Embryonic Zebrafish as a Model for Investigating the Interaction between Environmental Pollutants and Neurodegenerative Disorders
Abstract
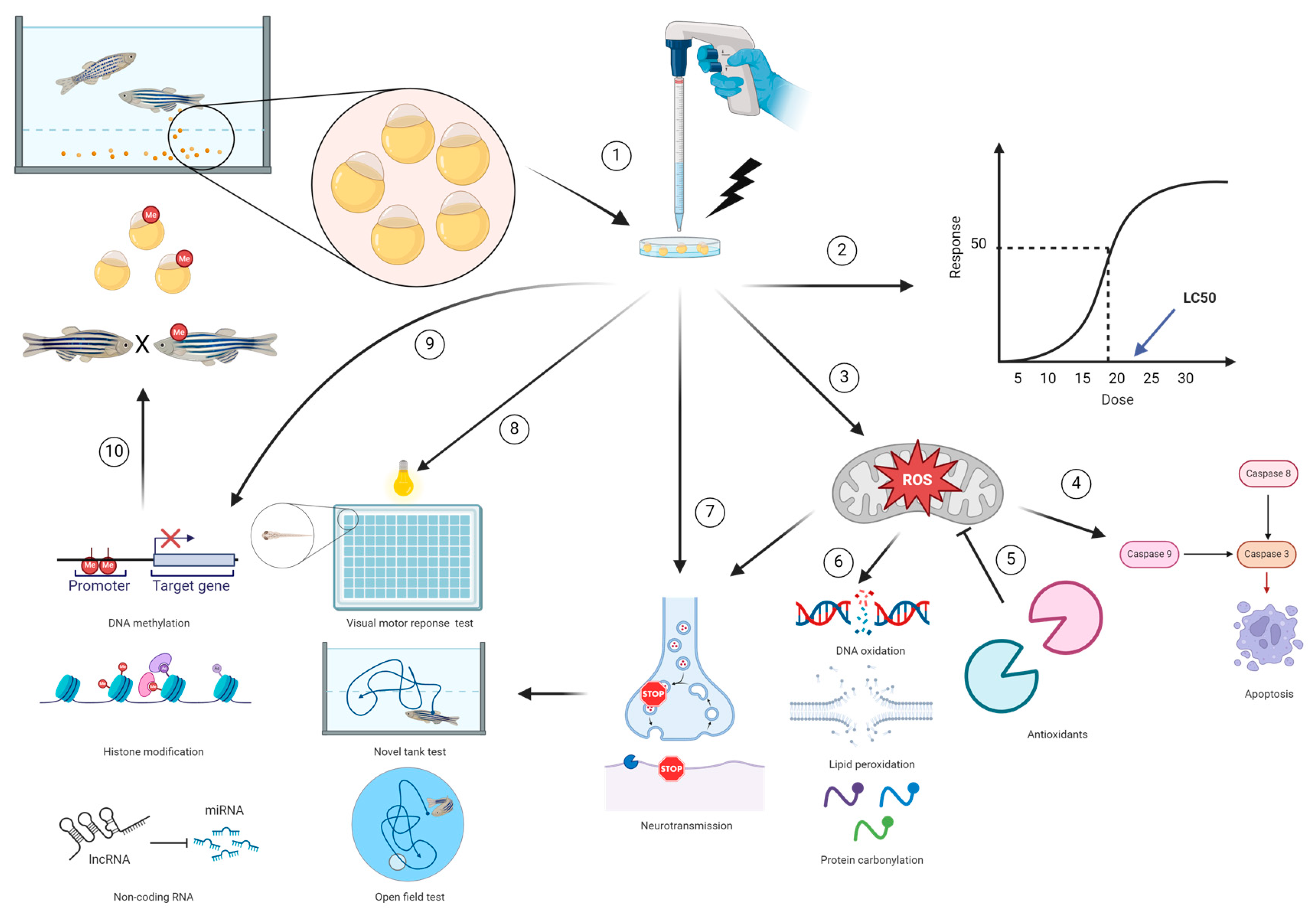
1. Introduction
2. The Zebrafish Model as a High-Throughput Platform in Neurologic Studies
3. The Fish Embryo Acute Toxicity Test as a Method to Determine the Lethal Effect of Environmental Pollutants
4. Investigating the Exposure to Environmental Pollutants as a Risk Factor for Neurodegenerative Disorders in the Zebrafish Model
4.1. Oxidative Stress
Zebrafish as a Model in Oxidative Stress Studies
4.2. Apoptosis
Zebrafish as a Model in Apoptosis Studies
4.3. Neurotransmission
Zebrafish as a Model for Neurotransmission
4.4. Epigenetic Modification
Zebrafish as a Model of Epigenetic Modification
5. Zebrafish Neurobehavior
5.1. Zebrafish Embryonic and Larval Locomotor Activity
5.2. Adult Zebrafish Behavior
6. Limitations in Using Embryonic Zebrafish as Models in Assessing Neurotoxicity Associated with Environmental Pollutants
7. Future Direction
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in europe: An analysis for the global burden of disease study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Gawas, C.; Ahmad, I.Z.; Wani, M.; Tabassum, H. Neurodegenerative disorders: Assessing the impact of natural vs drug-induced treatment options. Aging Med. 2023, 6, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Labadorf, A.; Choi, S.H.; Myers, R.H. Evidence for a pan-neurodegenerative disease response in huntington’s and parkinson’s disease expression profiles. Front. Mol. Neurosci. 2017, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared mechanisms of neurodegeneration in alzheimer’s disease and parkinson’s disease. Biomed. Res. Int. 2014, 2014, 648740. [Google Scholar] [CrossRef] [PubMed]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The pathogenesis of parkinson’s disease: A complex interplay between astrocytes, microglia, and t lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Johnston, J.; Cushing, L. Chemical exposures, health, and environmental justice in communities living on the fenceline of industry. Curr. Environ. Health Rep. 2020, 7, 48–57. [Google Scholar] [CrossRef]
- US EPA. Summary of the Toxic Substances Control Act; US EPA: Washington, DC, USA, 2023. [Google Scholar]
- Fitzgerald, J.A.; Könemann, S.; Krümpelmann, L.; Županič, A.; Vom Berg, C. Approaches to test the neurotoxicity of environmental contaminants in the zebrafish model: From behavior to molecular mechanisms. Environ. Toxicol. Chem. 2021, 40, 989–1006. [Google Scholar] [CrossRef]
- Weichbrod, R.H.; Thompson, G.A.; Norton, J.N. Management of Animal Care and Use Programs in Research, Education, and Testing; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2018. [Google Scholar]
- Wolfle, T.L. 50 years of the institute for laboratory animal research (ilar): 1953–2003. ILAR J. 2003, 44, 324–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2018. [Google Scholar]
- Smith, A.J.; Lilley, E. The role of the three rs in improving the planning and reproducibility of animal experiments. Animals 2019, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 86/609/eec of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. 1986; pp. 1–28. Available online: https://www.legislation.gov.uk/eudr/1986/609/contents (accessed on 15 January 2024).
- European Parliament (EP); Council of the European Union Directive 2010/63/eu on the protection of animals used for scientific purposes. EU Off. J. 2010, L276, 33–79.
- ECHA. Echa and Alternatives to Animal Testing; ECHA: Helsinki, Finland. Available online: https://echa.europa.eu/animal-testing-under-reach/infographic (accessed on 15 January 2024).
- Kang, S.Y.; Joshi, P.; Lee, M.Y. High-throughput screening of compound neurotoxicity using 3d-cultured neural stem cells on a 384-pillar plate. Curr. Protoc. 2021, 1, e107. [Google Scholar] [CrossRef] [PubMed]
- Shou, W.Z. Current status and future directions of high-throughput adme screening in drug discovery. J. Pharm. Anal. 2020, 10, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Blaauboer, B.J.; Clewell, H.J. Quantitative in vitro to in vivo extrapolation (qivive): An essential element for in vitro-based risk assessment. Toxicology 2015, 332, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Campana, O. Toward high-throughput fish embryo toxicity tests in aquatic toxicology. Environ. Sci. Technol. 2021, 55, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 212: Fish, Short-Term Toxicity Test on Embryo and Sac-Fry Stages; OECD: Paris, France, 1998. [Google Scholar]
- OECD. Test No. 305: Bioaccumulation in Fish: AQUEOUS and Dietary Exposure; OECD: Paris, France, 2012. [Google Scholar]
- OECD. Test No. 210: Fish, Early-Life Stage Toxicity Test; OECD: Paris, France, 2013. [Google Scholar]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Jarema, K.A.; Hunter, D.L.; Hill, B.N.; Olin, J.K.; Britton, K.N.; Waalkes, M.R.; Padilla, S. Developmental neurotoxicity and behavioral screening in larval zebrafish with a comparison to other published results. Toxics 2022, 10, 256. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Genario, R.; Giacomini, A.; Demin, K.A.; Lakstygal, A.M.; Amstislavskaya, T.G.; Fontana, B.D.; Parker, M.O.; Kalueff, A.V. Zebrafish as a model of neurodevelopmental disorders. Neuroscience 2020, 445, 3–11. [Google Scholar] [CrossRef]
- d’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef]
- Ali, S.; van Mil, H.G.; Richardson, M.K. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS ONE 2011, 6, e21076. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (Fet) Test; OECD: Paris, France, 2013. [Google Scholar]
- Vaughan, M.; van Egmond, R. The use of the zebrafish (Danio rerio) embryo for the acute toxicity testing of surfactants, as a possible alternative to the acute fish test. Altern. Lab. Anim. 2010, 38, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ahkin Chin Tai, J.K.; Horzmann, K.A.; Franco, J.; Jannasch, A.S.; Cooper, B.R.; Freeman, J.L. Developmental atrazine exposure in zebrafish produces the same major metabolites as mammals along with altered behavioral outcomes. Neurotoxicol. Teratol. 2021, 85, 106971. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today 2013, 99, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Bailone, R.L.; Fukushima, H.C.S.; Fernandes, B.H.V.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Setti, P.G.; Roça, R.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, A.; Riley, M.; Koyama, M.; Monen, J.; Satou, C.; Kimura, Y.; Higashijima, S.; Fetcho, J. A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Choudhary, P.R.; Nirmal, N.K.; Syed, F.; Verma, R. Neurotransmitter Systems in Zebrafish Model as a Target for Neurobehavioural Studies. Mater. Today Proc. 2022, 69, 1565–1580. Available online: https://www.sciencedirect.com/science/article/pii/S221478532204771X (accessed on 15 January 2024). [CrossRef]
- Lal, P.; Kawakami, K. Integrated behavioral, genetic and brain circuit visualization methods to unravel functional anatomy of zebrafish amygdala. Front. Neuroanat. 2022, 16, 837527. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Freeman, J.L. Zebrafish get connected: Investigating neurotransmission targets and alterations in chemical toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Espinosa, P.; Franco-Esclapez, C.; Robles-Gómez, L.; Silva, W.; Romero, A.; Immler, S.; Gómez-Torres, M.J. Morphological and ultrastructural alterations of zebrafish (Danio rerio) spermatozoa after motility activation. Theriogenology 2022, 188, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish—From embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Fraser, S.E. Order and coherence in the fate map of the zebrafish nervous system. Development 1995, 121, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Gutzman, J.H.; Graeden, E.G.; Lowery, L.A.; Holley, H.S.; Sive, H. Formation of the zebrafish midbrain-hindbrain boundary constriction requires laminin-dependent basal constriction. Mech. Dev. 2008, 125, 974–983. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Carmean, V.; Ribera, A.B. Genetic analysis of the touch response in zebrafish (Danio rerio). Int. J. Comp. Psychol. 2010, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Lehtinen, M.K.; Sive, H. Zebrafish cerebrospinal fluid mediates cell survival through a retinoid signaling pathway. Dev. Neurobiol. 2016, 76, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Silvero, C.; Hübner, K.; Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 2020, 457, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef] [PubMed]
- Neely, S.A.; Lyons, D.A. Insights into central nervous system glial cell formation and function from zebrafish. Front. Cell Dev. Biol. 2021, 9, 754606. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Chu, J.; Sadler, K.C. New school in liver development: Lessons from zebrafish. Hepatology 2009, 50, 1656–1663. [Google Scholar] [CrossRef]
- Sales Cadena, M.R.; Cadena, P.G.; Watson, M.R.; Sarmah, S.; Ii, S.L.B.; Marrs, J.A. Zebrafish (Danio rerio) larvae show behavioral and embryonic development defects when exposed to opioids at embryo stage. Neurotoxicol. Teratol. 2021, 85, 106964. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish larvae behavior models as a tool for drug screenings and pre-clinical trials: A review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- McLean, D.L.; Fetcho, J.R. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 2004, 480, 38–56. [Google Scholar] [CrossRef]
- Wolf, J.C.; Segner, H.E. Hazards of current concentration-setting practices in environmental toxicology studies. Crit. Rev. Toxicol. 2023, 53, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Ld50/lc50 (lethal dosage 50/lethal concentration 50). In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 58–60. [Google Scholar]
- Braunbeck, T.; Kais, B.; Lammer, E.; Otte, J.; Schneider, K.; Stengel, D.; Strecker, R. The fish embryo test (fet): Origin, applications, and future. Environ. Sci. Pollut. Res. Int. 2015, 22, 16247–16261. [Google Scholar] [CrossRef] [PubMed]
- Belanger, S.E.; Rawlings, J.M.; Carr, G.J. Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ. Toxicol. Chem. 2013, 32, 1768–1783. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E. Braunbeck. Is the fish embryo toxicity test (fet) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Sobanska, M.; Scholz, S.; Nyman, A.M.; Cesnaitis, R.; Alonso, S.G.; Klüver, N.; Kühne, R.; Tyle, H.; de Knecht, J.; Dang, Z.; et al. Applicability of the fish embryo acute toxicity (fet) test (oecd 236) in the regulatory context of registration, evaluation, authorisation, and restriction of chemicals (reach). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef]
- Klüver, N.; König, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kühne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef]
- Glaberman, S.; Padilla, S.; Barron, M.G. Evaluating the zebrafish embryo toxicity test for pesticide hazard screening. Environ. Toxicol. Chem. 2017, 36, 1221–1226. [Google Scholar] [CrossRef]
- Henn, K.; Braunbeck, T. Dechorionation as a tool to improve the fish embryo toxicity test (fet) with the zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 91–98. [Google Scholar] [CrossRef]
- Olivares, C.I.; Field, J.A.; Simonich, M.; Tanguay, R.L.; Sierra-Alvarez, R. Arsenic (iii, v), indium (iii), and gallium (iii) toxicity to zebrafish embryos using a high-throughput multi-endpoint in vivo developmental and behavioral assay. Chemosphere 2016, 148, 361–368. [Google Scholar] [CrossRef]
- Coral, J.A.; Heaps, S.; Glaholt, S.P.; Karty, J.A.; Jacobson, S.C.; Shaw, J.R.; Bondesson, M. Arsenic exposure induces a bimodal toxicity response in zebrafish. Environ. Pollut. 2021, 287, 117637. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.P.; Chandra, S.; Chapman, J. Parkinson’s disease and the environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V. Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol. 2008, 22, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Ménage à trois. Mutat. Res. 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal Cell Death Mechanisms in Alzheimer’s Disease: An Insight. Front. Mol. Neurosci. 2022, 15, 937133. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9454331/pdf/fnmol-15-937133.pdf (accessed on 15 January 2024). [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of alzheimer’s disease. Oxidative Med. Cell Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Landreth, G.E. The microglial nadph oxidase complex as a source of oxidative stress in alzheimer’s disease. J. Neuroinflamm. 2006, 3, 30. [Google Scholar] [CrossRef][Green Version]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress 2018, 9, 9–21. Available online: https://www.sciencedirect.com/science/article/pii/S2352289518300079 (accessed on 15 January 2024). [CrossRef]
- Exley, C. What is the risk of aluminium as a neurotoxin? Expert Rev. Neurother. 2014, 14, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Flaten, T.P. Aluminium as a risk factor in alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef]
- Martyn, C.N.; Barker, D.J.; Osmond, C.; Harris, E.C.; Edwardson, J.A.; Lacey, R.F. Geographical relation between alzheimer’s disease and aluminum in drinking water. Lancet 1989, 1, 59–62. [Google Scholar] [CrossRef]
- Oshima, E.; Ishihara, T.; Yokota, O.; Nakashima-Yasuda, H.; Nagao, S.; Ikeda, C.; Naohara, J.; Terada, S.; Uchitomi, Y. Accelerated tau aggregation, apoptosis and neurological dysfunction caused by chronic oral administration of aluminum in a mouse model of tauopathies. Brain Pathol. 2013, 23, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Narang, R.K.; Singh, S. Alcl(3) induced learning and memory deficit in zebrafish. Neurotoxicology 2022, 92, 67–76. [Google Scholar] [CrossRef]
- Lee, J.; Peterson, S.M.; Freeman, J.L. Sex-specific characterization and evaluation of the alzheimer’s disease genetic risk factor sorl1 in zebrafish during aging and in the adult brain following a 100 ppb embryonic lead exposure. J. Appl. Toxicol. 2017, 37, 400–407. [Google Scholar] [CrossRef]
- Tanner, C.M.; Goldman, S.M. Epidemiology of parkinson’s disease. Neurol. Clin. 1996, 14, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s disease and parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Borghammer, P. The α-synuclein origin and connectome model (soc model) of parkinson’s disease: Explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinsons Dis. 2021, 11, 455–474. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of parkinson’s disease: Present and future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Hartmann, A.; Hunot, S.; Michel, P.P.; Muriel, M.P.; Vyas, S.; Faucheux, B.A.; Mouatt-Prigent, A.; Turmel, H.; Srinivasan, A.; Ruberg, M.; et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2875–2880. [Google Scholar] [CrossRef]
- Hartmann, A.; Troadec, J.D.; Hunot, S.; Kikly, K.; Faucheux, B.A.; Mouatt-Prigent, A.; Ruberg, M.; Agid, Y.; Hirsch, E.C. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in parkinson’s disease, but pathway inhibition results in neuronal necrosis. J. Neurosci. 2001, 21, 2247–2255. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, J.C.; Liu, P.; Zhang, S.F.; Xu, J.Y.; Bai, L.M. Pathogenesis of parkinson’s disease: Oxidative stress, environmental impact factors and inflammatory processes. Neurosci. Bull. 2007, 23, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Freyberger, A.; Lawrenz, B.; Schladt, L.; Schmuck, G.; Ellinger-Ziegelbauer, H. Mechanistic investigations of the mitochondrial complex i inhibitor rotenone in the context of pharmacological and safety evaluation. Sci. Rep. 2017, 7, 45465. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Choi, D.Y.; Hunter, R.L.; Pandya, J.D.; Cass, W.A.; Sullivan, P.G.; Kim, H.C.; Gash, D.M.; Bing, G. Trichloroethylene induces dopaminergic neurodegeneration in fisher 344 rats. J. Neurochem. 2010, 112, 773–783. [Google Scholar] [CrossRef]
- Wang, X.H.; Souders, C.L., 2nd; Zhao, Y.H.; Martyniuk, C.J. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2018, 191, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; Jin, Y. Differential responses of larval zebrafish to the fungicide propamocarb: Endpoints at development, locomotor behavior and oxidative stress. Sci. Total Environ. 2020, 731, 139136. [Google Scholar] [CrossRef] [PubMed]
- Kalyn, M.; Lee, H.; Curry, J.; Tu, W.; Ekker, M.; Mennigen, J.A. Effects of pfos, f-53b and obs on Locomotor Behaviour, the Dopaminergic System and Mitochondrial Function in Developing Zebrafish (Danio rerio). Environ. Pollut. 2023, 326, 121479. Available online: https://www.sciencedirect.com/science/article/pii/S0269749123004815?via%3Dihub (accessed on 15 January 2024). [CrossRef] [PubMed]
- Alfalahi, H.; Dias, S.B.; Khandoker, A.H.; Chaudhuri, K.R.; Hadjileontiadis, L.J. A scoping review of neurodegenerative manifestations in explainable digital phenotyping. NPJ Parkinsons Dis. 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Percário, S.; da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; de Nazaré Araújo Moreira, T.; Dolabela, M.F. Oxidative stress in parkinson’s disease: Potential benefits of antioxidant supplementation. Oxidative Med. Cell Longev. 2020, 2020, 2360872. [Google Scholar] [CrossRef]
- Ni, A.; Ernst, C. Evidence that substantia nigra pars compacta dopaminergic neurons are selectively vulnerable to oxidative stress because they are highly metabolically active. Front. Cell Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- Abbate, F.; Maugeri, A.; Laurà, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germanà, A. Zebrafish as a useful model to study oxidative stress-linked disorders: Focus on flavonoids. Antioxidants 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41–R55. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of total reactive oxygen species in adherent cells by 2′,7′-dichlorodihydrofluorescein diacetate staining. J. Vis. Exp. 2020, 160, e60682. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxidative Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Bode, K.; Link, C.; Krammer, P.H.; Weyd, H. Flow-cytometric detection of low-level reactive oxygen species in cell lines and primary immune cells. Bio Protoc. 2020, 10, e3737. [Google Scholar] [CrossRef]
- Ng, N.S.; Ooi, L. A simple microplate assay for reactive oxygen species generation and rapid cellular protein normalization. Bio Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lauridsen, H.; Buels, K.; Chi, L.H.; La Du, J.; Bruun, D.A.; Olson, J.R.; Tanguay, R.L.; Lein, P.J. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 2011, 121, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-ohdg): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodg) and 8-hydroxy-2′-deoxyguanosine (8-ohdg) as a potential biomarker for gestational diabetes mellitus (gdm) development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef]
- McKelvey-Martin, V.J.; Green, M.H.; Schmezer, P.; Pool-Zobel, B.L.; De Méo, M.P.; Collins, A. The single cell gel electrophoresis assay (comet assay): A european review. Mutat. Res. 1993, 288, 47–63. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Zhu, B.; He, W.; Hu, S.; Kong, R.; Yang, L. The fate and oxidative stress of different sized sio(2) nanoparticles in zebrafish (Danio rerio) larvae. Chemosphere 2019, 225, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Arockiaraj, J. Exposure to environmental pollutant bisphenol a causes oxidative damage and lipid accumulation in zebrafish larvae: Protective role of wl15 peptide derived from cysteine and glycine-rich protein 2. J. Biochem. Mol. Toxicol. 2023, 37, e23223. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Yildirim, S.; Ghosigharehaghaji, A.; Bolat, İ.; Sulukan, E.; Ceyhun, S.B. An approach to evaluating the potential teratogenic and neurotoxic mechanism of bha based on apoptosis induced by oxidative stress in zebrafish embryo (Danio rerio). Hum. Exp. Toxicol. 2021, 40, 425–438. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J. Eckschlager and R. Kizek. Redox status expressed as gsh:Gssg ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, J.Y.; Wang, Q.; Zhu, E.; Chen, W.; Lin, H.; Chen, J.; Hong, H. Environmentally relevant concentrations of arsenite induces developmental toxicity and oxidative responses in the early life stage of zebrafish. Environ. Pollut. 2019, 254, 113022. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.A.; da Cunha Martins-Junior, A.; Barbosa, F., Jr. Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 172–173, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gooneratne, R.; Huang, X.; Lai, R.; Wei, J.; Wang, W. A rapid in vivo zebrafish model to elucidate oxidative stress-mediated pcb126-induced apoptosis and developmental toxicity. Free Radic. Biol. Med. 2015, 84, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kusik, B.W.; Carvan, M.J., 3rd; Udvadia, A.J. Detection of mercury in aquatic environments using epre reporter zebrafish. Mar. Biotechnol. 2008, 10, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Mourabit, S.; Fitzgerald, J.A.; Ellis, R.P.; Takesono, A.; Porteus, C.S.; Trznadel, M.; Metz, J.; Winter, M.J.; Kudoh, T.; Tyler, C.R. New insights into organ-specific oxidative stress mechanisms using a novel biosensor zebrafish. Environ. Int. 2019, 133, 105138. [Google Scholar] [CrossRef] [PubMed]
- Klim, J.; Gładki, A.; Kucharczyk, R.; Zielenkiewicz, U.; Kaczanowski, S. Ancestral state reconstruction of the apoptosis machinery in the common ancestor of eukaryotes. G3 2018, 8, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- McGrath, P.; Seng, W.L. Use of zebrafish apoptosis assays for preclinical drug discovery. Expert Opin. Drug Discov. 2013, 8, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.; Toruno, C.; Stewart, R.A.; Jette, C. Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated caspase 3. J. Vis. Exp. 2013, 82, e51060. [Google Scholar] [CrossRef]
- Tucker, B.; Lardelli, M. A rapid apoptosis assay measuring relative acridine orange fluorescence in zebrafish embryos. Zebrafish 2007, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Banfalvi, G. Methods to detect apoptotic cell death. Apoptosis 2017, 22, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Butterick, T.A.; Duffy, C.M.; Lee, R.E.; Billington, C.J.; Kotz, C.M.; Nixon, J.P. Use of a caspase multiplexing assay to determine apoptosis in a hypothalamic cell model. J. Vis. Exp. 2014, 86, e51305. [Google Scholar] [CrossRef]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of apoptosis by tunel assay. Methods Mol. Biol. 2012, 887, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin v-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087205. [Google Scholar] [CrossRef]
- Frankfurt, O.S.; Robb, J.A.; Sugarbaker, E.V.; Villa, L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp. Cell Res. 1996, 226, 387–397. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Strugger, S.; Degode, F. Fluoreszenzmikroskopische untersuchungen über die aufnahme und speicherung des akridinorange durch lebende und tote pflanzenzellen. Protoplasma 1941, 36, 469. [Google Scholar]
- Pinheiro-da-Silva, J.; Luchiari, A.C. Embryonic ethanol exposure on zebrafish early development. Brain Behav. 2021, 11, e02062. [Google Scholar] [CrossRef] [PubMed]
- Parlak, V. Evaluation of apoptosis, oxidative stress responses, ache activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 2018, 207, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C.; Gao, X.; Wang, L.; Cao, S.; Wu, Q.; Qiao, S.; Zhang, Z.; Li, L. Comparative effects of mercury chloride and methylmercury exposure on early neurodevelopment in zebrafish larvae. RSC Adv. 2019, 9, 10766–10775. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in zebrafish embryos and adults after cadmium exposure. Eur. J. Histochem. 2017, 61, 2833. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Do tunel and other apoptosis assays detect cell death in preclinical studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, B.; Gan, J.; Wang, S.; Jiang, X.; Li, H. Apoptosis detection: A purpose-dependent approach selection. Cell Cycle 2021, 20, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- van Ham, T.J.; Mapes, J.; Kokel, D.; Peterson, R.T. Live imaging of apoptotic cells in zebrafish. FASEB J. 2010, 24, 4336–4342. [Google Scholar] [CrossRef]
- Yamashita, M.; Mizusawa, N.; Hojo, M.; Yabu, T. Extensive apoptosis and abnormal morphogenesis in pro-caspase-3 transgenic zebrafish during development. J. Exp. Biol. 2008, 211, 1874–1881. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.A.; De Rose, R.A.; Olson, E.S.; Halpern, M.E. The zebrafish annexin gene family. Genome Res. 2003, 13, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Oliveros, A.; Campusano, J.M. Studying the contribution of serotonin to neurodevelopmental disorders. Can this fly? Front. Behav. Neurosci. 2020, 14, 601449. [Google Scholar] [CrossRef] [PubMed]
- Ncube, D.; Tallafuss, A.; Serafin, J.; Bruckner, J.; Farnsworth, D.R.; Miller, A.C.; Eisen, J.S.; Washbourne, P. A conserved transcriptional fingerprint of multi-neurotransmitter neurons necessary for social behavior. BMC Genom. 2022, 23, 675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of gabaergic neurotransmission in alzheimer’s disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, I.; Barata, C. Characterization of neurotransmitters and related metabolites in daphnia magna juveniles deficient in serotonin and exposed to neuroactive chemicals that affect its behavior: A targeted lc-ms/ms method. Chemosphere 2021, 263, 127814. [Google Scholar] [CrossRef]
- Pal, M.M. Glutamate: The master neurotransmitter and its implications in chronic stress and mood disorders. Front. Hum. Neurosci. 2021, 15, 722323. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007s–1015s. [Google Scholar] [CrossRef]
- Reddy-Thootkur, M.; Kraguljac, N.V.; Lahti, A.C. The role of glutamate and gaba in cognitive dysfunction in schizophrenia and mood disorders—A systematic review of magnetic resonance spectroscopy studies. Schizophr. Res. 2022, 249, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is there more to gaba than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.N.; Triller, A.; Sieghart, W. Gaba(a) receptors: Post-synaptic co-localization and cross-talk with other receptors. Front. Cell Neurosci. 2011, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, L.B.; Tantirigama, M.L.S.; Bekkers, J.M. Pre- and postsynaptic activation of gaba(b) receptors modulates principal cell excitation in the piriform cortex. Front. Cell Neurosci. 2018, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Chiapponi, C.; Piras, F.; Caltagirone, C.; Spalletta, G. Gaba system in schizophrenia and mood disorders: A mini review on third-generation imaging studies. Front. Psychiatry 2016, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Schür, R.R.; Draisma, L.W.; Wijnen, J.P.; Boks, M.P.; Koevoets, M.G.; Joëls, M.; Klomp, D.W.; Kahn, R.S.; Vinkers, C.H. Brain gaba levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) h-mrs studies. Hum. Brain Mapp. 2016, 37, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and gaba homeostasis and neurometabolism in major depressive disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar] [CrossRef]
- Jung-Klawitter, S.; Hübschmann, O.K. Analysis of catecholamines and pterins in inborn errors of monoamine neurotransmitter metabolism-from past to future. Cells 2019, 8, 867. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Izquierdo, I.; Blandina, P.; Passani, M.B. Brain histamine modulates recognition memory: Possible implications in major cognitive disorders. Br. J. Pharmacol. 2020, 177, 539–556. [Google Scholar] [CrossRef]
- Passani, M.B.; Bacciottini, L.; Mannaioni, P.F.; Blandina, P. Central histaminergic system and cognition. Neurosci. Biobehav. Rev. 2000, 24, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.V.; Porkka-Heiskanen, T.; Panula, P. On the role of histamine receptors in the regulation of circadian rhythms. PLoS ONE 2015, 10, e0144694. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Nomura, S.; Hosoda, H.; Kangawa, K.; Watanabe, T.; Yamatodani, A. A role of the histaminergic system for the control of feeding by orexigenic peptides. Physiol. Behav. 2006, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Lu, B. Interaction between bdnf and serotonin: Role in mood disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. The human hypothalamus in mood disorders: The hpa axis in the center. IBRO Rep. 2019, 6, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chaouloff, F. Serotonin, stress and corticoids. J. Psychopharmacol. 2000, 14, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes Castro, V.H.; Valenzuela, C.L.L.; Sánchez, J.C.S.; Peña, K.P.; Pérez, S.J.L.; Ibarra, J.O.; Villagrán, A.M. An update of the classical and novel methods used for measuring fast neurotransmitters during normal and brain altered function. Curr. Neuropharmacol. 2014, 12, 490–508. [Google Scholar] [CrossRef] [PubMed]
- Tufi, S.; Leonards, P.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in neurotransmitter profiles during early zebrafish (Danio rerio) development and after pesticide exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Sepúlveda, M.S.; Xiao, C.; Cannon, J.R.; Freeman, J.L. Developmental origins of neurotransmitter and transcriptome alterations in adult female zebrafish exposed to atrazine during embryogenesis. Toxicology 2015, 333, 156–167. [Google Scholar] [CrossRef]
- Weber, G.J.; Sepúlveda, M.S.; Peterson, S.M.; Lewis, S.S.; Freeman, J.L. Transcriptome alterations following developmental atrazine exposure in zebrafish are associated with disruption of neuroendocrine and reproductive system function, cell cycle, and carcinogenesis. Toxicol. Sci. 2013, 132, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Wirbisky, S.E.; Weber, G.J.; Lee, J.W.; Cannon, J.R.; Freeman, J.L. Novel dose-dependent alterations in excitatory gaba during embryonic development associated with lead (pb) neurotoxicity. Toxicol. Lett. 2014, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Twaddle, N.C.; Silva, C.; Robinson, B.; Wolle, M.; Conklin, S.; MacMahon, S.; Gu, Q.; Edhlund, I.; Benjamin, L.; et al. Inorganic arsenic alters the development of dopaminergic neurons but not serotonergic neurons and induces motor neuron development via sonic hedgehog pathway in zebrafish. Neurosci. Lett. 2023, 795, 137042. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Xiang, C.; Li, X.; Chen, H.; Shi, X.; Huang, C.; Yu, Y.; Qi, J.; Li, A.J.; Zhang, L.; et al. Photoaged microplastics induce neurotoxicity via oxidative stress and abnormal neurotransmission in zebrafish larvae (Danio rerio). Sci. Total Environ. 2023, 881, 163480. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Paska, A.V.; Konjevod, M.; Kouter, K.; Strac, D.S.; Erjavec, G.N.; Pivac, N. Epigenetics of alzheimer’s disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Almatarneh, M.H.; Kayed, G.G.; Abbad, S.S.A.; Alsunaidi, Z.H.A.; Al-Sheraideh, M.S.; Zhao, Y. Mechanistic Study on DNA Mutation of the Cytosine Methylation Reaction at c5 Position. Arab. J. Chem. 2022, 15, 103956. Available online: https://www.sciencedirect.com/science/article/pii/S1878535222002726 (accessed on 15 January 2024). [CrossRef]
- Elhamamsy, A.R. Role of DNA Methylation in Imprinting Disorders: An Updated Review. J. Assist. Reprod. Genet. 2017, 34, 549–562. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427654/pdf/10815_2017_Article_895.pdf (accessed on 15 January 2024). [CrossRef]
- Jin, B.; Robertson, K.D. DNA Methyltransferases, DNA Damage Repair, and Cancer. Adv. Exp. Med. Biol. 2013, 754, 3–29. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3707278/pdf/nihms486049.pdf (accessed on 15 January 2024). [CrossRef] [PubMed]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Santana, D.A.; Smith, M.A.C.; Chen, E.S. Histone modifications in alzheimer’s disease. Genes 2023, 14, 347. [Google Scholar] [CrossRef]
- Morris, K.V. Non-Coding RNAs, Epigenetic Memory and the Passage of Information to Progeny. RNA Biol. 2009, 6, 242–247. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754389/pdf/nihms-146963.pdf (accessed on 15 January 2024). [CrossRef] [PubMed]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc. Res. 2011, 90, 430–440. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096308/pdf/cvr097.pdf (accessed on 15 January 2024). [CrossRef] [PubMed]
- Cavalieri, V.; Spinelli, G. Environmental epigenetics in zebrafish. Epigenetics Chromatin 2017, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, C.; Li, X. Base modifications: Regulation of stem cell functions and diseases. Stem Cells Int. 2018, 2018, 5402384. [Google Scholar] [CrossRef] [PubMed]
- Nomura, W. Development of toolboxes for precision genome/epigenome editing and imaging of epigenetics. Chem. Rec. 2018, 18, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Waryah, C.B.; Moses, C.; Arooj, M.; Blancafort, P. Zinc fingers, tales, and crispr systems: A comparison of tools for epigenome editing. Methods Mol. Biol. 2018, 1767, 19–63. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Raghunath, A.; Perumal, E. Role of epigenetics in zebrafish development. Gene 2019, 718, 144049. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, J.H.; Aleström, P.; Kooter, J.M.; Legler, J. Zebrafish as a model to study the role of DNA methylation in environmental toxicology. Environ. Sci. Pollut. Res. Int. 2015, 22, 16262–16276. [Google Scholar] [CrossRef]
- Li, Y. Modern epigenetics methods in biological research. Methods 2021, 187, 104–113. [Google Scholar] [CrossRef]
- Miyata, K.; Naito, M.; Miyata, T.; Mokuda, S.; Asahara, H. Bisulfite sequencing for DNA methylation analysis of primary muscle stem cells. Methods Mol. Biol. 2017, 1668, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tollefsbol, T.O. DNA methylation detection: Bisulfite genomic sequencing analysis. Methods Mol. Biol. 2011, 791, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Strömqvist, M.; Tooke, N.; Brunström, B. DNA methylation levels in the 5′ flanking region of the vitellogenin i gene in liver and brain of adult zebrafish (Danio rerio)—Sex and tissue differences and effects of 17alpha-ethinylestradiol exposure. Aquat. Toxicol. 2010, 98, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.C.; Laforest, L.; Akimenko, M.A.; Ekker, M. A role for DNA methylation in gastrulation and somite patterning. Dev. Biol. 1999, 206, 189–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macleod, D.; Clark, V.H.; Bird, A. Absence of genome-wide changes in DNA methylation during development of the zebrafish. Nat. Genet. 1999, 23, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Searle, B.; Müller, M.; Carell, T.; Kellett, A. Third-generation sequencing of epigenetic DNA. Angew. Chem. 2023, 62, e202215704. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tian, S.; Slager, S.L.; Sun, Z. Chip-seq in studying epigenetic mechanisms of disease and promoting precision medicine: Progresses and future directions. Epigenomics 2016, 8, 1239–1258. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, O.; Fernández-Miñán, A.; Tena, J.J.; de la Calle-Mustienes, E.; Gómez-Skarmeta, J.L. The developmental epigenomics toolbox: Chip-seq and methylcap-seq profiling of early zebrafish embryos. Methods 2013, 62, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Terrazas-Salgado, L.; García-Gasca, A.; Betancourt-Lozano, M.; Llera-Herrera, R.; Alvarado-Cruz, I.; Yáñez-Rivera, B. Epigenetic transgenerational modifications induced by xenobiotic exposure in zebrafish. Front. Cell Dev. Biol. 2022, 10, 832982. [Google Scholar] [CrossRef]
- O’Neill, L.P.; VerMilyea, M.D.; Turner, B.M. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat. Genet. 2006, 38, 835–841. [Google Scholar] [CrossRef]
- Wang, S.; Bryan, C.; Xie, J.; Zhao, H.; Lin, L.F.; Tai, J.A.C.; Horzmann, K.A.; Sanchez, O.F.; Zhang, M.; Freeman, J.L.; et al. Atrazine exposure in zebrafish induces aberrant genome-wide methylation. Neurotoxicol. Teratol. 2022, 92, 107091. [Google Scholar] [CrossRef] [PubMed]
- Lakstygal, A.M.; de Abreu, M.S.; Kalueff, A.V. Zebrafish models of epigenetic regulation of cns functions. Brain Res. Bull. 2018, 142, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Gao, Y. DNA methylation and gene expression alterations in zebrafish embryos exposed to cadmium. Environ. Sci. Pollut. Res. Int. 2021, 28, 30101–30110. [Google Scholar] [CrossRef] [PubMed]
- Wirbisky, S.E.; Weber, G.J.; Schlotman, K.E.; Sepúlveda, M.S.; Freeman, J.L. Embryonic atrazine exposure alters zebrafish and human mirnas associated with angiogenesis, cancer, and neurodevelopment. Food Chem. Toxicol. 2016, 98, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Anger, W.K. Neurobehavioural tests and systems to assess neurotoxic exposures in the workplace and community. Occup. Environ. Med. 2003, 60, 474, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T.; Hawkey, A.B.; Levin, E.D. Translating neurobehavioral toxicity across species from zebrafish to rats to humans: Implications for risk assessment. Front. Toxicol. 2021, 3, 629229. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Menelaou, E.; Husbands, E.E.; Pollet, R.G.; Coutts, C.A.; Ali, D.W.; Svoboda, K.R. Embryonic motor activity and implications for regulating motoneuron axonal pathfinding in zebrafish. Eur. J. Neurosci. 2008, 28, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- McKeown, K.A.; Downes, G.B.; Hutson, L.D. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish 2009, 6, 179–185. [Google Scholar] [CrossRef] [PubMed]
- McClenahan, P.; Troup, M.; Scott, E.K. Fin-tail coordination during escape and predatory behavior in larval zebrafish. PLoS ONE 2012, 7, e32295. [Google Scholar] [CrossRef] [PubMed]
- Winata, C.L.; Korzh, S.; Kondrychyn, I.; Zheng, W.; Korzh, V.; Gong, Z. Development of Zebrafish Swimbladder: The Requirement of Hedgehog Signaling in Specification and Organization of the Three Tissue Layers. Dev. Biol. 2009, 331, 222–236. Available online: https://www.sciencedirect.com/science/article/pii/S0012160609002978 (accessed on 15 January 2024). [CrossRef] [PubMed]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Carbaugh, C.M.; Widder, M.W.; Phillips, C.S.; Jackson, D.A.; DiVito, V.T.; van der Schalie, W.H.; Glover, K.P. Assessment of zebrafish embryo photomotor response sensitivity and phase-specific patterns following acute- and long-duration exposure to neurotoxic chemicals and chemical weapon precursors. J. Appl. Toxicol. 2020, 40, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, V.C.; Neuhauss, S.C. Visual behavior in zebrafish. Zebrafish 2006, 3, 191–201. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, J.; Altenburger, R.; Scholz, S.; Luckenbach, T. Photomotor response data analysis approach to assess chemical neurotoxicity with the zebrafish embryo. Altex 2022, 39, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R.L. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Kokel, D.; Dunn, T.W.; Ahrens, M.B.; Alshut, R.; Cheung, C.Y.; Saint-Amant, L.; Bruni, G.; Mateus, R.; van Ham, T.J.; Shiraki, T.; et al. Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J. Neurosci. 2013, 33, 3834–3843. [Google Scholar] [CrossRef]
- Kokel, D.; Peterson, R.T. Using the zebrafish photomotor response for psychotropic drug screening. Methods Cell Biol. 2011, 105, 517–524. [Google Scholar] [CrossRef]
- Burton, C.E.; Zhou, Y.; Bai, Q.; Burton, E.A. Spectral properties of the zebrafish visual motor response. Neurosci. Lett. 2017, 646, 62–67. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Portales, A.M.; Batcho, K.G.; Freeman, J.L. Developmental toxicity of trichloroethylene in zebrafish (Danio rerio). Environ. Sci. Process Impacts 2020, 22, 728–739. [Google Scholar] [CrossRef]
- Huang, M.; Ivantsova, E.; Konig, I.; Patel, N.; English, C.; Souders, C.L., 2nd; Martyniuk, C.J. Developmental and mitochondrial toxicity assessment of perfluoroheptanoic acid (pfhpa) in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2023, 97, 104037. [Google Scholar] [CrossRef]
- Petrovic, M.; Farré, M.; Eljarrat, E.; Diaz, S.; Barceló, D. Chapter 14—Environmental analysis: Emerging pollutants. In Liquid Chromatography; Fanali, S., Haddad, P.R., Poole, C.F., Schoenmakers, P., Lloyd, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 389–410. [Google Scholar]
- Gyimah, E.; Zhu, X.; Zhang, Z.; Guo, M.; Xu, H.; Mensah, J.K.; Dong, X.; Gyimah, G.N.W. Oxidative stress and apoptosis in bisphenol af-induced neurotoxicity in zebrafish embryos. Environ. Toxicol. Chem. 2022, 41, 2273–2284. [Google Scholar] [CrossRef]
- Lockwood, B.; Bjerke, S.; Kobayashi, K.; Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 2004, 77, 647–654. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the Locomotor Activities of Zebrafish Larvae under the Influence of Various Neuroactive Drugs. Ann. Transl. Med. 2018, 6, 173. Available online: https://atm.amegroups.org/article/view/19504 (accessed on 15 January 2024). [CrossRef]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef]
- Kenney, J.W. Chapter 12—Associative and nonassociative learning in adult zebrafish. In Behavioral and Neural Genetics of Zebrafish; Gerlai, R.T., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 187–204. [Google Scholar]
- Tan, J.K.; Nazar, F.H.; Makpol, S.; Teoh, S.L. Zebrafish: A pharmacological model for learning and memory research. Molecules 2022, 27, 7374. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses. In Zebrafish Neurobehavioral Protocols; Kalueff, A.V., Cachat, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–14. [Google Scholar]
- Wang, J.; Wang, D.; Hu, G.; Yang, L.; Yan, D.; Wang, M.; Serikuly, N.; Alpyshov, E.; Amstislavskaya, T.G.; Demin, K.A.; et al. A new method for vibration-based neurophenotyping of zebrafish. J. Neurosci. Methods 2020, 333, 108563. [Google Scholar] [CrossRef]
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 2012, 1451, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Richardson, M.K. Exploratory behaviour in the open field test adapted for larval zebrafish: Impact of environmental complexity. Behav. Process. 2013, 92, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, R.; Marcon, M.; Gallas-Lopes, M.; de Mello, A.J.; Herrmann, A.P.; Piato, A. Swimming in the Maze: An Overview of Maze Apparatuses and Protocols to Assess Zebrafish Behavior. Neurosci. Biobehav. Rev. 2021, 127, 761–778. Available online: https://www.sciencedirect.com/science/article/pii/S0149763421002372?via%3Dihub (accessed on 15 January 2024). [CrossRef] [PubMed]
- Mathur, P.; Guo, S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 2011, 219, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, S.; Zhang, F.; Liu, H.; Zhang, H.; Kang, X. Effects of Atrazine on the Development of Neural System of Zebrafish, Danio rerio. Biomed. Res. Int. 2015, 2015, 976068. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4465686/pdf/BMRI2015-976068.pdf (accessed on 15 January 2024). [CrossRef] [PubMed]
- Glazer, L.; Brennan, C.H. Developmental exposure to low concentrations of methylmercury causes increase in anxiety-related behaviour and locomotor impairments in zebrafish. Int. J. Mol. Sci. 2021, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Thomas, M.; Colin, I.; Galant, S.; Machado, E.; Affaticati, P.; Jenett, A.; Yamamoto, K. Mesencephalic origin of the inferior lobe in zebrafish. BMC Biol. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Krasnikov, B.F.; Joshee, L.; Pinto, J.T.; Hallen, A.; Li, J.; Zalups, R.K.; Cooper, A.J. New insights into the metabolism of organomercury compounds: Mercury-containing cysteine s-conjugates are substrates of human glutamine transaminase k and potent inactivators of cystathionine γ-lyase. Arch. Biochem. Biophys. 2012, 517, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.C.; Carvalho, I.; Santos, C.; Herdeiro, M.T.; Fardilha, M.; Pavlaki, M.D.; Loureiro, S. Unveiling the molecular mechanisms and developmental consequences of mercury (hg) toxicity in zebrafish embryo-larvae: A comprehensive approach. Neurotoxicol. Teratol. 2023, 100, 107302. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Jing, M.; Jia, X.; Tian, L.; Tao, J. Environmentally relevant concentrations of organic (benzophenone-3) and inorganic (titanium dioxide nanoparticles) uv filters co-exposure induced neurodevelopmental toxicity in zebrafish. Ecotoxicol. Environ. Saf. 2023, 249, 114343. [Google Scholar] [CrossRef]
- Yu, T.; Xu, X.; Mao, H.; Han, X.; Liu, Y.; Zhang, H.; Lai, J.; Gu, J.; Xia, M.; Hu, C.; et al. Fenpropathrin exposure induces neurotoxicity in zebrafish embryos. Fish Physiol. Biochem. 2022, 48, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Xia, Y.; Tang, K.; Xu, J.; Wang, A.; Hu, S.; Wen, L.; Wang, B.; Yao, W.; et al. Toxic effects of isofenphos-methyl on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2023, 254, 114723. [Google Scholar] [CrossRef] [PubMed]
- Eghan, K.; Lee, S.; Yoo, D.; Kim, C.H.; Kim, W.K. Adverse effects of bifenthrin exposure on neurobehavior and neurodevelopment in a zebrafish embryo/larvae model. Chemosphere 2023, 341, 140099. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Deng, P.; Xing, D.; Liu, H.; Shi, F.; Hu, L.; Zou, X.; Nie, H.; Zuo, J.; Zhuang, Z.; et al. Developmental neurotoxicity of difenoconazole in zebrafish embryos. Toxics 2023, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Chen, Y.; Chen, J.; Qiu, Y.; Zhao, Y.; Li, H.; Xia, S.; Chen, S.; Zhu, J. The adverse effects of fluxapyroxad on the neurodevelopment of zebrafish embryos. Chemosphere 2022, 307, 135751. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, H.; Chen, Y.; Zheng, N.; Li, X.; Wang, X.; Ding, W.; Zhang, B. Developmental neurotoxicity of trichlorfon in zebrafish larvae. Int. J. Mol. Sci. 2023, 24, 11099. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.; Dong, M.; Li, M.; Hu, C.; Xu, X. Chlorphoxim induces neurotoxicity in zebrafish embryo through activation of oxidative stress. Environ. Toxicol. 2023, 38, 566–578. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, M.; Liu, C.; Wang, J.; Zou, L.; Yang, F.; Zhu, R. Curcumin protects against fenvalerate-induced neurotoxicity in zebrafish (Danio rerio) larvae through inhibition of oxidative stress. Ecotoxicol. Environ. Saf. 2023, 264, 115484. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Guo, L.; Zhu, Y.; Qian, L.; Shi, L.; Zhang, H.; Ji, G. Neurodevelopmental toxicity of emamectin benzoate to the early life stage of zebrafish larvae (Danio rerio). Int. J. Mol. Sci. 2023, 24, 3757. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Liao, G.; Tu, H.; Huang, Y.; Peng, T.; Chen, X.; Huang, Z.; Zhang, Y.; Meng, X.; et al. The role of ambra1 in pb-induced developmental neurotoxicity in zebrafish. Biochem. Biophys. Res. Commun. 2022, 594, 139–145. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.; Xu, Y.; Song, Z.; Lan, X.; Pan, C.; Zhang, S.; Foulkes, N.S.; Zhao, H. Ferroptosis contributes to nickel-induced developmental neurotoxicity in zebrafish. Sci. Total Environ. 2023, 858, 160078. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, J.; Gao, M.; Zhu, Y.; Hong, Y. Excessive selenium affects neural development and locomotor behavior of zebrafish embryos. Ecotoxicol. Environ. Saf. 2022, 238, 113611. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Yang, L.; Zheng, Q.; Liu, Q.; Wawryk, N.J.P.; Li, X.F. Neurotoxicity and transcriptome changes in embryonic zebrafish induced by halobenzoquinone exposure. J. Environ. Sci. 2022, 117, 129–140. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, W.; Li, R.; Chen, B.; Wu, X.; Magnuson, J.T.; Xu, B.; Luo, S.; Xu, E.G.; Zheng, C. Perfluorononanoic acid induces neurotoxicity via synaptogenesis signaling in zebrafish. Environ. Sci. Technol. 2023, 57, 3783–3793. [Google Scholar] [CrossRef]
- Mahapatra, A.; Gupta, P.; Suman, A.; Ray, S.S.; Malafaia, G.; Singh, R.K. Unraveling the mechanisms of perfluorooctanesulfonic acid-induced dopaminergic neurotoxicity and microglial activation in developing zebrafish. Sci. Total Environ. 2023, 887, 164030. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, L.; Gao, G.; He, L.; Zhao, K.; Shang, X.; Liu, C. 2, 5-dichloro-1, 4-benuinone exposure to zebrafish embryos/larvae causes neurodevelopmental toxicity. Ecotoxicol. Environ. Saf. 2022, 243, 114007. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; de Alba González, M.; Morales, M.; Martin-Folgar, R.; González, M.C.; Cañas-Portilla, A.I.; De la Vieja, A. Neurotoxicity and Endocrine Disruption Caused by Polystyrene Nanoparticles in Zebrafish Embryo. Sci. Total Environ. 2023, 874, 162406. Available online: https://www.sciencedirect.com/science/article/pii/S0048969723010227 (accessed on 15 January 2024). [CrossRef]
- Xiang, C.; Chen, H.; Liu, X.; Dang, Y.; Li, X.; Yu, Y.; Li, B.; Li, X.; Sun, Y.; Ding, P.; et al. Uv-aged microplastics induces neurotoxicity by affecting the neurotransmission in larval zebrafish. Chemosphere 2023, 324, 138252. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, H.; Zheng, Y.; Zheng, N.; Lei, L.; Li, X.; Ding, W. Neurotoxicity of an emerging organophosphorus flame retardant, resorcinol bis(diphenyl phosphate), in zebrafish larvae. Chemosphere 2023, 334, 138944. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, B.; Zhou, S.; Zhao, M.; Li, R.; Zhou, Y.; Shi, X.; Han, J.; Zhang, W.; Zhou, B. Mitochondrial dysfunction was involved in decabromodiphenyl ethane-induced glucolipid metabolism disorders and neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2023, 57, 11043–11055. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Dai, J.; Zhang, K. Environmentally relevant concentrations of antidepressant mirtazapine impair the neurodevelopment of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2023, 262, 115335. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bang, J.; Ryu, B.; Kim, C.Y.; Park, J.H. Flubendazole exposure disrupts neural development and function of zebrafish embryos (Danio rerio). Sci. Total Environ. 2023, 898, 165376. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Ye, X.; Qin, Y.; Wang, H.; Liang, Z.; Zhu, L.; Zhou, L.; Martyniuk, C.J.; Yan, B. Dopaminergic and serotoninergic neurotoxicity of lanthanide phosphate (tbpo(4)) in developing zebrafish. Chemosphere 2023, 340, 139861. [Google Scholar] [CrossRef]
- Gayathri, M.; Sutha, J.; Mohanthi, S.; Ramesh, M.; Poopal, R.K. Ecotoxicological evaluation of the uv-filter octocrylene (oc) in embryonic zebrafish (Danio rerio): Developmental, biochemical and cellular biomarkers. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109688. [Google Scholar] [CrossRef] [PubMed]
| Environmental Pollutants | Concentrations | Exposure Duration | Results | Reference | |
|---|---|---|---|---|---|
| Insecticide | Fenpropathrin | 0.016 mg/L, 0.032 mg/L, 0.064 mg/L | 96 hpf |
| [266] |
| Isofenphos-methyl | 2, 4, and 8 mg/L | 6–96 hpf |
| [267] | |
| Bifenthrin | 103.9 and 362.1 μg/L | <3–120 hpf |
| [268] | |
| Fungicide | Difenoconazole | 0.25, 0.5, and 1 mg/L | 120 hpf |
| [269] |
| Fluxapyroxad | 0.5, 0.75, and 1 mg/L | 96 hpf |
| [270] | |
| Pesticide | Trichlorfon | 0, 0.1, 2 and 5 mg/L | 144 hpf |
| [271] |
| Chlorphoxim | 2.5, 5, 7.5, 10, and 12.5 mg/L | 96 hpf |
| [272] | |
| Fenvalerate | 0, 3.5, 7 and 14 μg/L | 4–96 hpf |
| [273] | |
| Emamectin benzoate (EMB) | 0.1, 0.25, 0.5, 1, 2, 4 and 8 μg/mL | 4–144 hpf |
| [274] | |
| Metal and mineral | Lead | 0.1, 1, and 10 μM | 120 hpf |
| [275] |
| Nickel | 0, 10, 50, 100, 500, and 1000 μM | 144 hpf |
| [276] | |
| Selenium | 0.125, 0.25, 0.5, and 1 µM | 96 hpf |
| [277] | |
| Disinfectant | Halobenzoquinones | 0–8 μmol/L | 120 hpf |
| [278] |
| Perfluorononanoic Acid | 0.01, 0.1, 1, 10, and 100 μg/L | 120 hpf |
| [279] | |
| 0, 100, 500, and 1000 μg/L | 4–120 hpf |
| [280] | ||
| 2,5-dichloro-1,4-benuinone (2,5-DCBQ) | 0.2, 0.4, and 0.6 mg/L | 4–120 hpf |
| [281] | |
| Nanoparticles | polystyrene nanoparticles | 0.2, 1, and 5 mg/L | 120 hpf |
| [282] |
| Microplastics | 0.1 to 100 μg/L | 120 hpf |
| [283] | |
| Flame retardant | Resorcinol bis(diphenyl phosphate) | 0, 0.3, 3, 90, 300 and 900 nM | 2–144 hpf |
| [284] |
| Decabromodiphenyl ethane | 50–400 μg/L | 120 hpf |
| [285] | |
| Drug | Mirtazapine | 3.9 and 43.5 ng/L | 2.5–96 hpf |
| [286] |
| Flubendazole | 0.05, 0.1, 0.2, 0.4, and 0.8 mg/mL | 96 hpf |
| [287] | |
| Others | Bisphenol AF | 0.03, 0.1, 0.3, and 1.0 µM | 144 hpf |
| [248] |
| Lanthanide phosphate (TbPO4) | 10, 20, and 50 mg/L | 144 hpf |
| [288] | |
| Octocrylene (OC) | 5, 50 and 500 μg/L | 3–96 hpf |
| [289] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.-H.; Horzmann, K.A. Embryonic Zebrafish as a Model for Investigating the Interaction between Environmental Pollutants and Neurodegenerative Disorders. Biomedicines 2024, 12, 1559. https://doi.org/10.3390/biomedicines12071559
Yin J-H, Horzmann KA. Embryonic Zebrafish as a Model for Investigating the Interaction between Environmental Pollutants and Neurodegenerative Disorders. Biomedicines. 2024; 12(7):1559. https://doi.org/10.3390/biomedicines12071559
Chicago/Turabian StyleYin, Ji-Hang, and Katharine A. Horzmann. 2024. "Embryonic Zebrafish as a Model for Investigating the Interaction between Environmental Pollutants and Neurodegenerative Disorders" Biomedicines 12, no. 7: 1559. https://doi.org/10.3390/biomedicines12071559
APA StyleYin, J.-H., & Horzmann, K. A. (2024). Embryonic Zebrafish as a Model for Investigating the Interaction between Environmental Pollutants and Neurodegenerative Disorders. Biomedicines, 12(7), 1559. https://doi.org/10.3390/biomedicines12071559






