Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1−/−) Mice
Abstract
:1. Introduction

2. Materials and Methods
2.1. Ethics
2.2. Sample Collection and Generation of Dab1 Null Conventional Mutants
2.3. Immunofluorescence
2.4. Data Acquisition and Analysis
2.5. Statistical Analyses
3. Results
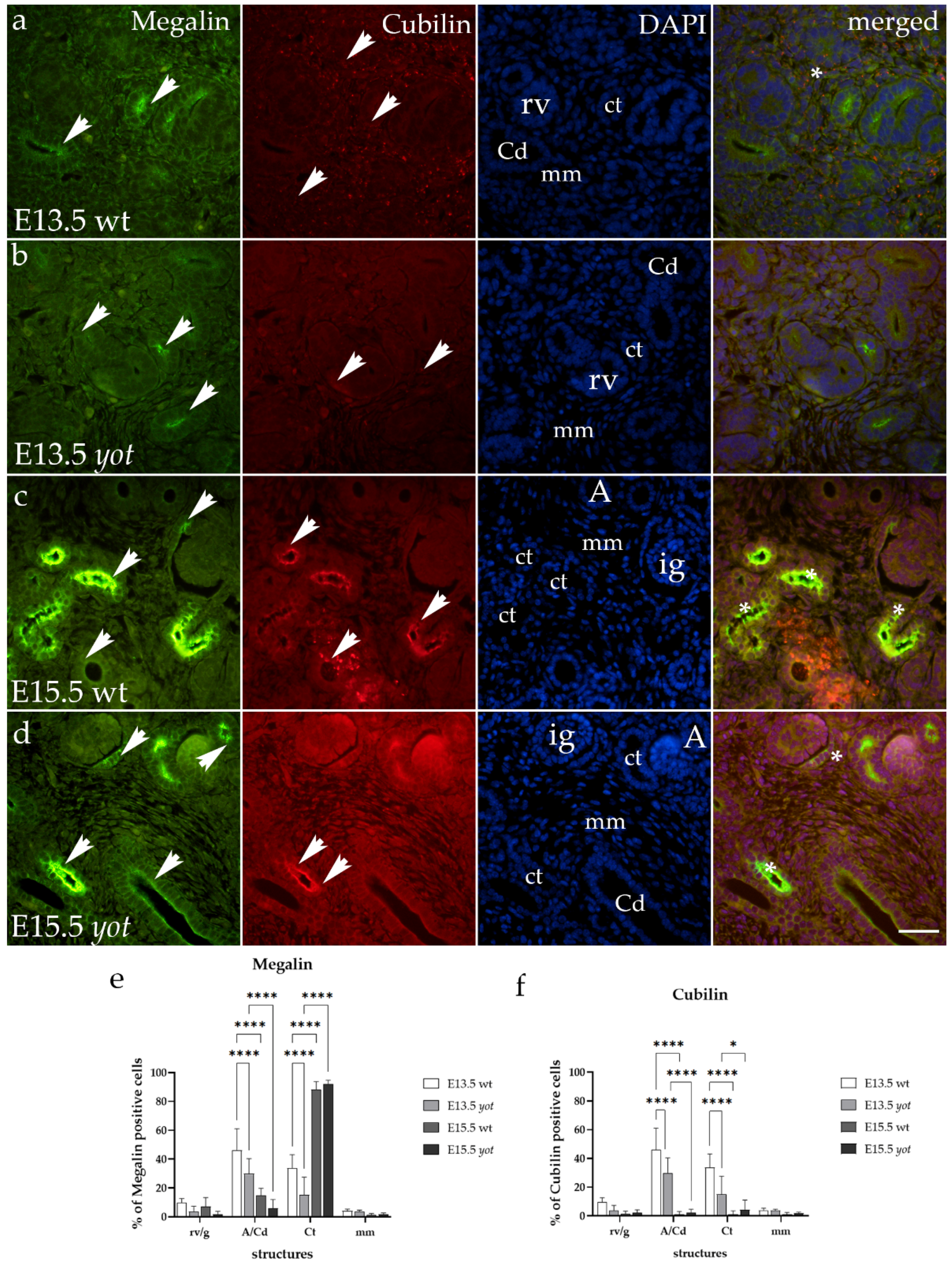
3.1. Megalin and Cubilin Immunoexpression
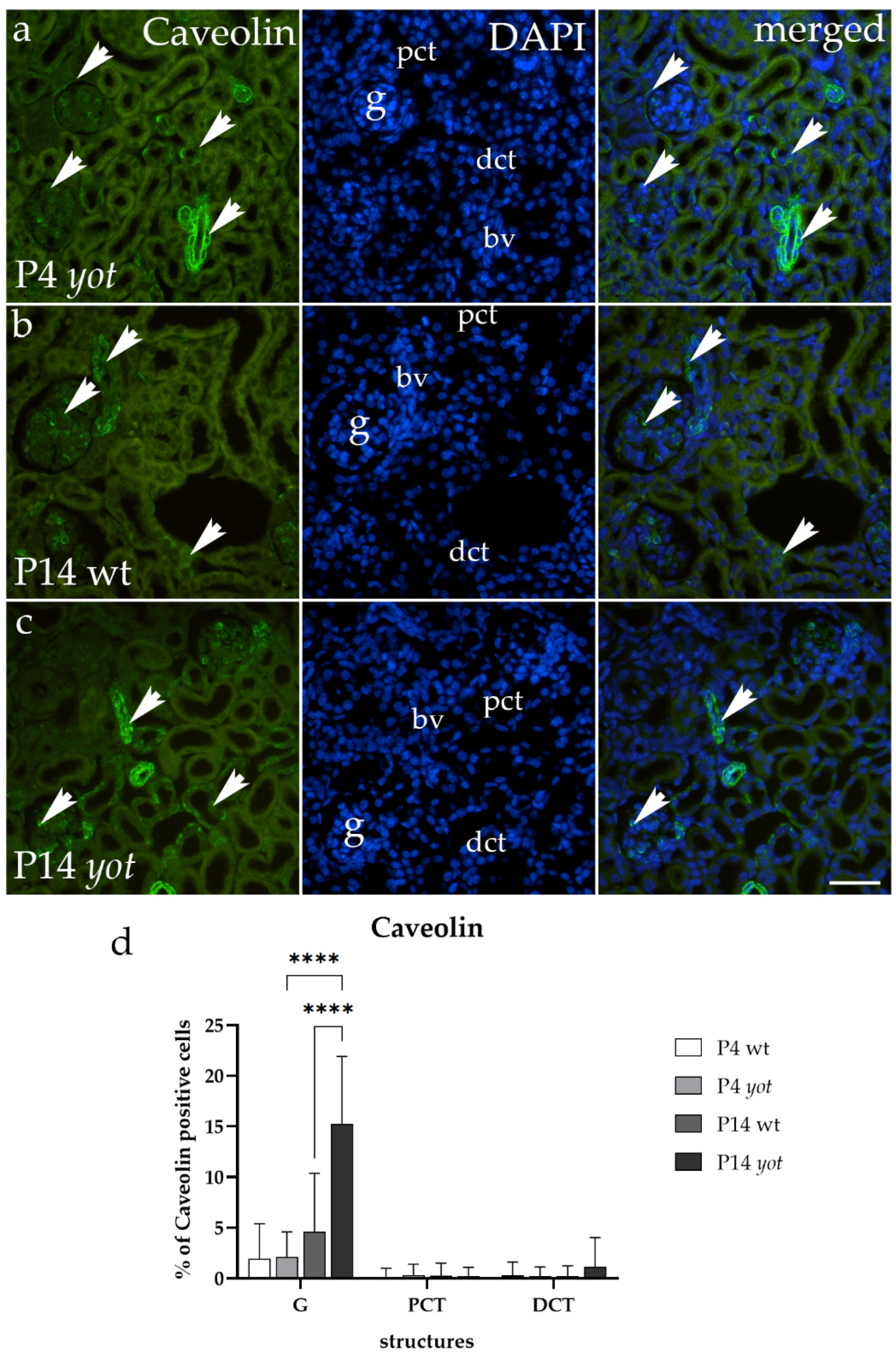
3.2. Caveolin-1 Immunoexpression
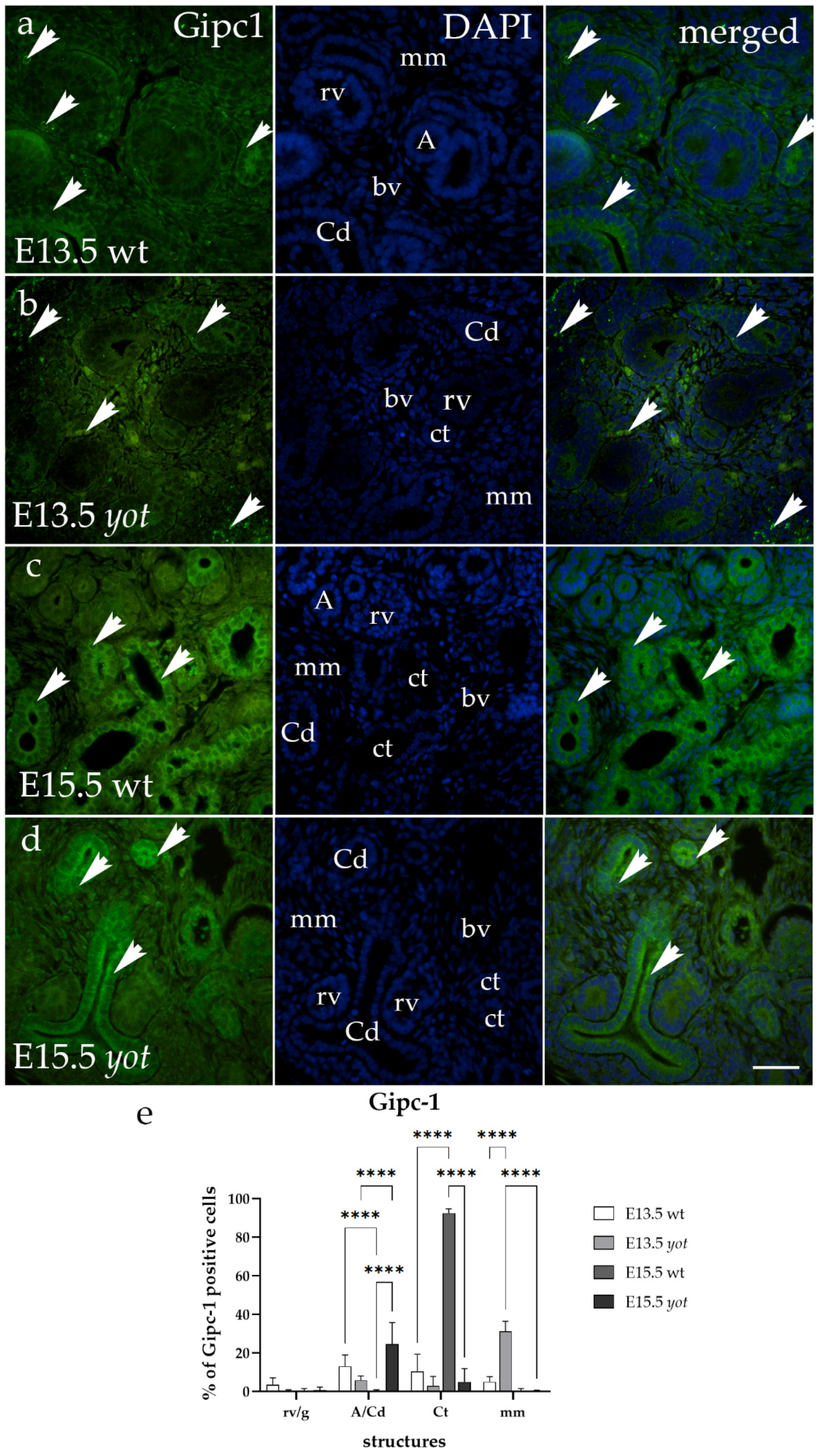
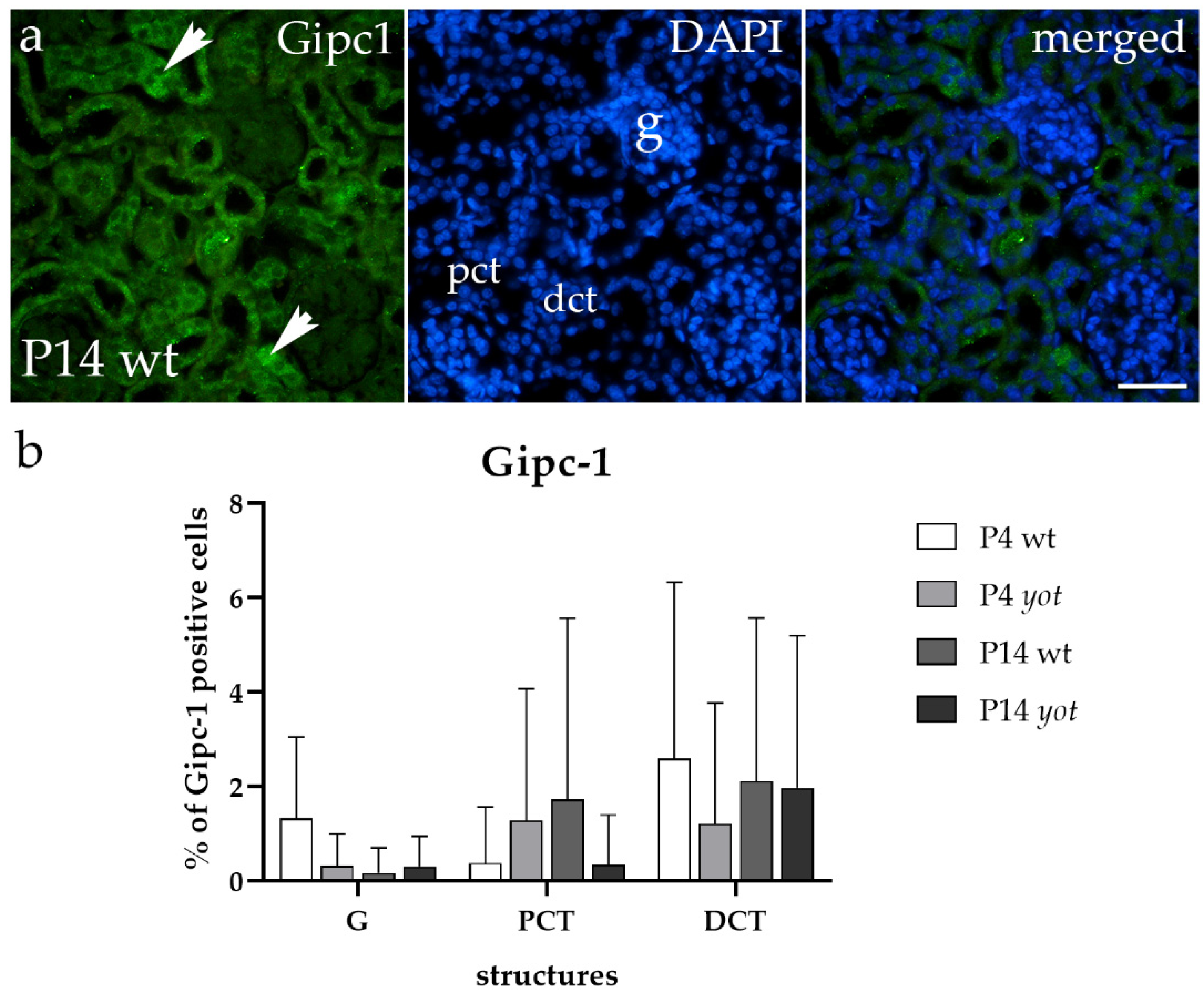
3.3. Gipc1 Immunoexpression
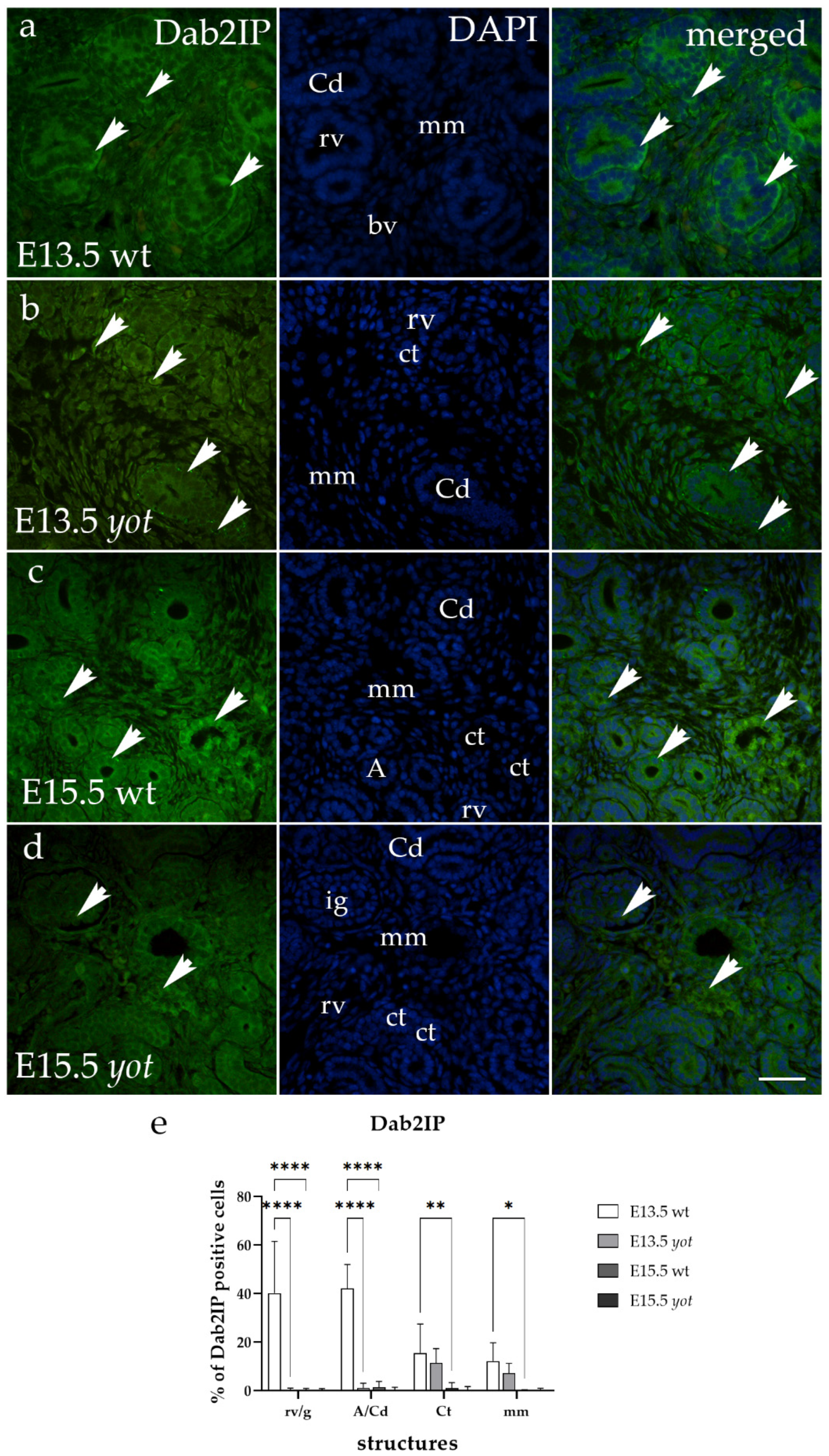
3.4. Dab2IP Immunoexpression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozić, M.; Filipović, N.; Jurić, M.; Kosović, I.; Benzon, B.; Šolić, I.; Kelam, N.; Racetin, A.; Watanabe, K.; Katsuyama, Y.; et al. Alteration of Cx37, Cx40, Cx43, Cx45, Panx1, and Renin Expression Patterns in Postnatal Kidneys of Dab1−/− (yotari) Mice. Int. J. Mol. Sci. 2021, 22, 1284. [Google Scholar] [CrossRef] [PubMed]
- Yoneshima, H.; Nagata, E.; Matsumoto, M.; Yamada, M.; Nakajima, K.; Miyata, T.; Ogawa, M.; Mikoshiba, K. A novel neurological mutant mouse, yotari, which exhibits reeler-like phenotype but expresses CR-50 antigen/Reelin. Neurosci. Res. 1997, 29, 217–223. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Setsu, T.; Katsuyama, Y.; Kikkawa, S.; Terashima, T.; Maeda, K. Cortical layer V neurons in the auditory and visual cortices of normal, reeler, and yotari mice. Kobe J. Med. Sci. 2010, 56, E50–E59. [Google Scholar] [PubMed]
- Perutina, I.; Kelam, N.; Maglica, M.; Racetin, A.; Ogorevc, M.; Filipović, N.; Katsuyama, Y.; Mišković, J.; Vukojević, K. Disturbances in Switching between Canonical and Non-Canonical Wnt Signaling Characterize Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. Biomedicines 2023, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendoza, M.J.; Lorda-Diez, C.I.; Montero, J.A.; Garcia-Porrero, J.A.; Hurle, J.M. Reelin/DAB-1 signaling in the embryonic limb regulates the chondrogenic differentiation of digit mesodermal progenitors. J. Cell. Physiol. 2014, 229, 1397–1404. [Google Scholar] [CrossRef]
- Lesko, J.; Rastović, P.; Mišković, J.; Šoljić, V.; Paštar, V.; Zovko, Z.; Filipović, N.; Katsuyama, Y.; Saraga-Babić, M.; Vukojević, K. The Interplay of Cx26, Cx32, Cx37, Cx40, Cx43, Cx45, and Panx1 in Inner-Ear Development of Yotari (dab1−/−) Mice and Humans. Biomedicines 2022, 10, 589. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, X.; Zhu, K.; Zeng, P.; Ding, G. Dab1 Contributes to Angiotensin II-Induced Apoptosis via p38 Signaling Pathway in Podocytes. BioMed Res. Int. 2017, 2017, 2484303. [Google Scholar] [CrossRef]
- Racetin, A.; Jurić, M.; Filipović, N.; Šolić, I.; Kosović, I.; Durdov, M.G.; Kunac, N.; Tomaš, S.Z.; Saraga, M.; Šoljić, V.; et al. Expression and localization of DAB1 and Reelin during normal human kidney development. Croat. Med. J. 2019, 60, 521–531. [Google Scholar] [CrossRef]
- Racetin, A.; Filipović, N.; Lozić, M.; Ogata, M.; Ensor, L.G.; Kelam, N.; Kovačević, P.; Watanabe, K.; Katsuyama, Y.; Saraga-Babić, M.; et al. A Homozygous Dab1−/− Is a Potential Novel Cause of Autosomal Recessive Congenital Anomalies of the Mice Kidney and Urinary Tract. Biomolecules 2021, 11, 609. [Google Scholar] [CrossRef]
- Kelam, N.; Racetin, A.; Katsuyama, Y.; Vukojević, K.; Kostić, S. Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. Int. J. Mol. Sci. 2022, 23, 2025. [Google Scholar] [CrossRef]
- Saito, A.; Pietromonaco, S.; Loo, A.K.; Farquhar, M.G. Complete cloning and sequencing of rat gp330/”megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc. Natl. Acad. Sci. USA 1994, 91, 9725–9729. [Google Scholar] [CrossRef] [PubMed]
- I Christensen, E.; Nielsen, S.; Moestrup, S.K.; Borre, C.; Maunsbach, A.B.; De Heer, E.; Ronco, P.; Hammond, T.G.; Verroust, P. Segmental distribution of the endocytosis receptor gp330 in renal proximal tubules. Eur. J. Cell Biol. 1995, 66, 349–364. [Google Scholar]
- Goto, S.; Hosojima, M.; Kabasawa, H.; Saito, A. The endocytosis receptor megalin: From bench to bedside. Int. J. Biochem. Cell Biol. 2023, 157, 106393. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 258–267. [Google Scholar] [CrossRef]
- Willnow, T.E.; Hilpert, J.; Armstrong, S.A.; Rohlmann, A.; Hammer, R.E.; Burns, D.K.; Herz, J. Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. USA 1996, 93, 8460–8464. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Verroust, P.J. Megalin and cubilin, role in proximal tubule function and during development. Pediatr. Nephrol. 2002, 17, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Gburek, J.; Verroust, P.J.; Willnow, T.E.; Fyfe, J.C.; Nowacki, W.; Jacobsen, C.; Moestrup, S.K.; Christensen, E.I. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J. Am. Soc. Nephrol. 2002, 13, 423–430. [Google Scholar] [CrossRef]
- Seetharam, B.; Levine, J.S.; Ramasamy, M.; Alpers, D.H. Purification, properties, and immunochemical localization of a receptor for intrinsic factor-cobalamin complex in the rat kidney. J. Biol. Chem. 1988, 263, 4443–4449. [Google Scholar] [CrossRef]
- Eshbach, M.L.; Weisz, O.A. Receptor-Mediated Endocytosis in the Proximal Tubule. Annu. Rev. Physiol. 2017, 79, 425–448. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef]
- Kozyraki, R.; Fyfe, J.; Verroust, P.J.; Jacobsen, C.; Dautry-Varsat, A.; Gburek, J.; Willnow, T.E.; Christensen, E.I.; Moestrup, S.K. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc. Natl. Acad. Sci. USA 2001, 98, 12491–12496. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R.; Seetharam, S.; Seetharam, B. Cubilin and megalin expression and their interaction in the rat intestine: Effect of thyroidectomy. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E900–E907. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, M.; Carter, J.E.; Chadwick, R.B.; Johnson, C.; Gräsbeck, R.; Abdelaal, M.A.; Broch, H.; Jenner, L.B.; Verroust, P.J.; Moestrup, S.K.; et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat. Genet. 1999, 21, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, Z.C.; Ebert, M.P.; Dooley, S.; Meyer, C. Caveolin-1 in the regulation of cell metabolism: A cancer perspective. Mol. Cancer 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Shvets, E.; Ludwig, A.; Nichols, B.J. News from the caves: Update on the structure and function of caveolae. Curr. Opin. Cell Biol. 2014, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Shihata, W.A.; Putra, M.R.A.; Chin-Dusting, J.P.F. Is There a Potential Therapeutic Role for Caveolin-1 in Fibrosis? Front. Pharmacol. 2017, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yang, M.; Zhao, H.; Han, Y.; Jiang, N.; Yang, J.; Chen, W.; Li, C.; Liu, Y.; Zhao, C.; et al. Caveolin-1 Regulates Cellular Metabolism: A Potential Therapeutic Target in Kidney Disease. Front. Pharmacol. 2021, 12, 768100. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Mythreye, K.; Lee, N.Y. Strength and duration of GIPC-dependent signaling networks as determinants in cancer. Neoplasia 2021, 23, 181–188. [Google Scholar] [CrossRef]
- Katoh, M. Functional proteomics, human genetics and cancer biology of GIPC family members. Exp. Mol. Med. 2013, 45, e26. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, J.; Wang, W. DAB2IP Plays Important Clinical Significance and Correlates With Immune Infiltration in Renal Cell Carcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820936682. [Google Scholar] [CrossRef]
- Wiley, J.W.; Higgins, G.A.; Hong, S. Chronic psychological stress alters gene expression in rat colon epithelial cells promoting chromatin remodeling, barrier dysfunction and inflammation. PeerJ 2022, 10, e13287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L.; Zhang, H.; Zhou, H.J.; Ji, W.; Min, W. Short AIP1 (ASK1-Interacting Protein-1) Isoform Localizes to the Mitochondria and Promotes Vascular Dysfunction. Arter. Thromb. Vasc. Biol. 2020, 40, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Shoji, H.; Ogata, M.; Kagawa, Y.; Owada, Y.; Miyakawa, T.; Sakimura, K.; Terashima, T.; Katsuyama, Y. Dorsal Forebrain-Specific Deficiency of Reelin-Dab1 Signal Causes Behavioral Abnormalities Related to Psychiatric Disorders. Cereb. Cortex 2017, 27, 3485–3501. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Robert, J.A.; Orson, W.M.; Michael Caplan, S. Giebisch’s the Kidney, 5th ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 3215–3278. ISBN 9780123814623. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 723–731. [Google Scholar] [CrossRef] [PubMed]
- MMaglica, M.; Kelam, N.; Haque, E.; Perutina, I.; Racetin, A.; Filipović, N.; Katsuyama, Y.; Vukojević, K. Immunoexpression Pattern of Autophagy Markers in Developing and Postnatal Kidneys of Dab1−/−(yotari) Mice. Biomolecules 2023, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Kuwahara, S.; Saito, A. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes 2014, 4, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Birn, H.; Fyfe, J.C.; Jacobsen, C.; Mounier, F.; Verroust, P.J.; Ørskov, H.; Willnow, T.E.; Moestrup, S.K.; Christensen, E.I. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J. Clin. Investig. 2000, 105, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, T.; Christensen, E.I.; Nielsen, R.; Verroust, P.J. Cubilin is expressed in rat and human glomerular podocytes. Nephrol. Dial. Transplant. 2012, 27, 3156–3159. [Google Scholar] [CrossRef]
- Motoyoshi, Y.; Matsusaka, T.; Saito, A.; Pastan, I.; Willnow, T.E.; Mizutani, S.; Ichikawa, I. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int. 2008, 74, 1262–1269. [Google Scholar] [CrossRef]
- Tan, V.P.; Miyamoto, S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 2015, 11, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Eddy, S.; Taroni, J.N.; Lightfoot, Y.L.; Mariani, L.; Parikh, H.; Lindenmeyer, M.T.; Ju, W.; Greene, C.S.; Godfrey, B.; et al. Vasculitis Clinical Research Consortium, the European Renal cDNA Bank cohort, and the Nephrotic Syndrome Study Network.Metabolic pathways and immunometabolism in rare kidney diseases. Ann. Rheum. Dis. 2018, 77, 1226–1233. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, K.; Sharma, A.K.; Dutta, S.K.; Lau, J.S.; Yan, I.K.; Wang, E.; Elkhanany, A.; Alkharfy, K.M.; Sanyal, A.; et al. GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways. PLoS ONE 2014, 9, e114409. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; McQuistan, T.; Orlando, R.A.; Farquhar, M.G. GAIP, GIPC and Galphai3 are concentrated in endocytic compartments of proximal tubule cells: Putative role in regulating megalin’s function. J. Am. Soc. Nephrol. 2002, 13, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tseng, C.P.; Pong, R.C.; Chen, H.; McConnell, J.D.; Navone, N.; Hsieh, J.T. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J. Biol. Chem. 2002, 277, 12622–12631. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, C.; Hsieh, J.-T.; Gong, J.; Xie, D. DAB2IP in cancer. Oncotarget 2016, 7, 3766–3776. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wen, Z.; Gao, W.; Lin, Z.; Zhong, J.; Jiu, Y. Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections. Viruses 2020, 12, 487. [Google Scholar] [CrossRef]
- Dai, X.; North, B.J.; Inuzuka, H. Negative regulation of DAB2IP by Akt and SCFFbw7 pathways. Oncotarget 2014, 5, 3307–3315. [Google Scholar] [CrossRef]



| Antibodies | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-Lrp2/Megalin antibody | ab76969 | Rabbit | 1:250 | Abcam, Cambridge, UK |
| Human/Mouse/Rat Cubilin Antibody | #AF3700 | Sheep | 1:50 | R&D Systems, Inc., Minneapolis, MN, USA | |
| Caveolin-1 (D46G3) XP® Rabbit mAb | #3267S | Rabbit | 1:300 | Cell Signaling Technology (CST), Danvers, MA, USA | |
| Gipc1 Polyclonal antibody | 14822-1-AP | Rabbit | 1:100 | Proteintech Group, Inc., Rosemont, IL, USA | |
| Dab2IP Polyclonal antibody | 23582-1-AP | Rabbit | 1:50 | Proteintech Group, Inc., Rosemont, IL, USA | |
| Anti-Aquaporin 2/AQP2 (E-2) | sc-515770 | Mouse | 1:50 | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA | |
| Lectins | Fluorescein labeled Lotus Tetragonolobus lectin (LTL) | FL-1321 | N/A | 1:400 | Vector Laboratories Ltd., Peterborough, UK |
| Fluorescein labeled Dolichos biflorus agglutinin (DBA) | FL-1031 | N/A | 1:400 | Vector Laboratories Ltd., Peterborough, UK | |
| Secondary | Alexa Fluor® 488 AffiniPure™ Donkey Anti-Rabbit IgG (H + L) | 711-545-152 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
| Rhodamine Red™-X (RRX) AffiniPure™ Donkey Anti-Rabbit IgG (H + L) | 711-295-152 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Alexa Fluor® 488 AffiniPure™ Donkey Anti-Mouse IgG (H + L) | 715-545-150 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Rhodamine Red™-X (RRX) AffiniPure™ Donkey Anti-Sheep IgG (H + L) | 713-295-003 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Embryonic Day (E) | Animal | Structure | Megalin | Cubilin | Caveolin | Dab2IP | GIPC-1 |
|---|---|---|---|---|---|---|---|
| E13.5 | wild type | mm | + | +++ | +++ | + | ++ |
| rv/g | + | + | − | + | + | ||
| Ct | ++ | + | − | + | + | ||
| A/Cd | +++ | + | − | + | + | ||
| yotari | mm | + | + | ++ | + | +++ | |
| rv/g | ++ | + | − | − | − | ||
| Ct | ++ | + | − | + | + | ||
| A/Cd | ++ | + | − | + | + | ||
| E15.5 | wild type | mm | + | + | ++ | − | − |
| rv/g | + | + | + | − | − | ||
| Ct | +++ | + | + | + | ++ | ||
| A/Cd | + | + | + | + | − | ||
| yotari | mm | + | + | + | − | − | |
| rv/g | + | + | + | − | − | ||
| Ct | + | +++ | + | − | − | ||
| A/Cd | +++ | + | + | − | ++ |
| Postnatal Day (P) | Animal | Structure | Megalin | Cubilin | Caveolin | Dab2IP | GIPC |
|---|---|---|---|---|---|---|---|
| P4 | wild type | g | − | − | + | − | + |
| pct | +++ | +++ | − | − | − | ||
| dct | + | + | − | + | + | ||
| yotari | g | − | − | + | + | − | |
| pct | +++ | +++ | − | + | + | ||
| dct | + | + | − | + | + | ||
| P14 | wild type | g | − | − | + | − | − |
| pct | +++ | +++ | − | − | + | ||
| dct | + | + | − | + | + | ||
| yotari | g | − | − | + | − | − | |
| pct | +++ | +++ | − | − | − | ||
| dct | − | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žužul, S.; Kelam, N.; Racetin, A.; Kovačević, P.; Konjevoda, S.; Filipović, N.; Pavlović, N.; Vukojević, K. Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1−/−) Mice. Biomedicines 2024, 12, 1542. https://doi.org/10.3390/biomedicines12071542
Žužul S, Kelam N, Racetin A, Kovačević P, Konjevoda S, Filipović N, Pavlović N, Vukojević K. Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1−/−) Mice. Biomedicines. 2024; 12(7):1542. https://doi.org/10.3390/biomedicines12071542
Chicago/Turabian StyleŽužul, Sani, Nela Kelam, Anita Racetin, Petra Kovačević, Suzana Konjevoda, Natalija Filipović, Nikola Pavlović, and Katarina Vukojević. 2024. "Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1−/−) Mice" Biomedicines 12, no. 7: 1542. https://doi.org/10.3390/biomedicines12071542
APA StyleŽužul, S., Kelam, N., Racetin, A., Kovačević, P., Konjevoda, S., Filipović, N., Pavlović, N., & Vukojević, K. (2024). Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1−/−) Mice. Biomedicines, 12(7), 1542. https://doi.org/10.3390/biomedicines12071542







