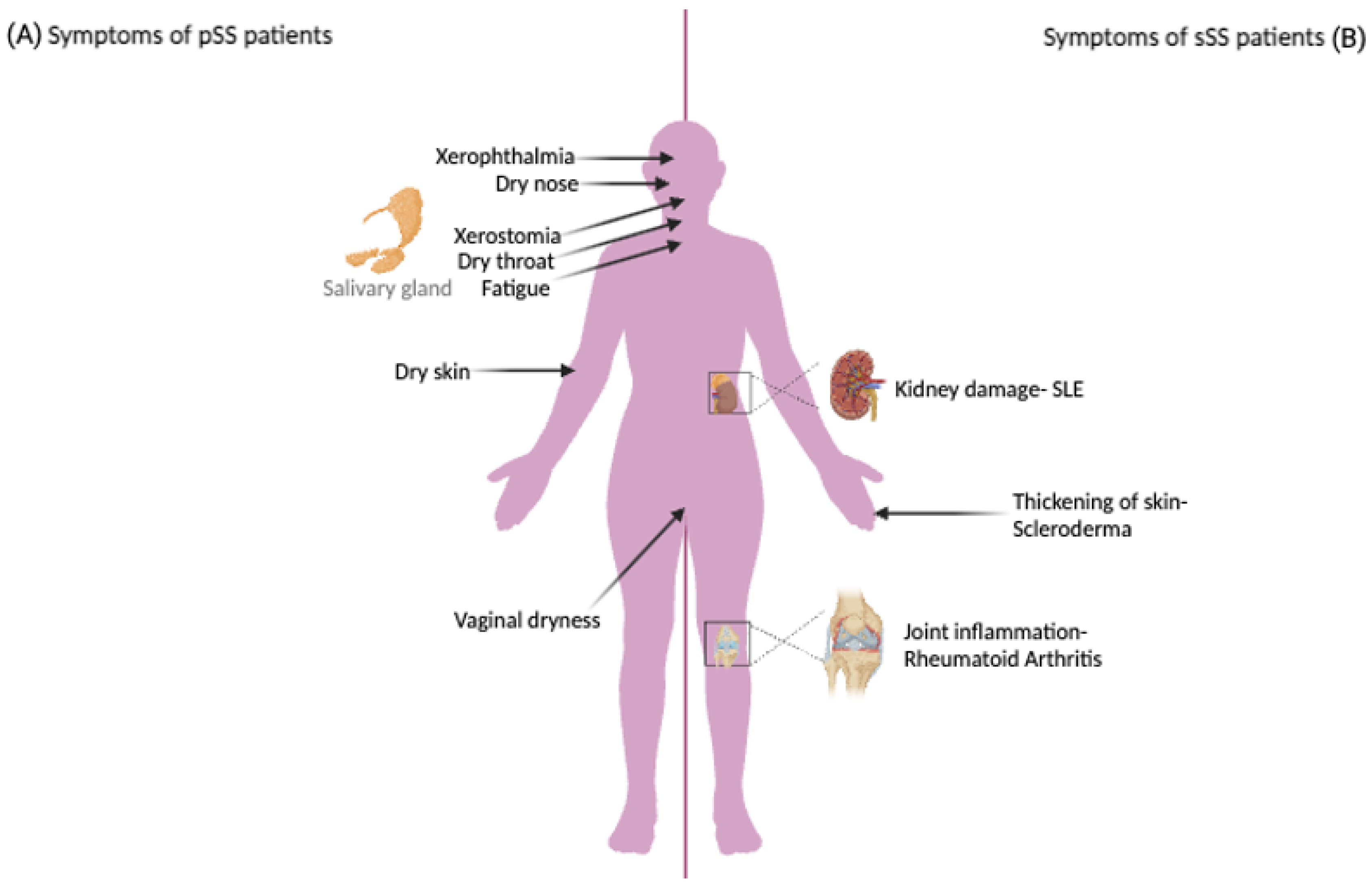
1.1. Sjögren’s Syndrome
1.2. Non-Coding RNAs
2. miRNAs in Sjögren’s Syndrome
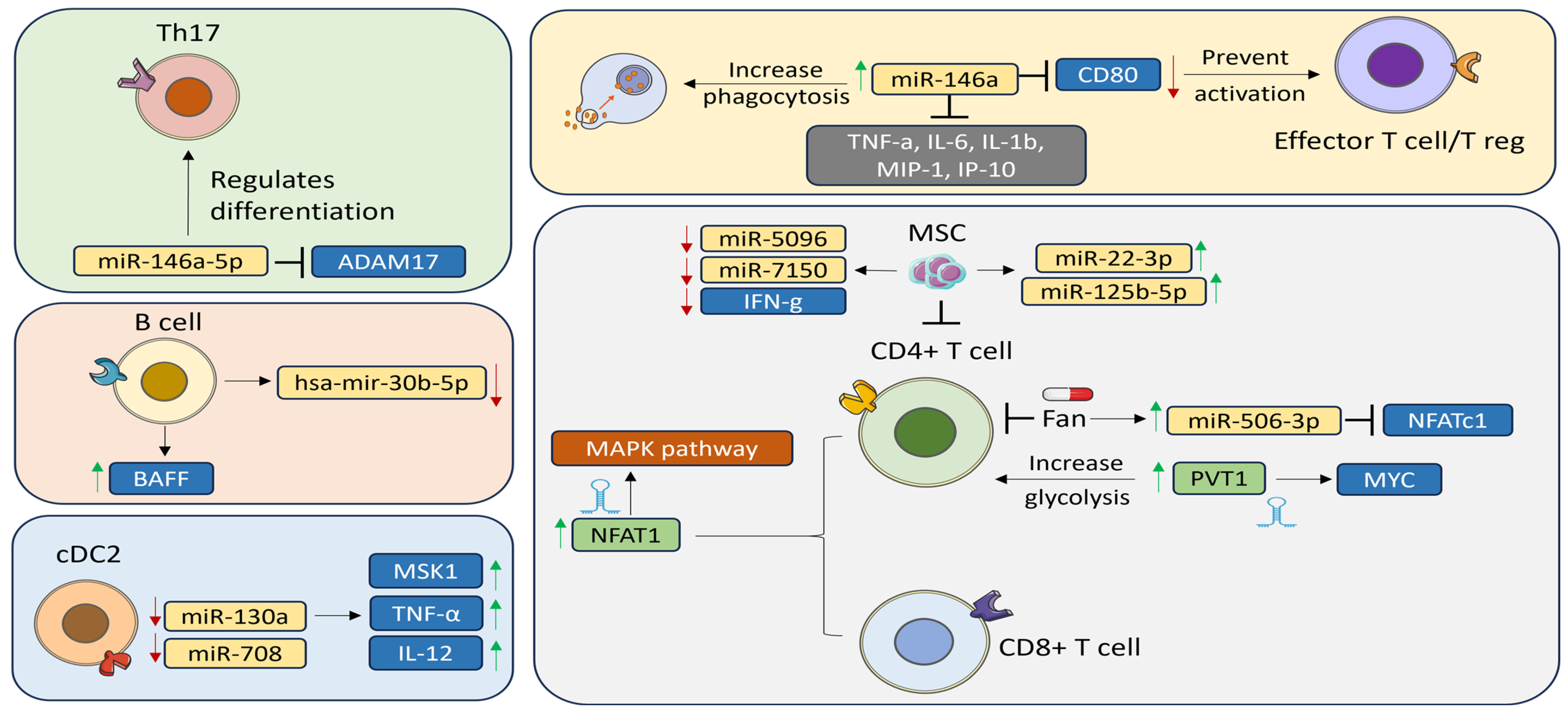
2.1. miRNA Expression in the Exocrine Glands
2.2. miRNAs in Peripheral Blood
2.3. Targeting miRNAs
3. LncRNAs in Sjögren’s Syndrome
| LncRNA | Immune Response | Actions | Associated Diseases | Ref. |
|---|---|---|---|---|
| PVT1 | Upregulated | Interacted with Myc to increase glycolysis upon CD4+ T cell activation | SS | [32] |
| Downregulated | Bound to miR-543, which negatively regulated SCUBE2 expression | RA | ||
| NEAT1 | Upregulated | Stimulated chemokine and interleukin secretion to affect monocyte–macrophage functions and T cell differentiation | pSS | [33] |
| SLE | ||||
| Improved CD4+ T cell differentiation into Th17 cells by increasing STAT3 expression | RA | |||
| NRIR and BISPR | Upregulated | Strongly correlated with JAK–STAT signaling, impacting as a negative regulator of IFN responses and resulting in an increase in the type 1 IFN-stimulated transcription | pSS | [34] |
| LINC00426 | Downregulated | Had an impact on HMGB1 located downstream that could activate the innate immune cells (macrophages/monocytes) through interaction with TLR-2/4 and trigger the release of cytokines like IL-8 through the NF-κB or MAPK signaling pathways | pSS | [34] |
| ENST00000455309.1 | Upregulated | Correlated with the β2 microglobulin expression. Involved in chemokine signaling, TNF signaling, NF-κB signaling, and natural killer cell-mediated cytotoxicity | pSS | |
| LINC00657 | Upregulated | Regulated large number of genes involved in cell adhesion, epithelial cell polarization, and apoptosis to regulate genes involved in T cell development and activation including the genes related to B cell activity | pSS | [60] |
| LINC00511 | Upregulated | Regulated a large number of transcripts involved in apoptosis | pSS | [60] |
| CTD-2020K17.1 | Upregulated | Regulated BAK1, which is frequently overexpressed in B cell lymphomas | pSS | [60] |
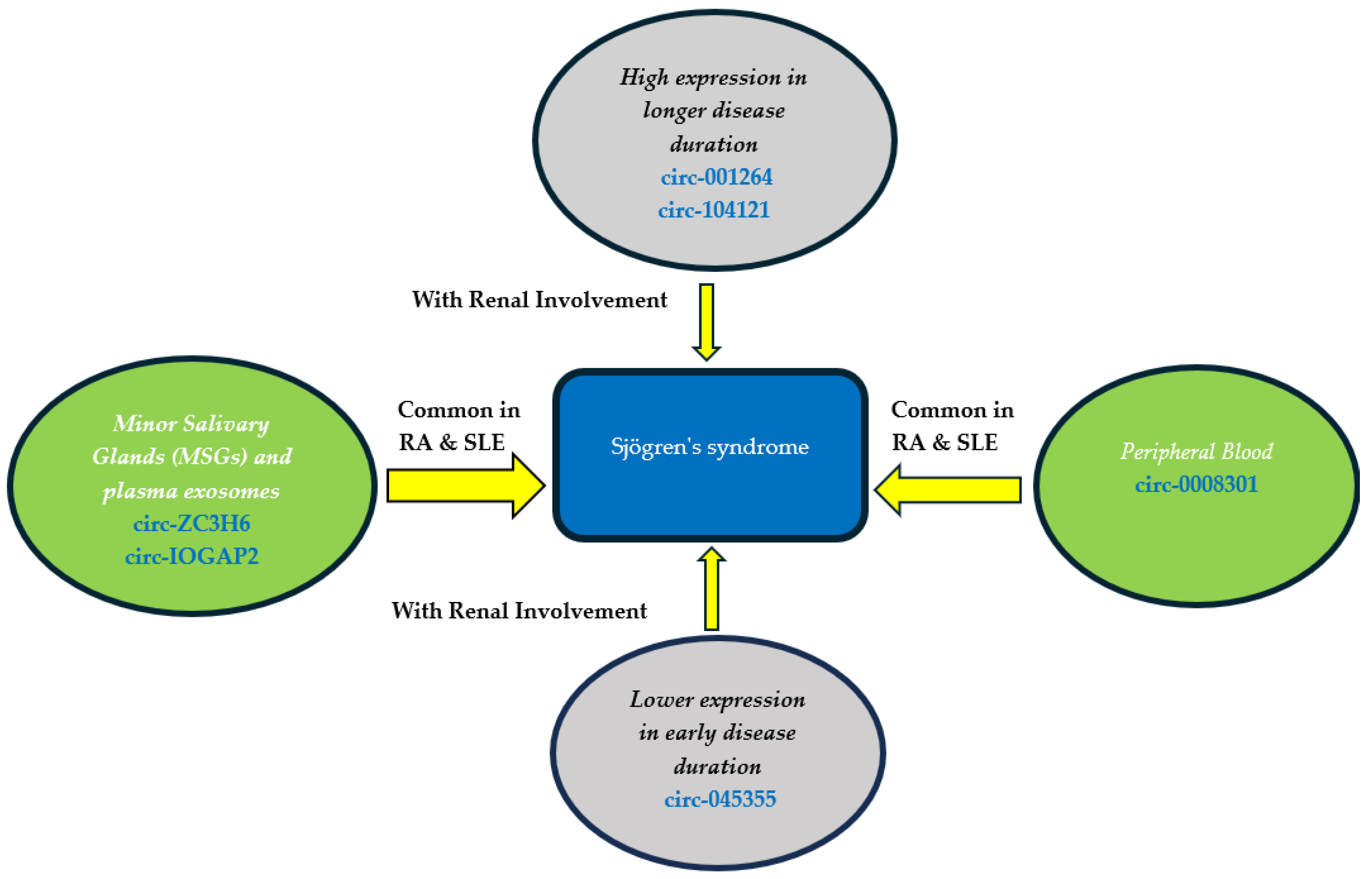
4. Circular RNAs in Sjogren’s Syndrome
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Imgenberg-Kreuz, J.; Rasmussen, A.; Sivils, K.; Nordmark, G. Genetics and Epigenetics in Primary Sjögren’s Syndrome. Rheumatol. Oxf. Engl. 2021, 60, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Noll, B.; Beckman, M.; Bahrani Mougeot, F.; Mougeot, J.-L. Exploring Salivary Epithelial Dysfunction in Sjögren’s Disease. Int. J. Mol. Sci. 2024, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Joachims, M.L.; Khatri, B.; Li, C.; Tessneer, K.L.; Ice, J.A.; Stolarczyk, A.M.; Means, N.; Grundahl, K.M.; Glenn, S.B.; Kelly, J.A.; et al. Dysregulated Long Non-Coding RNA in Sjögren’s Disease Impacts Both Interferon and Adaptive Immune Responses. RMD Open 2022, 8, e002672. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s Syndrome: A Systemic Autoimmune Disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting Non-Coding RNAs to Overcome Cancer Therapy Resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, G.; Ciccacci, C.; Latini, A.; Novelli, L.; Novelli, G.; Borgiani, P. Emerging Role of microRNAs and Long Non-Coding RNAs in Sjögren’s Syndrome. Genes 2021, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef]
- Cross, T.; Haug, K.B.F.; Brusletto, B.S.; Ommundsen, S.K.; Trøseid, A.-M.S.; Aspelin, T.; Olstad, O.K.; Aass, H.C.D.; Galtung, H.K.; Utheim, T.P.; et al. Non-Coding RNA in Salivary Extracellular Vesicles: A New Frontier in Sjögren’s Syndrome Diagnostics? Int. J. Mol. Sci. 2023, 24, 13409. [Google Scholar] [CrossRef] [PubMed]
- Yura, Y.; Hamada, M. Outline of Salivary Gland Pathogenesis of Sjögren’s Syndrome and Current Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11179. [Google Scholar] [CrossRef]
- Reale, M.; D’Angelo, C.; Costantini, E.; Laus, M.; Moretti, A.; Croce, A. MicroRNA in Sjögren’s Syndrome: Their Potential Roles in Pathogenesis and Diagnosis. J. Immunol. Res. 2018, 2018, 7510174. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A Signature for Cancer Progression. Biomed. Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Eghtedarian, R.; Dinger, M.E.; Ghafouri-Fard, S. Dysregulation of Non-Coding RNAs in Autoimmune Thyroid Disease. Exp. Mol. Pathol. 2020, 117, 104527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, X.; Li, H.; Hui, S.; Zhang, Z.; Xiao, Y.; Peng, W. Non-Coding RNAs as Novel Regulators of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2022, 13, 908076. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.; Ali, H.; Secco, I.; Giacca, M. Non-Coding RNA Therapeutics for Cardiac Regeneration. Cardiovasc. Res. 2021, 117, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Mycko, M.P.; Baranzini, S.E. microRNA and Exosome Profiling in Multiple Sclerosis. Mult. Scler. J. 2020, 26, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Lin, J.; Wang, M.; Zhao, Y.; Liao, Z.; Wei, P. Non-Coding RNA as Biomarkers for Type 2 Diabetes Development and Clinical Management. Front. Endocrinol. 2021, 12, 630032. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-I.; Tandon, M.; Teos, L.; Zheng, C.; Warner, B.M.; Alevizos, I. Dual Function of miR-1248 Links Interferon Induction and Calcium Signaling Defects in Sjögren’s Syndrome. eBioMedicine 2019, 48, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, P.; Aguilera, S.; Jara, D.; Indo, S.; Barrera, M.-J.; González, S.; Molina, C.; Heathcote, B.; Hermoso, M.; Castro, I.; et al. Hsa-miR-424–5p and Hsa-miR-513c-3p Dysregulation Mediated by IFN-γ Is Associated with Salivary Gland Dysfunction in Sjögren’s Syndrome Patients. J. Autoimmun. 2023, 138, 103037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Zhang, L.; Zhao, M.; Huang, H. Decreased microRNA-181a and -16 Expression Levels in the Labial Salivary Glands of Sjögren Syndrome Patients. Exp. Ther. Med. 2018, 15, 426–432. [Google Scholar] [CrossRef]
- Gallo, A.; Vella, S.; Tuzzolino, F.; Cuscino, N.; Cecchettini, A.; Ferro, F.; Mosca, M.; Alevizos, I.; Bombardieri, S.; Conaldi, P.G.; et al. MicroRNA-Mediated Regulation of Mucin-Type O-Glycosylation Pathway: A Putative Mechanism of Salivary Gland Dysfunction in Sjögren Syndrome. J. Rheumatol. 2019, 46, 1485–1494. [Google Scholar] [CrossRef]
- Castro, I.; Carvajal, P.; Jara, D.; Aguilera, S.; Heathcote, B.; Barrera, M.-J.; Aliaga-Tobar, V.; Maracaja-Coutinho, V.; Urzúa, U.; Quest, A.F.G.; et al. Small RNA Expression Profiling Reveals Hsa-miR-181d-5p Downregulation Associated With TNF-α Overexpression in Sjögren’s Syndrome Patients. Front. Immunol. 2022, 13, 870094. [Google Scholar] [CrossRef]
- Jara, D.; Carvajal, P.; Castro, I.; Barrera, M.-J.; Aguilera, S.; González, S.; Molina, C.; Hermoso, M.; González, M.-J. Type I Interferon Dependent Hsa-miR-145-5p Downregulation Modulates MUC1 and TLR4 Overexpression in Salivary Glands from Sjögren’s Syndrome Patients. Front. Immunol. 2021, 12, 685837. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ye, Z.; Liu, T.; Jiang, J.; Jiang, Z.; Cao, D.; Zhao, L. Hsa-miR -3202 Attenuates Jurkat Cell Infiltration via MMP2 in Primary Sjögren’s Syndrome. J. Oral Pathol. Med. 2022, 51, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Nyberg, W.A.; Sjöstrand, M.; Moruzzi, N.; Bergman, P.; Khademi, M.; Andersson, M.; Piehl, F.; Berggren, P.-O.; Covacu, R.; et al. miR-31 Regulates Energy Metabolism and Is Suppressed in T Cells from Patients with Sjögren’s Syndrome. Eur. J. Immunol. 2019, 49, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xin, S.; Wang, Y.; Ju, D.; Wu, Q.; Qiu, Y.; Niu, X.; Liu, W.; Li, J.; Ji, P. MicroRNA-146a-5p Enhances T Helper 17 Cell Differentiation via Decreasing a Disintegrin and Metalloprotease 17 Level in Primary Sjögren’s Syndrome. Bioengineered 2021, 12, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Pauley, K.M.; Stewart, C.M.; Gauna, A.E.; Dupre, L.C.; Kuklani, R.; Chan, A.L.; Pauley, B.A.; Reeves, W.H.; Chan, E.K.L.; Cha, S. Altered miR-146a Expression in Sjögren’s Syndrome and Its Functional Role in Innate Immunity. Eur. J. Immunol. 2011, 41, 2029–2039. [Google Scholar] [CrossRef]
- Gauna, A.E.; Park, Y.-J.; Nayar, G.; Onate, M.; Jin, J.; Stewart, C.M.; Yu, Q.; Cha, S. Dysregulated Co-Stimulatory Molecule Expression in a Sjögren’s Syndrome Mouse Model with Potential Implications by microRNA-146a. Mol. Immunol. 2015, 68, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Wang, L.; Sun, C.; Xie, C.; Li, Z. MiR-Let-7d-3p Regulates IL-17 Expression through Targeting AKT1/mTOR Signaling in CD4+ T Cells. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 67–74. [Google Scholar] [CrossRef]
- Lopes, A.P.; van Roon, J.A.G.; Blokland, S.L.M.; Wang, M.; Chouri, E.; Hartgring, S.A.Y.; van der Wurff-Jacobs, K.M.G.; Kruize, A.A.; Burgering, B.M.T.; Rossato, M.; et al. MicroRNA-130a Contributes to Type-2 Classical DC-Activation in Sjögren’s Syndrome by Targeting Mitogen- and Stress-Activated Protein Kinase-1. Front. Immunol. 2019, 10, 1335. [Google Scholar] [CrossRef]
- Wang-Renault, S.-F.; Boudaoud, S.; Nocturne, G.; Roche, E.; Sigrist, N.; Daviaud, C.; Bugge Tinggaard, A.; Renault, V.; Deleuze, J.-F.; Mariette, X.; et al. Deregulation of microRNA Expression in Purified T and B Lymphocytes from Patients with Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2018, 77, 133–140. [Google Scholar] [CrossRef]
- Pilson, Q.; Smith, S.; Jefferies, C.A.; Ní Gabhann-Dromgoole, J.; Murphy, C.C. miR-744-5p Contributes to Ocular Inflammation in Patients with Primary Sjogrens Syndrome. Sci. Rep. 2020, 10, 7484. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shi, H.; Wang, B.; Zhan, T.; Shao, Y.; Ye, L.; Wu, S.; Yu, C.; Zheng, L. LncRNA PVT1 Links Myc to Glycolytic Metabolism upon CD4+ T Cell Activation and Sjögren’s Syndrome-like Autoimmune Response. J. Autoimmun. 2020, 107, 102358. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Shi, H.; Yu, C.; Fu, J.; Chen, C.; Wu, S.; Zhan, T.; Wang, B.; Zheng, L. LncRNA Neat1 Positively Regulates MAPK Signaling and Is Involved in the Pathogenesis of Sjögren’s Syndrome. Int. Immunopharmacol. 2020, 88, 106992. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Luo, X.; Chen, Y.; Peng, L.; Deng, C.; Fei, Y.; Zhang, W.; Zhao, Y. LncRNA and mRNA Expression Profile of Peripheral Blood Mononuclear Cells in Primary Sjögren’s Syndrome Patients. Sci. Rep. 2020, 10, 19629. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Z.; Zhang, B.; Jiang, S.; Wang, Q.; Du, L.; Xue, H.; Zhang, Y.; Jin, M.; Zhu, X.; et al. Circular RNA Sequencing Indicates Circ-IQGAP2 and Circ-ZC3H6 as Noninvasive Biomarkers of Primary Sjögren’s Syndrome. Rheumatol. Oxf. Engl. 2020, 59, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, X.; Ling, Y.; Tian, J.; Wang, Y.; Luo, Y.; Zhu, R.; Zhou, Y.; Zhu, T.; Wang, L.; et al. Hsa_circ_0008301 as a Potential Biomarker of Disease Activity for Primary Sjogren’s Syndrome: Increased Expression in Peripheral Blood of Patients with Primary Sjogren’s Syndrome. Int. Immunopharmacol. 2022, 112, 109231. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, N.; Pu, Y.; Xie, L.; Zheng, L.; Yu, C. Long Non-Coding RNA Expression Profile in Minor Salivary Gland of Primary Sjögren’s Syndrome. Arthritis Res. Ther. 2016, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ma, J.-F.; Chang, C.; Xu, T.; Gao, C.-Y.; Gershwin, M.E.; Lian, Z.-X. Immunobiology of T Cells in Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2021, 60, 111–131. [Google Scholar] [CrossRef]
- Chaudhury, N.M.A.; Proctor, G.B.; Karlsson, N.G.; Carpenter, G.H.; Flowers, S.A. Reduced Mucin-7 (Muc7) Sialylation and Altered Saliva Rheology in Sjögren’s Syndrome Associated Oral Dryness. Mol. Cell. Proteom. MCP 2016, 15, 1048–1059. [Google Scholar] [CrossRef]
- Cortés, J.; Hidalgo, J.; Aguilera, S.; Castro, I.; Brito, M.; Urra, H.; Pérez, P.; Barrera, M.-J.; Carvajal, P.; Urzúa, U.; et al. Synaptotagmin-1 Overexpression under Inflammatory Conditions Affects Secretion in Salivary Glands from Sjögren’s Syndrome Patients. J. Autoimmun. 2019, 97, 88–99. [Google Scholar] [CrossRef]
- Cascio, S.; Zhang, L.; Finn, O.J. MUC1 Protein Expression in Tumor Cells Regulates Transcription of Proinflammatory Cytokines by Forming a Complex with Nuclear Factor-κB P65 and Binding to Cytokine Promoters: Importance of Extracellular Domain. J. Biol. Chem. 2011, 286, 42248–42256. [Google Scholar] [CrossRef]
- Kim, S.; Joe, Y.; Surh, Y.-J.; Chung, H.T. Differential Regulation of Toll-Like Receptor-Mediated Cytokine Production by Unfolded Protein Response. Oxid. Med. Cell. Longev. 2018, 2018, 9827312. [Google Scholar] [CrossRef]
- Alevizos, I.; Alexander, S.; Turner, R.J.; Illei, G.G. MicroRNA Expression Profiles as Biomarkers of Minor Salivary Gland Inflammation and Dysfunction in Sjögren’s Syndrome. Arthritis Rheum. 2011, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T Cells in Primary Sjögren’s Syndrome: Targets for Early Intervention. Rheumatol. Oxf. Engl. 2021, 60, 3088–3098. [Google Scholar] [CrossRef]
- Ríos-Ríos, W.d.J.; Sosa-Luis, S.A.; Torres-Aguilar, H. T Cells Subsets in the Immunopathology and Treatment of Sjogren’s Syndrome. Biomolecules 2020, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.; Schröder, J.; Monhasery, N.; Ackfeld, T.; Hummel, T.M.; Rabe, B.; Garbers, C.; Becker-Pauly, C.; Floss, D.M.; Scheller, J. Human and Murine Interleukin 23 Receptors Are Novel Substrates for A Disintegrin and Metalloproteases ADAM10 and ADAM17. J. Biol. Chem. 2016, 291, 10551–10561. [Google Scholar] [CrossRef]
- Talotta, R.; Mercurio, V.; Bongiovanni, S.; Vittori, C.; Boccassini, L.; Rigamonti, F.; Batticciotto, A.; Atzeni, F.; Trabattoni, D.; Sarzi-Puttini, P.; et al. Evaluation of Salivary and Plasma microRNA Expression in Patients with Sjögren’s Syndrome, and Correlations with Clinical and Ultrasonographic Outcomes. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S118), 70–77. [Google Scholar] [PubMed]
- Ibrahim, M.R.K.; Waly, N.G.; Moness, H.; Ahmed, S.S.; Ibrahem, R. Serum miRNA-21, miRNA-146a and Plasma Cell Free DNA as Novel Biomarkers for Assessing Systemic Lupus Erythematosus Activity. Mol. Biol. Rep. 2023, 50, 10025–10036. [Google Scholar] [CrossRef] [PubMed]
- Mielle, J.; Tison, A.; Cornec, D.; Le Pottier, L.; Daien, C.; Pers, J.-O. B Cells in Sjögren’s Syndrome: From Pathophysiology to Therapeutic Target. Rheumatology 2021, 60, 2545–2560. [Google Scholar] [CrossRef]
- Zollars, E.; Bienkowska, J.; Czerkowicz, J.; Allaire, N.; Ranger, A.M.; Magder, L.; Petri, M. BAFF (B Cell Activating Factor) Transcript Level in Peripheral Blood of Patients with SLE Is Associated with Same-Day Disease Activity as Well as Global Activity over the next Year. Lupus Sci. Med. 2015, 2, e000063. [Google Scholar] [CrossRef]
- Mackay, F.; Woodcock, S.A.; Lawton, P.; Ambrose, C.; Baetscher, M.; Schneider, P.; Tschopp, J.; Browning, J.L. Mice Transgenic for BAFF Develop Lymphocytic Disorders along with Autoimmune Manifestations. J. Exp. Med. 1999, 190, 1697–1710. [Google Scholar] [CrossRef]
- Nandula, S.-R.; Scindia, Y.M.; Dey, P.; Bagavant, H.; Deshmukh, U.S. Activation of Innate Immunity Accelerates Sialoadenitis in a Mouse Model for Sjögren’s Syndrome-like Disease. Oral Dis. 2011, 17, 801–807. [Google Scholar] [CrossRef]
- Shao, Y.; Fu, J.; Zhan, T.; Yin, J.; Xu, J.; Lu, Y.; Luo, Q.; Yu, C. Inhibition of CD4 + T Cells by Fanchinoline via miR506-3p/NFATc1 in Sjögren’s Syndrome. Inflammopharmacology 2023, 31, 2431–2443. [Google Scholar] [CrossRef]
- Chihaby, N.; Orliaguet, M.; Le Pottier, L.; Pers, J.-O.; Boisramé, S. Treatment of Sjögren’s Syndrome with Mesenchymal Stem Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10474. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, L.; Lu, Z.; Huang, J.; Pu, J.; Pan, S.; Zhang, M.; Liu, J.; Tang, J. Mesenchymal Stem Cells Negatively Regulate CD4+ T Cell Activation in Patients with Primary SjöGren Syndrome through the miRNA-125b and miRNA-155 TCR Pathway. Mol. Med. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Bian, X.; Peng, H.; Wang, Y.; Guo, H.; Shi, G. MicroRNA-22-3p Alleviates Atherosclerosis by Mediating Macrophage M2 Polarization as Well as Inhibiting NLRP3 Activation. J. Int. Med. Res. 2023, 51, 3000605231197071. [Google Scholar] [CrossRef]
- Wang, J.; Kong, X.; Hu, H.; Shi, S. Knockdown of Long Non-Coding RNA PVT1 Induces Apoptosis of Fibroblast-like Synoviocytes through Modulating miR-543-Dependent SCUBE2 in Rheumatoid Arthritis. J. Orthop. Surg. 2020, 15, 142. [Google Scholar] [CrossRef]
- Mohammed, A.; Shaker, O.G.; Khalil, M.A.F.; Elsabagh, Y.A.; Gomaa, M.; Ahmed, A.M.; Erfan, R. Association of Long Non-Coding RNAs NEAT1, and MALAT1 Expression and Pathogenesis of Behçet’s Disease among Egyptian Patients. Saudi J. Biol. Sci. 2022, 29, 103344. [Google Scholar] [CrossRef]
- Sequí-Sabater, J.M.; Beretta, L. Defining the Role of Monocytes in Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 12765. [Google Scholar] [CrossRef]
- Dolcino, M.; Tinazzi, E.; Vitali, C.; Del Papa, N.; Puccetti, A.; Lunardi, C. Long Non-Coding RNAs Modulate Sjögren’s Syndrome Associated Gene Expression and Are Involved in the Pathogenesis of the Disease. J. Clin. Med. 2019, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-C.; Xu, W.-D.; Liu, X.-Y.; Fu, L.; Huang, A.-F. Altered Expression of Circular RNA in Primary Sjögren’s Syndrome. Clin. Rheumatol. 2019, 38, 3425–3433. [Google Scholar] [CrossRef]
| Non-Coding RNA | Expression in SS Patients | Target/Association | Tissue/Cell | Comments | Ref. |
|---|---|---|---|---|---|
| miR-1248 | Upregulated | IFN | Salivary gland | Overexpression affects both interferon and calcium signaling pathways | [17] |
| miR-513c-3p | Upregulated | XBP-1s and GRP78 | LSG | Impact cellular proteostasis which regulate secretory function in LSG | [18] |
| miR-424–5p | Downregulated | ATF6α and SEL1L | LSG | [18] | |
| miR-181a and miR-16 | Downregulated | labial salivary pathological focus scores | LSG | Involved in SS pathogenesis through regulating La/SSB and Ro/SSA | [19] |
| miR-18b, miR-20a, miR-106a, miR-146b, miR-30, miR-17/92, miR-200, miR-let-7 | Upregulated | Mucin O-glycosylation pathway | MSGBs | Cause dysfunction of salivary flow rates | [20] |
| miR-635, miR-372 | Downregulated | ||||
| miR-181d-5p | Downregulated | TNF-α | LSG | Dysregulation could impact the glandular pro-inflammatory environment | [21] |
| miR-145-5p | Downregulated | Type 1 IFN, MUC1, TLR4 | LSG | Has anti-inflammatory role | [22] |
| miR-3202 | Downregulated | MMP2 | PBMCs and Jurkat cell line | Has a protective function via inhibiting T cell infiltration from peripheral blood into the gland | [23] |
| miR-31-5p | Downregulated | PBMCs (CD4+) | Associated with energy metabolism | [24] | |
| miR-146a-5p | Upregulated | ADAM17 | PBMCs (Th17) | Plays a role in Th17 differentiation | [25] |
| miR-146a | Upregulated | CD80 TNF-α, IL-6, IL-1β MIP-1α, IP-10 | PBMCs and Salivary gland | Elevates the phagocytic activity, suppresses the production of inflammatory cytokine, and could potentially modifies the regulation of T cells in autoimmune processes | [26,27] |
| miR-let-7d-3p | Downregulated | IL-17 | PBMCs (CD4+) | Targeting the AKT1/mTOR signaling pathway, revealing a unique mechanism in pSS that may inform future treatment | [28] |
| miR-130a | Downregulated | MSK1 | cDC2s | Inhibiting MSK1 can decrease the activity of cDC2s which consequently lowers the production of pro-inflammatory cytokines | [29] |
| mir-30b-5p | Downregulated | BAFF | PBMCs (B cells) | Correlated with decrease salivary flow in the gland | [30] |
| miR-744-5p | Upregulated | PELI3 | PECs | Regulating eye inflammation in pSS | [31] |
| PVT1 | Upregulated | Myc | PBMCs (CD19+, CD4+) | Engaged in altering the glycolytic metabolism and promoting cell growth | [32] |
| NEAT1 | Upregulated | MAPK pathway | PBMCs (CD4+) | Elevated activation of the TCR pathway by PMA/ionomycin, suggested to be a contributing factor to the increased expression | [33] |
| LINC00426, TPTEP1-202 | Downregulate | KATNAL1, HMGB1, UBE2L5, CCT8L2 | PBMCs | Have a substantial correlation with markers of disease activity and regulate essential immunological processes | [34] |
| NRIR | Upregulated | RSAD2, CMPK2, RNF144A | |||
| BISPR | Upregulated | PGLS, GTPBP3, MRPL34, PLVAP, CCDC194, NXNL1, DDA1, BST2, TMEM221 | |||
| circ-ZC3H6 and circ-IQGAP2 | Upregulated | TFEC | MSGBs | Play important roles in pSS as non-invasive biomarkers associated with clinical characteristics | [35] |
| circ_0008301 | Upregulated | TOLLIP | PBMCs | The expression level is high in patients associated with thrombocytopenia | [36] |
| lncRNAs | 890 Upregulated 353 Downregulated | - | LSG | Demonstrating their potential as biomarkers and therapeutic targets for pSS, as well as the importance of their role in immunity response | [37] |
| mRNAs | 1141 Upregulated 316 Downregulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Haidose, A.; Hassan, S.; Elhassan, M.; Ahmed, E.; Al-Riashi, A.; Alharbi, Y.M.; Ghunaim, M.; Alhejaili, T.; Abdallah, A.M. Role of ncRNAs in the Pathogenesis of Sjögren’s Syndrome. Biomedicines 2024, 12, 1540. https://doi.org/10.3390/biomedicines12071540
Al-Haidose A, Hassan S, Elhassan M, Ahmed E, Al-Riashi A, Alharbi YM, Ghunaim M, Alhejaili T, Abdallah AM. Role of ncRNAs in the Pathogenesis of Sjögren’s Syndrome. Biomedicines. 2024; 12(7):1540. https://doi.org/10.3390/biomedicines12071540
Chicago/Turabian StyleAl-Haidose, Amal, Sondoss Hassan, Mahmoud Elhassan, Eiman Ahmed, Abdulla Al-Riashi, Yazeed M. Alharbi, Monther Ghunaim, Talal Alhejaili, and Atiyeh M. Abdallah. 2024. "Role of ncRNAs in the Pathogenesis of Sjögren’s Syndrome" Biomedicines 12, no. 7: 1540. https://doi.org/10.3390/biomedicines12071540
APA StyleAl-Haidose, A., Hassan, S., Elhassan, M., Ahmed, E., Al-Riashi, A., Alharbi, Y. M., Ghunaim, M., Alhejaili, T., & Abdallah, A. M. (2024). Role of ncRNAs in the Pathogenesis of Sjögren’s Syndrome. Biomedicines, 12(7), 1540. https://doi.org/10.3390/biomedicines12071540






