Cordycepin Augments the Efficacy of Anti-PD1 against Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Analysis
2.3. Cell Cycle Analysis
2.4. Morphological Observation by Light Microscopy
2.5. Western Blotting Analysis
2.6. mRNA Expression Assessment
2.7. Syngeneic Tumor Implantation Model
2.8. Evaluation of the Tumor Volume and Toxicity
2.9. Flow Cytometry Analysis of Immune Cells
2.10. Statistical Analyses
3. Results
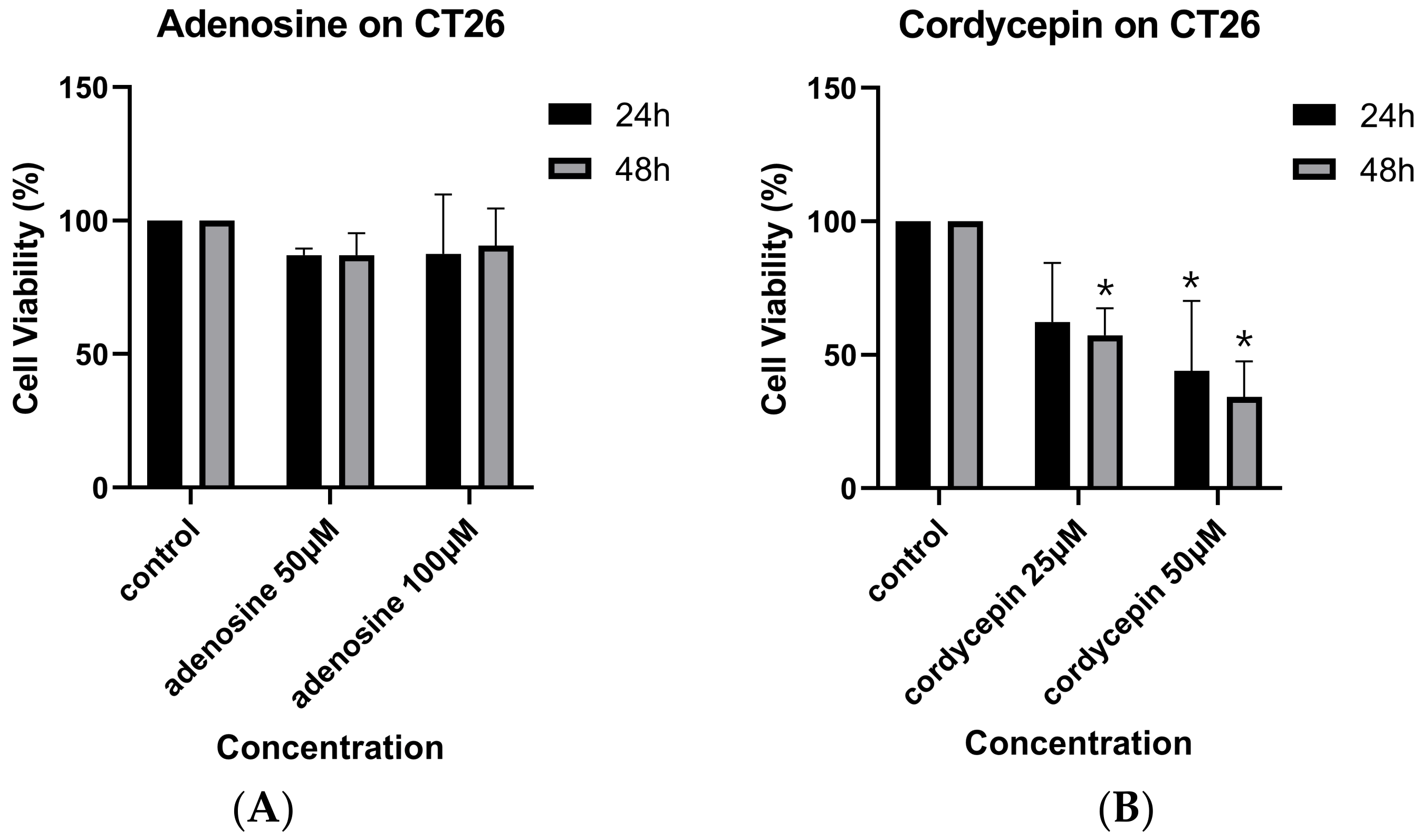
3.1. Cordycepin and Adenosine on Mouse Colorectal Carcinoma Cell Growth
3.2. Cordycepin and Adenosine on Cell Cycle of Mouse Colonrectal Carcinoma Cells
3.3. Cordycepin and Adenosine on Morphology of Colorectal Carcinoma Cells
3.4. Cordycepin and Adenosine on Adenosine Receptor mRNA Expression in Mouse Colorectal Carcinoma Cells
3.5. Cordycepin Inhibited Molecular Target Protein Expression of A2AR and A2BR in Mouse Colorectal Carcinoma Cells
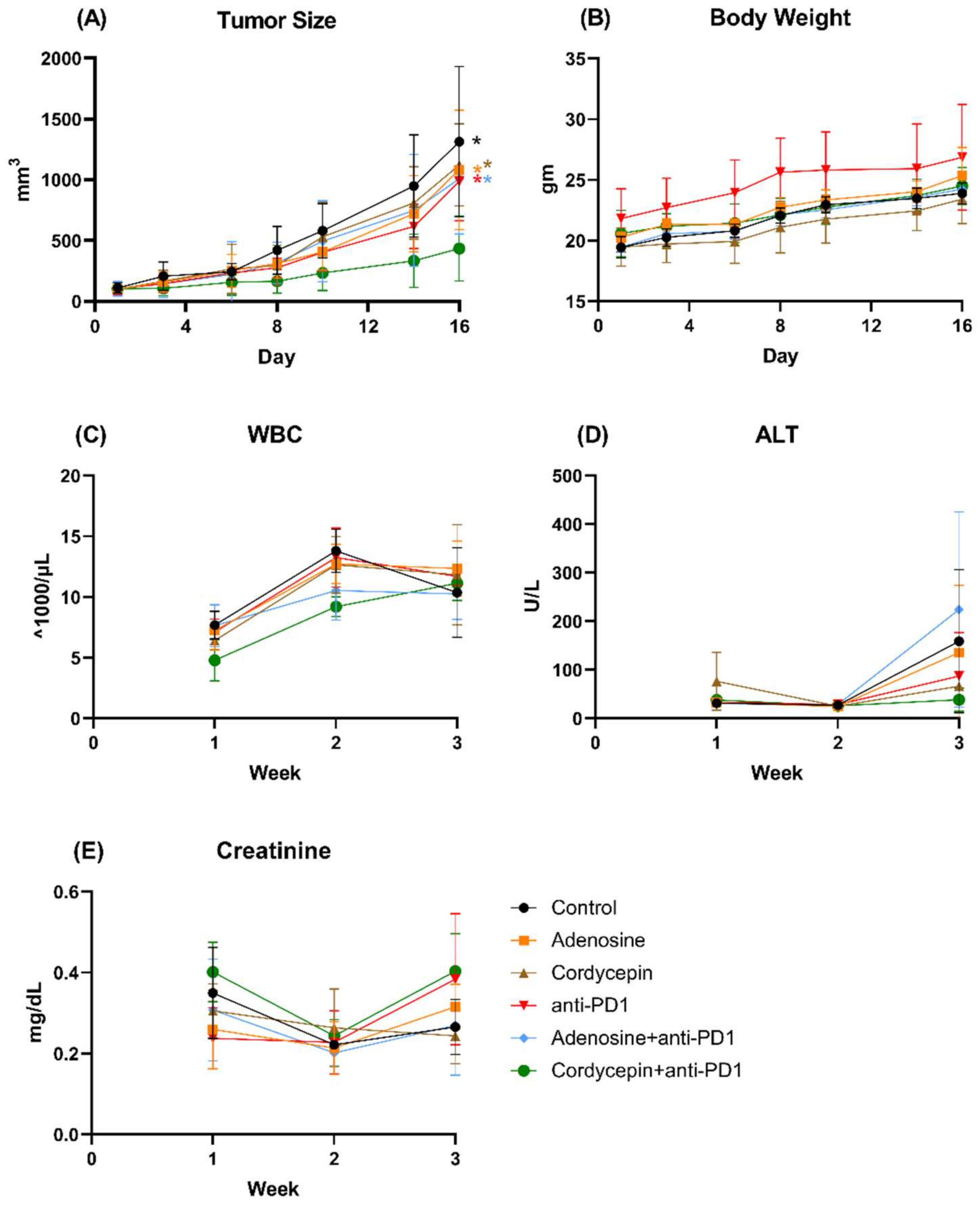
3.6. Combination of Cordycepin and Anti-PD1 against Syngeneic CT26 Implanted Tumor In Vivo
3.7. Analysis of Immune Cell Profiles in Tumor Microenvironment and Spleen In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, K.X.; Istl, A.C.; Quan, D.; Skaro, A.; Tang, E.; Zheng, X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: Challenges and strategies. Cancer Immunol. Immunother. 2023, 72, 3875–3893. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, J.; Reddy, S.; Verma, N.; Huang, E.H.; Kazmi, S.M. Immune Checkpoint Inhibitors in pMMR/MSS Colorectal Cancer. J. Gastrointest. Cancer 2023, 54, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; He, W.; Duan, Y.; Yao, Q.; Zhao, Y.; Fan, X.; Liu, S.; Chen, H.; He, F.; Liu, T.; et al. PD-1 blockade enhances the effect of targeted chemotherapy on locally advanced pMMR/MSS colorectal cancer. Cancer Med. 2024, 13, e7224. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.H.; Kim, T.I.; Kim, Y.J.; Pak, M.E.; Jeon, C.H.; Park, Y.J.; Li, W.; Kim, Y.S.; Choi, J.G.; et al. Sanguisorbae Radix Suppresses Colorectal Tumor Growth Through PD-1/PD-L1 Blockade and Synergistic Effect with Pembrolizumab in a Humanized PD-L1-Expressing Colorectal Cancer Mouse Model. Front. Immunol. 2021, 12, 737076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; He, W.; Zhou, X.; Lv, Q.; Xu, X.; Yang, S.; Zhao, C.; Guo, L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur. J. Pharmacol. 2011, 664, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hong, X.; Liu, L.; Wu, Y.; Xie, X.; Fang, G.; Zhi, S. Cordycepin Decreases Ischemia/Reperfusion Injury in Diabetic Hearts via Upregulating AMPK/Mfn2-dependent Mitochondrial Fusion. Front. Pharmacol. 2021, 12, 754005. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Wang, Z.L.; Qi, W.; Lei, W.; Zhao, G.Y. Cordycepin (3’-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation 2014, 37, 1044–1049. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jeong, Y.K.; Choi, Y.J.; Yang, K.M.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Park, C.; Jeong, J.W.; Kim, M.J.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, G.Y.; Choi, B.T.; Choi, Y.H.; et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen species-mediated mitochondrial death pathway. Int. J. Oncol. 2013, 42, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. 2010, 24, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Kunitomo, M.; Kagota, S.; Shinozuka, K.; Nakamura, K. Inhibitory effect of cordycepin on hematogenic metastasis of B16-F1 mouse melanoma cells accelerated by adenosine-5’-diphosphate. Anticancer Res. 2009, 29, 3857–3860. [Google Scholar] [PubMed]
- Chen, Y.H.; Wang, J.Y.; Pan, B.S.; Mu, Y.F.; Lai, M.S.; So, E.C.; Wong, T.S.; Huang, B.M. Cordycepin enhances cisplatin apoptotic effect through caspase/MAPK pathways in human head and neck tumor cells. Onco Targets Ther. 2013, 6, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Hu, Z.M.; Shang, M.J.; Zhao, D.J.; Zhang, C.W.; Hong, D.F.; Huang, D.S. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int. J. Mol. Sci. 2014, 15, 12778–12790. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin Suppresses the Malignant Phenotypes of Colon Cancer Cells through The GSK3ß/ß-catenin/cyclin D1 Signaling Pathway. Cell J. 2022, 24, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, Z.J.; Chang, Z. Single-cell RNA sequencing indicates cordycepin remodels the tumor immune microenvironment to enhance TIGIT blockade’s anti-tumor effect in colon cancer. Int. Immunopharmacol. 2024, 126, 111268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, K.; Zheng, Z.; Liu, Y. Cordycepin inhibits colon cancer proliferation by suppressing MYC expression. BMC Pharmacol. Toxicol. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Nishiuchi, A.; Kubo, E.; Yamaguchi, Y.; Kunitomo, M.; Kagota, S.; Shinozuka, K.; Nakamura, K. Cordyceps sinensis Acts as an Adenosine A3 Receptor Agonist on Mouse Melanoma and Lung Carcinoma Cells, and Human Fibrosarcoma and Colon Carcinoma Cells. Pharmacol. Pharm. 2011, 2, 5. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem. Biol. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef]
- Cao, H.L.; Liu, Z.J.; Chang, Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumor Biol. 2017, 39, 1010428317706915. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.; Bar-Yehuda, S.; Synowitz, M.; Powell, J.D.; Klotz, K.N.; Gessi, S.; Borea, P.A. Adenosine receptors and cancer. Handb. Exp. Pharmacol. 2009, 193, 399–441. [Google Scholar] [CrossRef]
- Wang, B.; Wang, T.; Yang, C.; Nan, Z.; Ai, D.; Wang, X.; Wang, H.; Qu, X.; Wei, F. Co-inhibition of adenosine 2b receptor and programmed death-ligand 1 promotes the recruitment and cytotoxicity of natural killer cells in oral squamous cell carcinoma. PeerJ 2023, 11, e15922. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.H.M.; Prieto-Díaz, R.; Neo, S.; Tong, L.; Chen, X.; Carannante, V.; Önfelt, B.; Hartman, J.; Haglund, F.; Majellaro, M.; et al. A(2B) adenosine receptor antagonists rescue lymphocyte activity in adenosine-producing patient-derived cancer models. J. Immunother. Cancer 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Beavis, P.A.; Milenkovski, N.; Henderson, M.A.; John, L.B.; Allard, B.; Loi, S.; Kershaw, M.H.; Stagg, J.; Darcy, P.K. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol. Res. 2015, 3, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.; Fang, C.; Li, X.; Zhang, J.; Xi, Q.; Li, Y.; Zhang, R. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharmacol. 2022, 107, 108695. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Zhao, X.; Sun, J.; Cheng, C.; Wang, C.; Tao, L.; Zong, L.; Yin, W.; Cong, J.; Li, J.; et al. A2AR limits IL-15-induced generation of CD39(+) NK cells with high cytotoxicity. Int. Immunopharmacol. 2023, 114, 109567. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fang, W.; Chen, L.; Feng, C.; Chen, R.; Ying, H.; Zheng, X.; Jiang, J. Cordycepin remodels the tumor microenvironment of colorectal cancer by down-regulating the expression of PD-L1. J. Cancer Res. Clin. Oncol. 2023, 149, 17567–17579. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Moon, S.; Park, Y.; Kwon, J.; Lee, S.; Lee, C.K.; Cho, K.; Ha, N.J.; Kim, K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009, 9, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jin, J.Y.; Zhang, B.; Liu, Z.Q.; Zheng, Y.G. Improvement of cordycepin production by an isolated Paecilomyces hepiali mutant from combinatorial mutation breeding and medium screening. Bioprocess Biosyst. Eng. 2021, 44. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-K.; Chen, Y.-T.; Lin, C.-P.; Wang, C.-J.; Shieh, H.-R.; Chi, C.-W.; Tsai, T.-H.; Chen, Y.-J. Cordycepin Augments the Efficacy of Anti-PD1 against Colon Cancer. Biomedicines 2024, 12, 1568. https://doi.org/10.3390/biomedicines12071568
Chang W-K, Chen Y-T, Lin C-P, Wang C-J, Shieh H-R, Chi C-W, Tsai T-H, Chen Y-J. Cordycepin Augments the Efficacy of Anti-PD1 against Colon Cancer. Biomedicines. 2024; 12(7):1568. https://doi.org/10.3390/biomedicines12071568
Chicago/Turabian StyleChang, Wen-Kuei, Yen-Ting Chen, Chin-Ping Lin, Chia-Jung Wang, Hui-Ru Shieh, Chih-Wen Chi, Tung-Hu Tsai, and Yu-Jen Chen. 2024. "Cordycepin Augments the Efficacy of Anti-PD1 against Colon Cancer" Biomedicines 12, no. 7: 1568. https://doi.org/10.3390/biomedicines12071568




