3-Hydroxy-3-Methylglutaric Acid Disrupts Brain Bioenergetics, Redox Homeostasis, and Mitochondrial Dynamics and Affects Neurodevelopment in Neonatal Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Ex Vivo Experiments
2.2.1. HMG Administration
2.2.2. Antioxidant Defenses
2.3. Bioenergetics
2.4. Western Blotting
2.5. Neurodevelopmental Reflex Assessment
2.6. Protein Determination
2.7. Statistical Analysis
3. Results
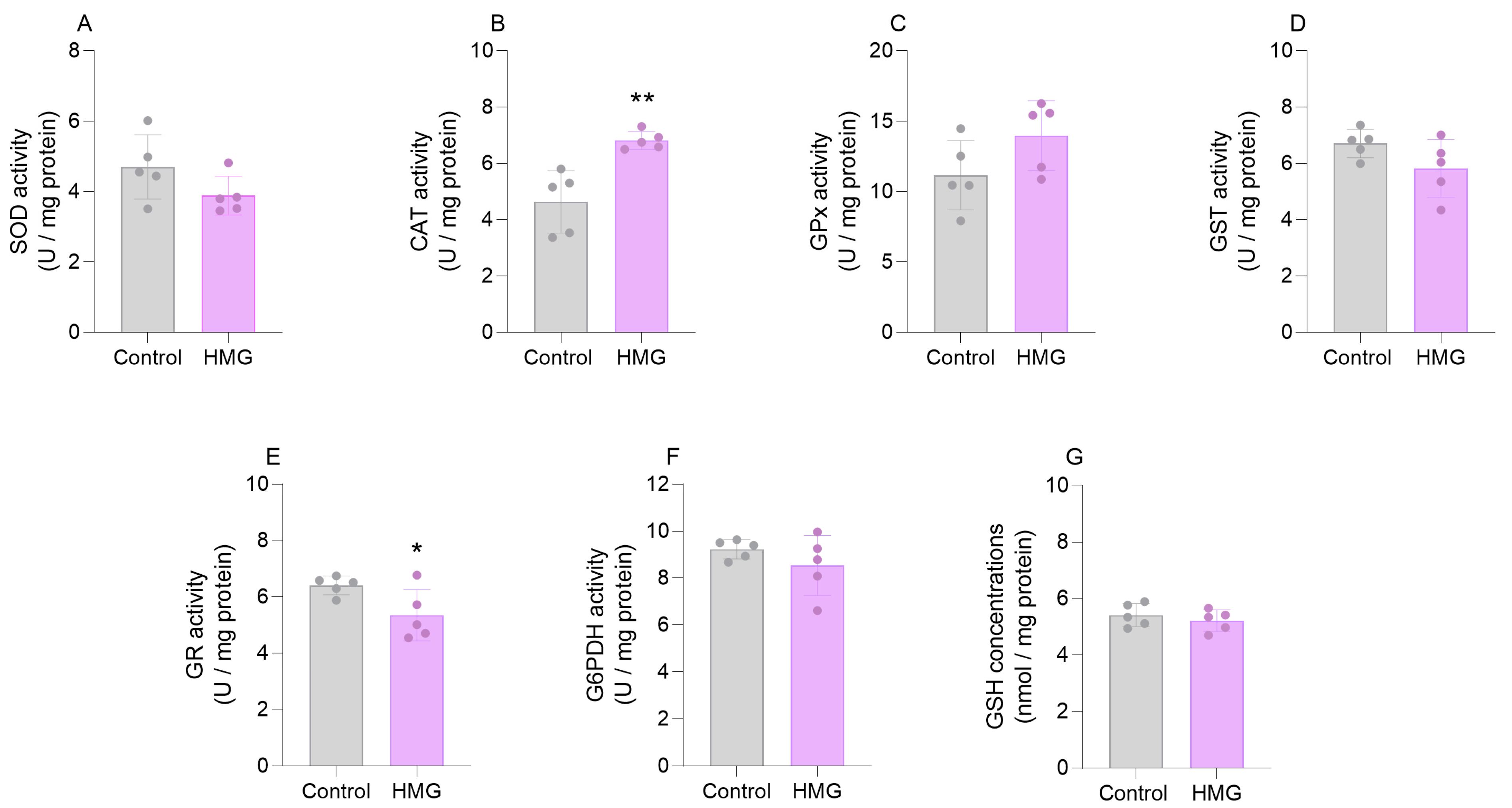
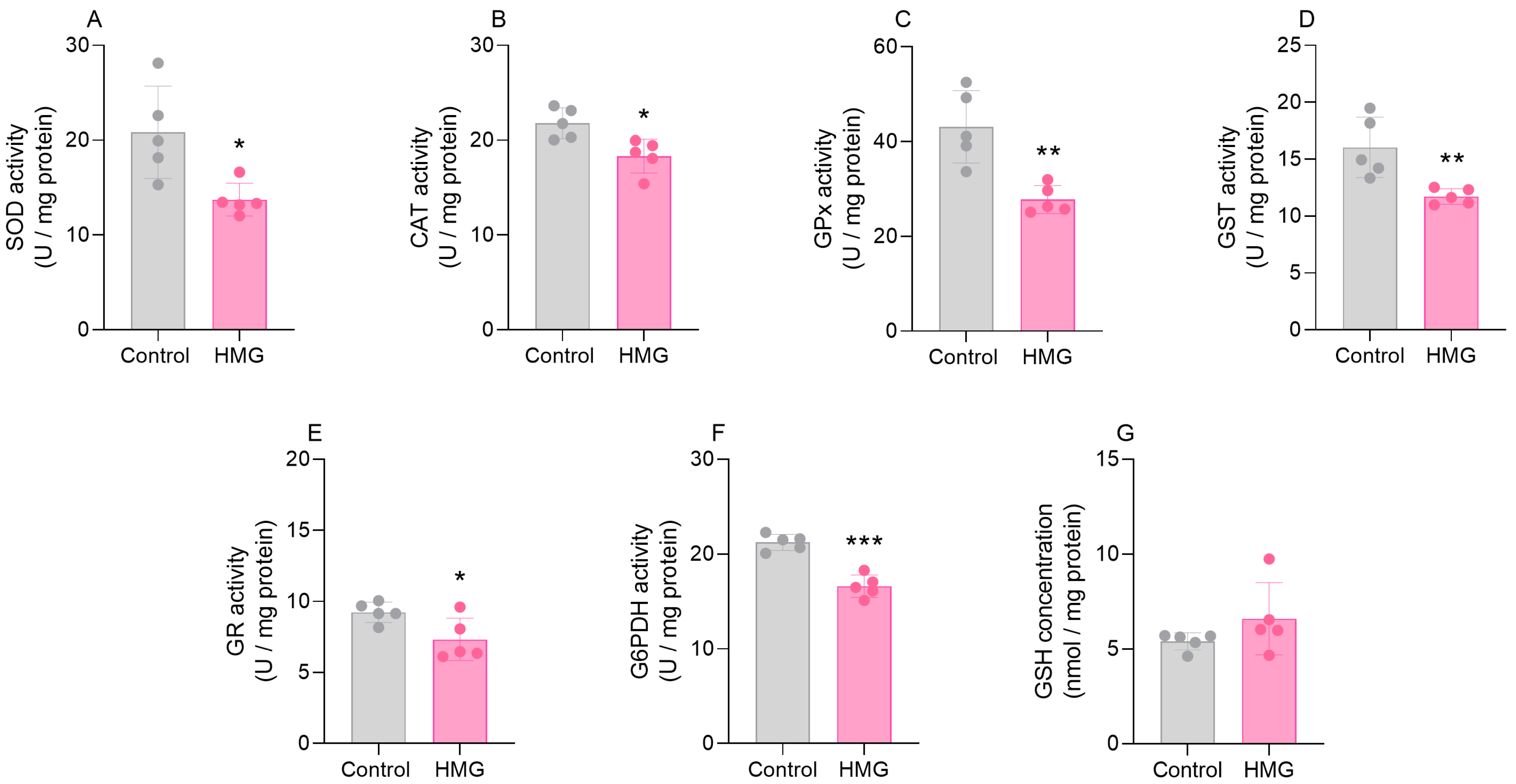
3.1. HMG Impairs Enzymatic Antioxidant Defenses in the Neonatal Brain
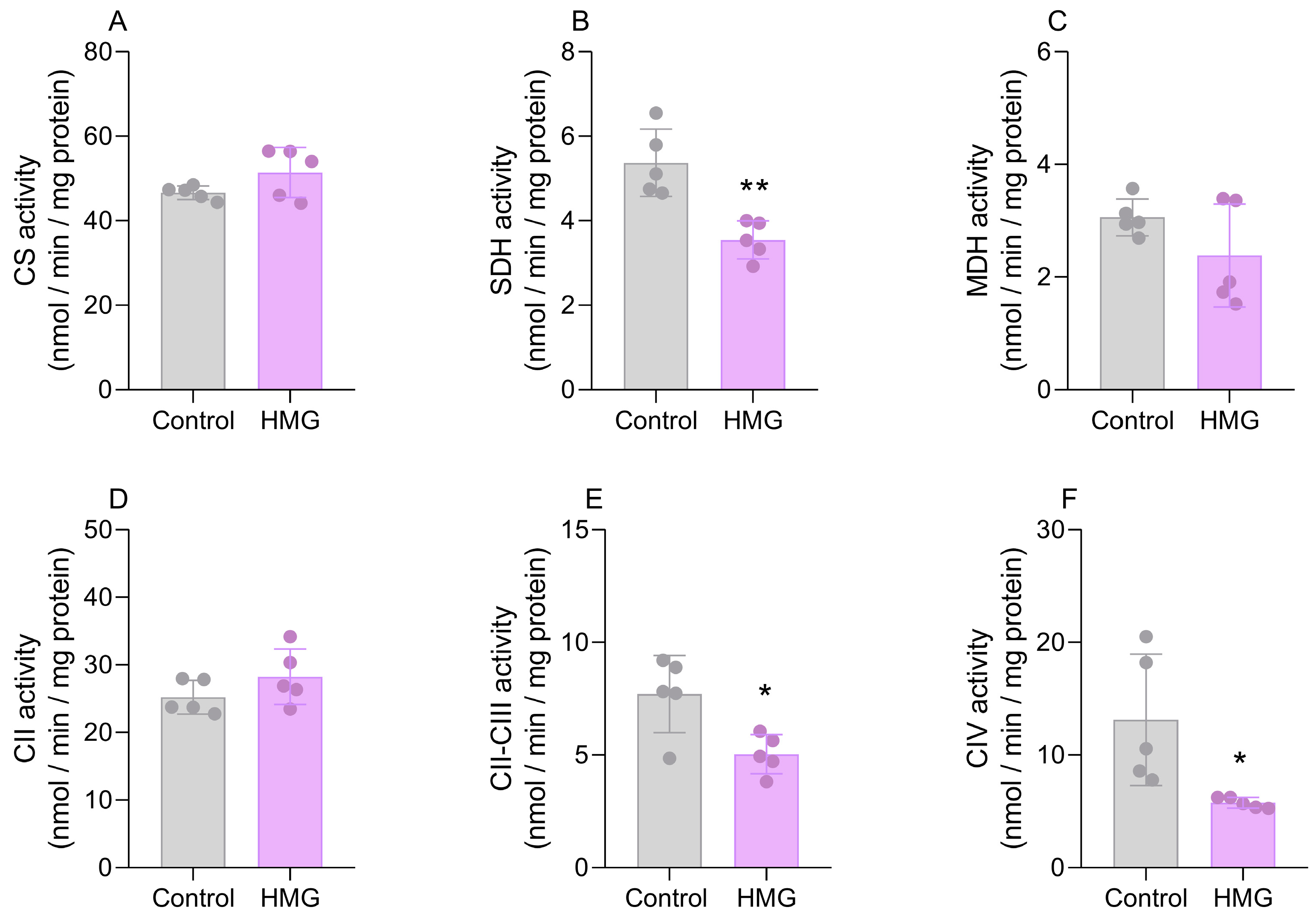
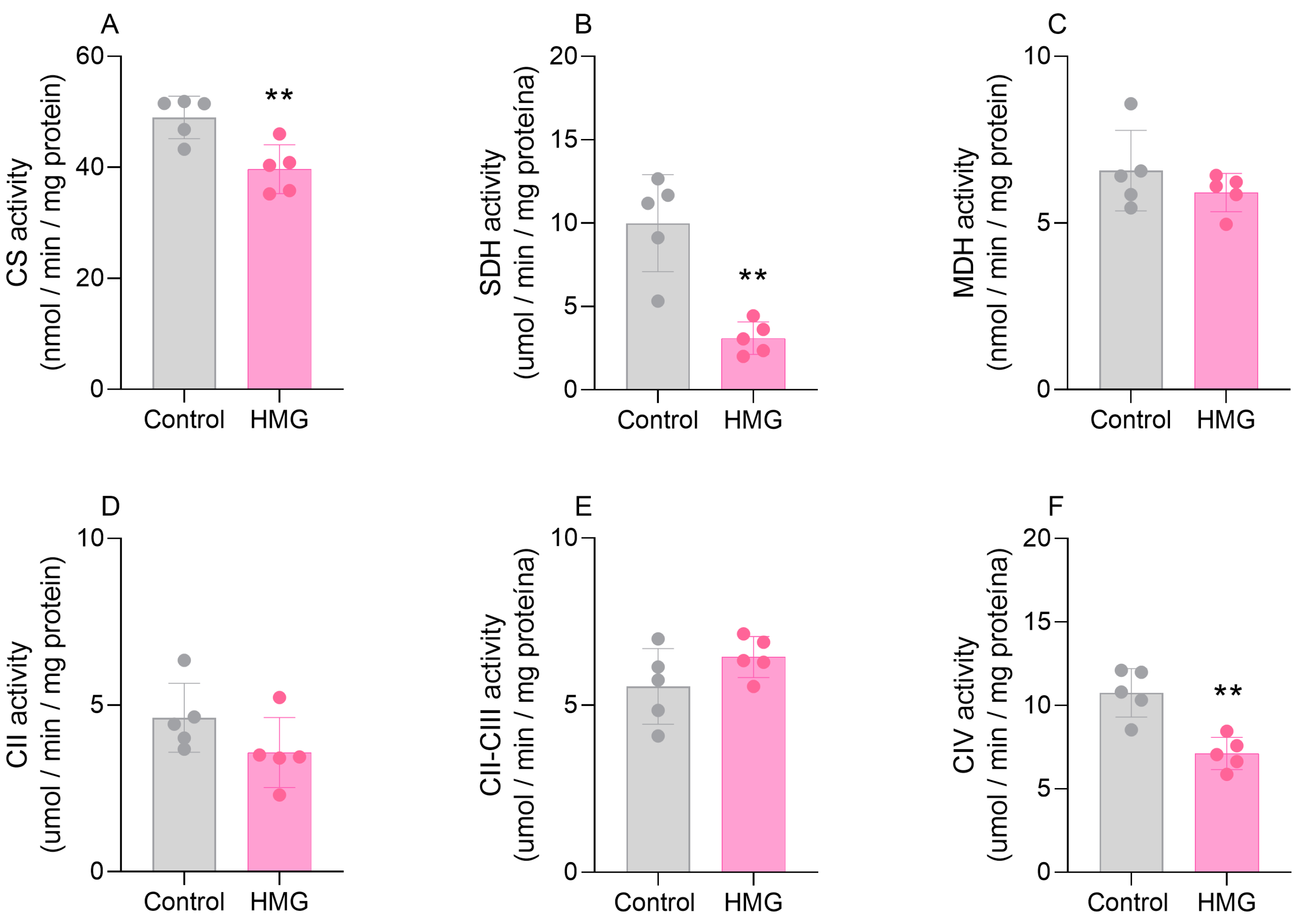
3.2. HMG Disrupts CAC and Mitochondrial Respiratory Chain Functioning in the Neonatal Brain
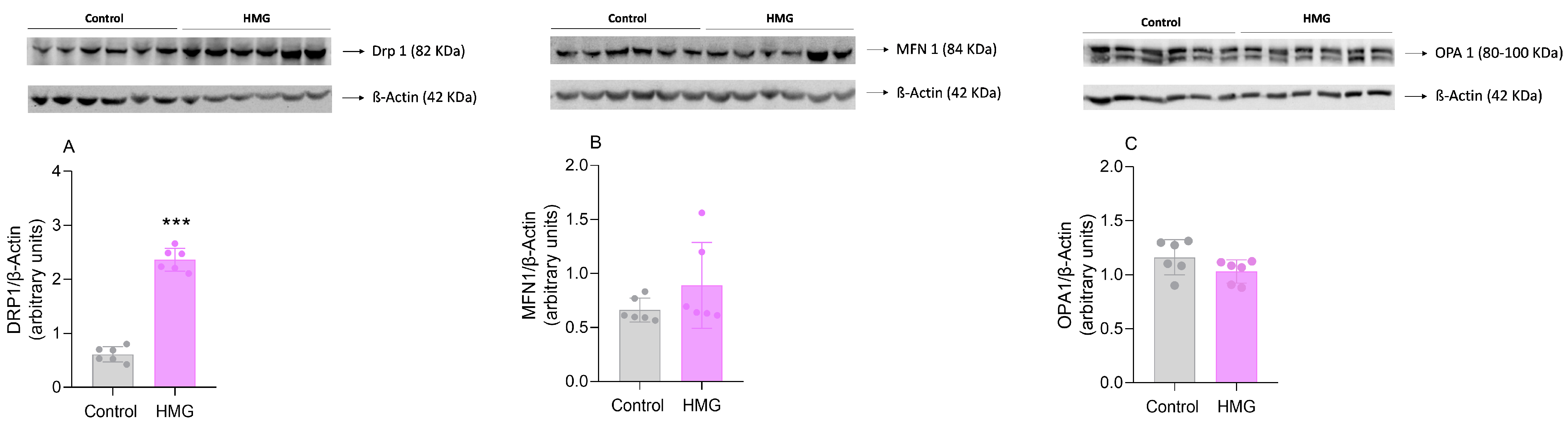
3.3. HMG Affects Mitochondrial Dynamics in the Neonatal Cerebral Cortex
3.4. HMG Impairs the Neurodevelopment of Neonatal Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roland, D.; Jissendi-Tchofo, P.; Briand, G.; Vamecq, J.; Fontaine, M.; Ultré, V.; Acquaviva-Bourdain, C.; Mention, K.; Dobbelaere, D. Coupled Brain and Urine Spectroscopy—In Vivo Metabolomic Characterization of HMG-CoA Lyase Deficiency in 5 Patients. Mol. Genet. Metab. 2017, 121, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Zschocke, J.; Knerr, I.; Vockley, C.W.; Gibson, K.M. Branched Chain Organic Acidurias. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Muñoz-Bonet, J.I.; Ortega-Sánchez, M.d.C.; León Guijarro, J.L. Management and Long-Term Evolution of a Patient with 3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase Deficiency. Ital. J. Pediatr. 2017, 43, 12. [Google Scholar] [CrossRef]
- Yilmaz, O.; Kitchen, S.; Pinto, A.; Daly, A.; Gerrard, A.; Hoban, R.; Santra, S.; Sreekantam, S.; Frost, K.; Pigott, A.; et al. Deficiencia de La 3-Hidroxi-3-Metilglutaril-Coa Liasa: Un Caso Clínico y Revisión de La Literatura. Nutr. Hosp. 2018, 35, 237–244. [Google Scholar] [PubMed]
- Grünert, S.C.; Sass, J.O. 3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase Deficiency: One Disease—Many Faces. Orphanet J. Rare Dis. 2020, 15, 48. [Google Scholar] [CrossRef]
- Grünert, S.C.; Schlatter, S.M.; Schmitt, R.N.; Gemperle-Britschgi, C.; Mrázová, L.; Balcı, M.C.; Bischof, F.; Çoker, M.; Das, A.M.; Demirkol, M.; et al. 3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase Deficiency: Clinical Presentation and Outcome in a Series of 37 Patients. Mol. Genet. Metab. 2017, 121, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Alfadhel, M.; Abadel, B.; Almaghthawi, H.; Umair, M.; Rahbeeni, Z.; Faqeih, E.; Almannai, M.; Alasmari, A.; Saleh, M.; Eyaid, W.; et al. HMG-CoA Lyase Deficiency: A Retrospective Study of 62 Saudi Patients. Front. Genet. 2022, 13, 880464. [Google Scholar] [CrossRef]
- Da Rosa, M.S.; Seminotti, B.; Amaral, A.U.; Fernandes, C.G.; Gasparotto, J.; Moreira, J.C.F.; Gelain, D.P.; Wajner, M.; Leipnitz, G. Redox Homeostasis Is Compromised in Vivo by the Metabolites Accumulating in 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency in Rat Cerebral Cortex and Liver. Free Radic. Res. 2013, 47, 1066–1075. [Google Scholar] [CrossRef]
- da Rosa, M.S.; Seminotti, B.; Ribeiro, C.A.J.; Parmeggiani, B.; Grings, M.; Wajner, M.; Leipnitz, G. 3-Hydroxy-3-Methylglutaric and 3-Methylglutaric Acids Impair Redox Status and Energy Production and Transfer in Rat Heart: Relevance for the Pathophysiology of Cardiac Dysfunction in 3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase Deficiency. Free Radic. Res. 2016, 50, 997–1010. [Google Scholar] [CrossRef]
- da Rosa, M.S.; da Rosa-Junior, N.T.; Parmeggiani, B.; Glänzel, N.M.; de Moura Alvorcem, L.; Ribeiro, R.T.; Grings, M.; Wajner, M.; Leipnitz, G. 3-Hydroxy-3-Methylglutaric Acid Impairs Redox and Energy Homeostasis, Mitochondrial Dynamics, and Endoplasmic Reticulum–Mitochondria Crosstalk in Rat Brain. Neurotox. Res. 2020, 37, 314–325. [Google Scholar] [CrossRef]
- Thompson, S.; Hertzog, A.; Selvanathan, A.; Batten, K.; Lewis, K.; Nisbet, J.; Mitchell, A.; Dalkeith, T.; Billmore, K.; Moore, F.; et al. Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases. Nutrients 2023, 15, 531. [Google Scholar] [CrossRef]
- Sizonenko, S.V.; Sirimanne, E.; Mayall, Y.; Gluckman, P.D.; Inder, T.; Williams, C. Selective Cortical Alteration after Hypoxic-Ischemic Injury in the Very Immature Rat Brain. Pediatr. Res. 2003, 54, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Bravo, S.; Fernández, A.; Sarlabós, M.N.; Rosillo, J.C.; Casanova, G.; Jiménez, M.; Barbeito, L. Neonatal Astrocyte Damage Is Sufficient to Trigger Progressive Striatal Degeneration in a Rat Model of Glutaric Acidemia-I. PLoS ONE 2011, 6, e20831. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Bravo, S.; Isasi, E.; Fernández, A.; Rosillo, J.C.; Jiménez, M.; Casanova, G.; Sarlabós, M.N.; Barbeito, L. White Matter Injury Induced by Perinatal Exposure to Glutaric Acid. Neurotox. Res. 2014, 25, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Browne Richard, W.; Armstrong, D. Reduced Glutathione and Glutathione Disulfide. In Free Radical and Antioxidant Protocols; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 1998; pp. 347–352. ISBN 978-1-59259-254-8. [Google Scholar]
- Marklund, S.L. Product of Extracellular-Superoxide Dismutase Catalysis. FEBS Lett. 1985, 184, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Wendel, A. Glutathione Peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [PubMed]
- Mannervik, B.; Guthenberg, C. Glutathione Transferase (Human Placenta). Methods Enzymol. 1981, 77, 231–235. [Google Scholar] [PubMed]
- Leong, S.F.; Clark, J.B. Regional Enzyme Development in Rat Brain. Enzymes Associated with Glucose Utilization. Biochem. J. 1984, 218, 131–138. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Grings, M.; Moura, A.P.; Parmeggiani, B.; Pletsch, J.T.; Cardoso, G.M.F.; August, P.M.; Matté, C.; Wyse, A.T.S.; Wajner, M.; Leipnitz, G. Bezafibrate prevents mitochondrial dysfunction, antioxidant system disturbance, glial reactivity and neuronal damage induced by sulfite administration in striatum of rats: Implications for a possible therapeutic strategy for sulfite oxidase deficiency. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2135–2148. [Google Scholar] [CrossRef]
- Fischer, J.C.; Ruitenbeek, W.; Berden, J.A.; Trijbels, J.M.F.; Veerkamp, J.H.; Stadhouders, A.M.; Sengers, R.C.A.; Janssen, A.J.M. Differential Investigation of the Capacity of Succinate Oxidation in Human Skeletal Muscle. Clin. Chim. Acta 1985, 153, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gérard, B.; Rötig, A.; Saudubray, J.M.; Munnich, A. Biochemical and Molecular Investigations in Respiratory Chain Deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Barrie Kitto, G. Intra- and Extramitochondrial Malate Dehydrogenases from Chicken and Tuna Heart: [EC 1.1.1.37 l-Malate: NAD Oxidoreductase]. Methods Enzymol. 1969, 13, 106–116. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- da Rosa-Junior, N.T.; Parmeggiani, B.; da Rosa, M.S.; Glänzel, N.M.; de Moura Alvorcem, L.; Wajner, M.; Leipnitz, G. Bezafibrate In Vivo Administration Prevents 3-Methylglutaric Acid-Induced Impairment of Redox Status, Mitochondrial Biogenesis, and Neural Injury in Brain of Developing Rats. Neurotox. Res. 2019, 35, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.T.; Carvalho, A.V.S.; Palavro, R.; Durán-Carabali, L.E.; Zemniaçak, Â.B.; Amaral, A.U.; Netto, C.A.; Wajner, M. L-2-Hydroxyglutaric Acid Administration to Neonatal Rats Elicits Marked Neurochemical Alterations and Long-Term Neurobehavioral Disabilities Mediated by Oxidative Stress. Neurotox. Res. 2023, 41, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.J.R.; Ballinger, S.; Dell’Italia, L.; Zhang, J.; Darley-Usmar, V.M. Integration of Cellular Bioenergetics with Mitochondrial Quality Control and Autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Shapiro, B.K.; Palmer, F.B.; Capute, A.J. The Diagnostic Value of the Neurodevelopmental Examination. Clin. Pediatr. (Phila) 1985, 24, 367–372. [Google Scholar] [CrossRef]
- Zafeiriou, D.I. Primitive Reflexes and Postural Reactions in the Neurodevelopmental Examination. Pediatr. Neurol. 2004, 31, 1–8. [Google Scholar] [CrossRef]
- Chakraborty, R.; Vijay Kumar, M.J.; Clement, J.P. Critical Aspects of Neurodevelopment. Neurobiol. Learn. Mem. 2021, 180, 107415. [Google Scholar] [CrossRef]
- Cisneros-Franco, J.M.; Voss, P.; Thomas, M.E.; de Villers-Sidani, E. Chapter 8—Critical Periods of Brain Development. Handb. Clin. Neurol. 2020, 173, 75–88. [Google Scholar] [PubMed]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial Complex I ROS Production and Redox Signaling in Hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Leipnitz, G.; Vargas, C.R.; Wajner, M. Disturbance of Redox Homeostasis as a Contributing Underlying Pathomechanism of Brain and Liver Alterations in 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency. J. Inherit. Metab. Dis. 2015, 38, 1021–1028. [Google Scholar] [CrossRef]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2022, 24, 1265–1276. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 9780198717478. [Google Scholar]
- Hill, J.M.; Switzer, R.C. The Regional Distribution and Cellular Localization of Iron in the Rat Brain. Neuroscience 1984, 11, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Pinero, D.J.; Connor, J.R.; Beard, J.L. Regional Brain Iron, Ferritin and Transferrin Concentrations during Iron Deficiency and Iron Repletion in Developing Rats1. J. Nutr. 1997, 127, 2030–2038. [Google Scholar] [CrossRef]
- Gu, M.; Wei, Z.; Wang, X.; Gao, Y.; Wang, D.; Liu, X.; Bai, C.; Su, G.; Yang, L.; Li, G. Myostatin Knockout Affects Mitochondrial Function by Inhibiting the AMPK/SIRT1/PGC1α Pathway in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 13703. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Cui, H.; Deng, H.; Zuo, Z.; Fang, J.; Guo, H. Effects of CuSO4 on Hepatic Mitochondrial Function, Biogenesis and Dynamics in Mice. Environ. Toxicol. 2024, 39, 2208–2217. [Google Scholar] [CrossRef]
- Hofmann, A.; Mishra, J.S.; Yadav, P.; Dangudubiyyam, V.; Blesson, C.S.; Kumar, S. PFOS Impairs Mitochondrial Biogenesis and Dynamics and Reduces Oxygen Consumption in Human Trophoblasts HHS Public Access. J. Environ. Sci. Public Health 2023, 7, 164–175. [Google Scholar] [CrossRef]
- Hayashi, M.; Kawarasaki, T.; Nakatsukasa, K. Degradation of Citrate Synthase Lacking the Mitochondrial Targeting Sequence Is Inhibited in Cells Defective in Hsp70/Hsp40 Chaperones under Heat Stress Conditions. FEMS Yeast Res. 2024, 24, foad054. [Google Scholar] [CrossRef]
- de Moura Alvorcem, L.; da Rosa, M.S.; Glänzel, N.M.; Parmeggiani, B.; Grings, M.; Schmitz, F.; Wyse, A.T.S.; Wajner, M.; Leipnitz, G. Disruption of Energy Transfer and Redox Status by Sulfite in Hippocampus, Striatum, and Cerebellum of Developing Rats. Neurotox. Res. 2017, 32, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wong, H.S.; Brand, M.D. Production of Superoxide and Hydrogen Peroxide in the Mitochondrial Matrix Is Dominated by Site IQ of Complex I in Diverse Cell Lines. Redox Biol. 2020, 37, 101722. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Wyse, A.T.S.; Grings, M.; Wajner, M.; Leipnitz, G. The Role of Oxidative Stress and Bioenergetic Dysfunction in Sulfite Oxidase Deficiency: Insights from Animal Models. Neurotox. Res. 2019, 35, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Yao, Z.; Sun, X.; Tong, X.; Dong, S. The Role of Mitochondrial Dynamics in Cerebral Ischemia-Reperfusion Injury. Biomed. Pharmacother. 2023, 162, 114671. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kapoor, R.; Sushma; Kanchan, S.; Jha, G.; Sharma, D.; Tomar, B.; Rath, S.K. Deoxynivalenol Induces Drp-1-Mediated Mitochondrial Dysfunction via Elevating Oxidative Stress. Chem. Res. Toxicol. 2024; in press. [Google Scholar] [CrossRef]
- Ho, D.; Sanches, E.F.; Sizonenko, S.V. Early Neurodevelopmental Reflex Impairments in a Rodent Model of Cerebral Palsy. Int. J. Dev. Neurosci. 2022, 82, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Buratti, P.; Covatti, C.; Centenaro, L.A.; Brancalhão, R.M.C.; Torrejais, M.M. Morphofunctional Characteristics of Skeletal Muscle in Rats with Cerebral Palsy. Int. J. Exp. Pathol. 2019, 100, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Armstrong, E.A.; Yager, J.Y. Neurodevelopmental Reflex Testing in Neonatal Rat Pups. J. Vis. Exp. 2017, 2017, 55261. [Google Scholar] [CrossRef]
- Arakawa, H.; Erzurumlu, R.S. Role of Whiskers in Sensorimotor Development of C57BL/6 Mice. Behav. Brain Res. 2015, 287, 146–155. [Google Scholar] [CrossRef]
- Pramio, J.; Grings, M.; da Rosa, A.G.; Ribeiro, R.T.; Glanzel, N.M.; Signori, M.F.; Marcuzzo, M.B.; Bobermin, L.D.; Wyse, A.T.S.; Quincozes-Santos, A.; et al. Sulfite Impairs Bioenergetics and Redox Status in Neonatal Rat Brain: Insights into the Early Neuropathophysiology of Isolated Sulfite Oxidase and Molybdenum Cofactor Deficiencies. Cell Mol. Neurobiol. 2023, 43, 2895–2907. [Google Scholar] [CrossRef]
- Moura, A.P.; Parmeggiani, B.; Grings, M.; Alvorcem, L.d.M.; Boldrini, R.M.; Bumbel, A.P.; Motta, M.M.; Seminotti, B.; Wajner, M.; Leipnitz, G. Intracerebral Glycine Administration Impairs Energy and Redox Homeostasis and Induces Glial Reactivity in Cerebral Cortex of Newborn Rats. Mol. Neurobiol. 2016, 53, 5864–5875. [Google Scholar] [CrossRef] [PubMed]
- Wajner, M. Neurological Manifestations of Organic Acidurias. Nat. Rev. Neurol. 2019, 15, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.W.; Opp, S.; Mahringer, A.; Kamiński, M.M.; Thiel, C.; Okun, J.G.; Fricker, G.; Morath, M.A.; Kölker, S. Glutaric Aciduria Type I and Methylmalonic Aciduria: Simulation of Cerebral Import and Export of Accumulating Neurotoxic Dicarboxylic Acids in in Vitro Models of the Blood–Brain Barrier and the Choroid Plexus. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 552–560. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, J.d.A.; Marcuzzo, M.B.; da Rosa, J.S.; Kist, N.S.; Hoffmann, C.I.H.; Carvalho, A.S.; Ribeiro, R.T.; Quincozes-Santos, A.; Netto, C.A.; Wajner, M.; et al. 3-Hydroxy-3-Methylglutaric Acid Disrupts Brain Bioenergetics, Redox Homeostasis, and Mitochondrial Dynamics and Affects Neurodevelopment in Neonatal Wistar Rats. Biomedicines 2024, 12, 1563. https://doi.org/10.3390/biomedicines12071563
Silveira JdA, Marcuzzo MB, da Rosa JS, Kist NS, Hoffmann CIH, Carvalho AS, Ribeiro RT, Quincozes-Santos A, Netto CA, Wajner M, et al. 3-Hydroxy-3-Methylglutaric Acid Disrupts Brain Bioenergetics, Redox Homeostasis, and Mitochondrial Dynamics and Affects Neurodevelopment in Neonatal Wistar Rats. Biomedicines. 2024; 12(7):1563. https://doi.org/10.3390/biomedicines12071563
Chicago/Turabian StyleSilveira, Josyane de Andrade, Manuela Bianchin Marcuzzo, Jaqueline Santana da Rosa, Nathalia Simon Kist, Chrístofer Ian Hernandez Hoffmann, Andrey Soares Carvalho, Rafael Teixeira Ribeiro, André Quincozes-Santos, Carlos Alexandre Netto, Moacir Wajner, and et al. 2024. "3-Hydroxy-3-Methylglutaric Acid Disrupts Brain Bioenergetics, Redox Homeostasis, and Mitochondrial Dynamics and Affects Neurodevelopment in Neonatal Wistar Rats" Biomedicines 12, no. 7: 1563. https://doi.org/10.3390/biomedicines12071563





