Changes of Target Essential Trace Elements in Multiple Sclerosis: A Systematic Review and Meta-Analysis
Abstract
1. Multiple Sclerosis and Trace Elements
2. Materials and Methods
2.1. Information Sources
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
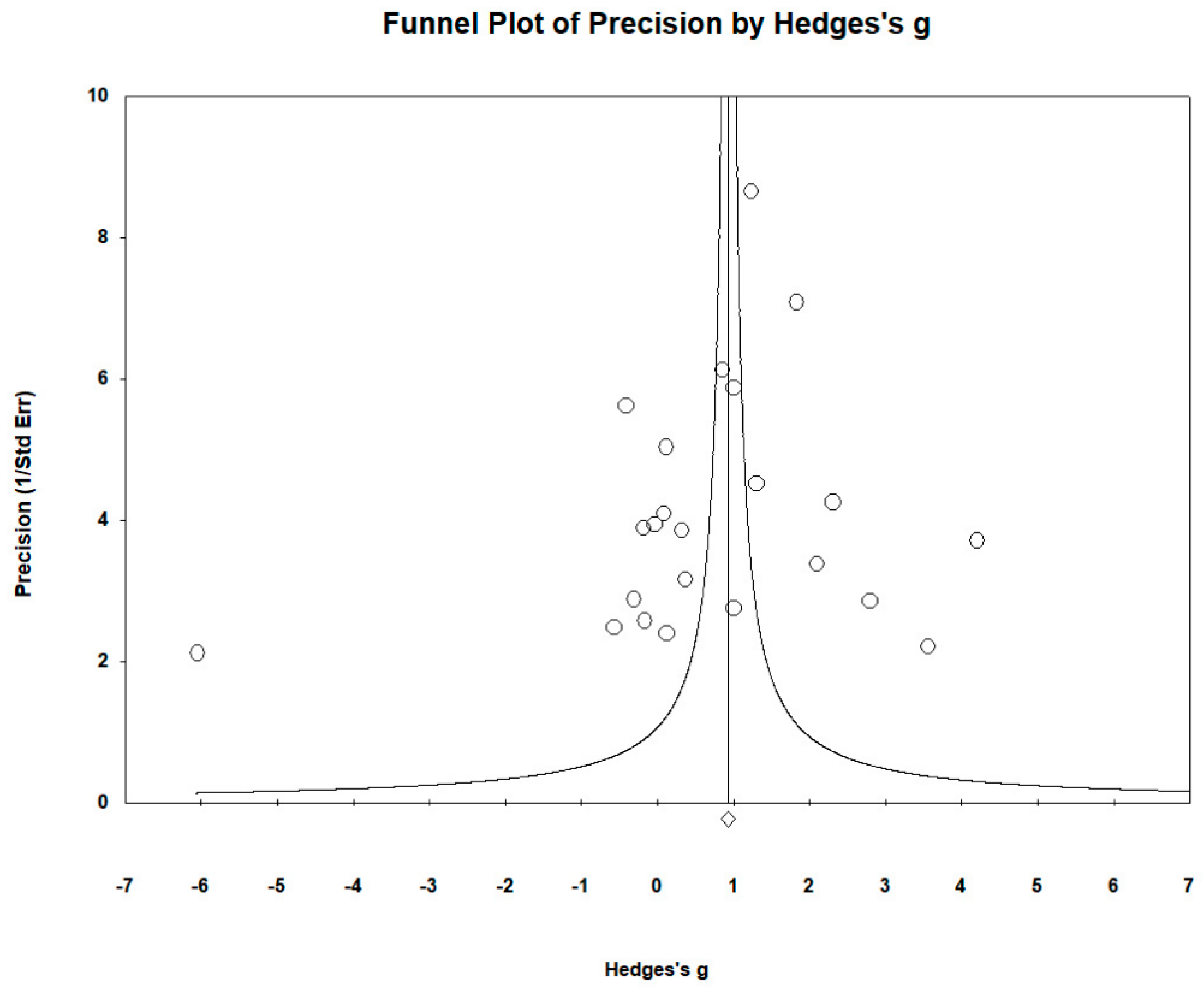
2.6. Publication Bias
2.7. Registration of Meta-Analysis
3. Results
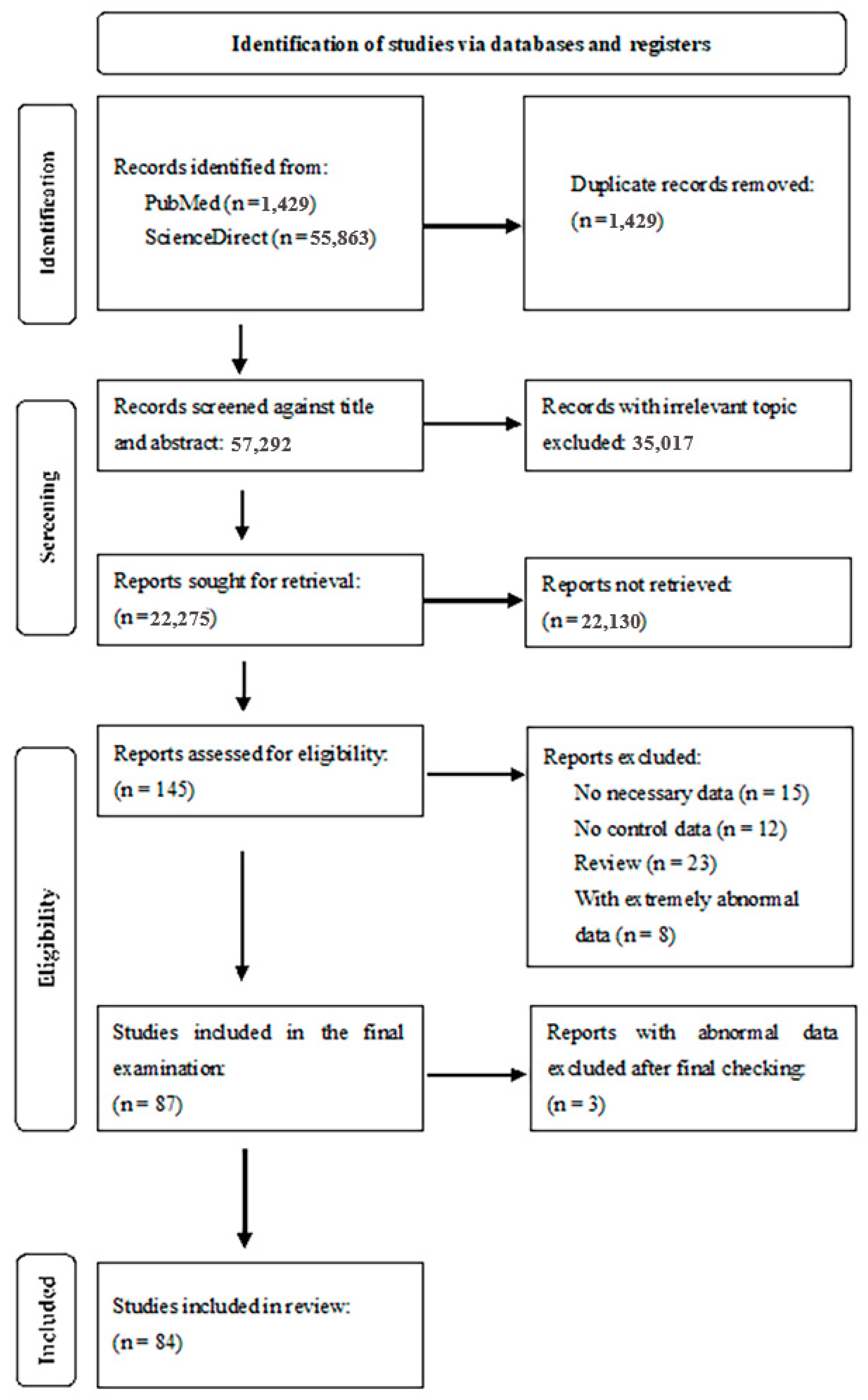
3.1. Selection and Identification of Studies
3.2. Characteristics of the Studies
3.3. Evaluation of the Quality Assessment
3.4. Meta-Analysis of Serum/Plasma Zn Levels
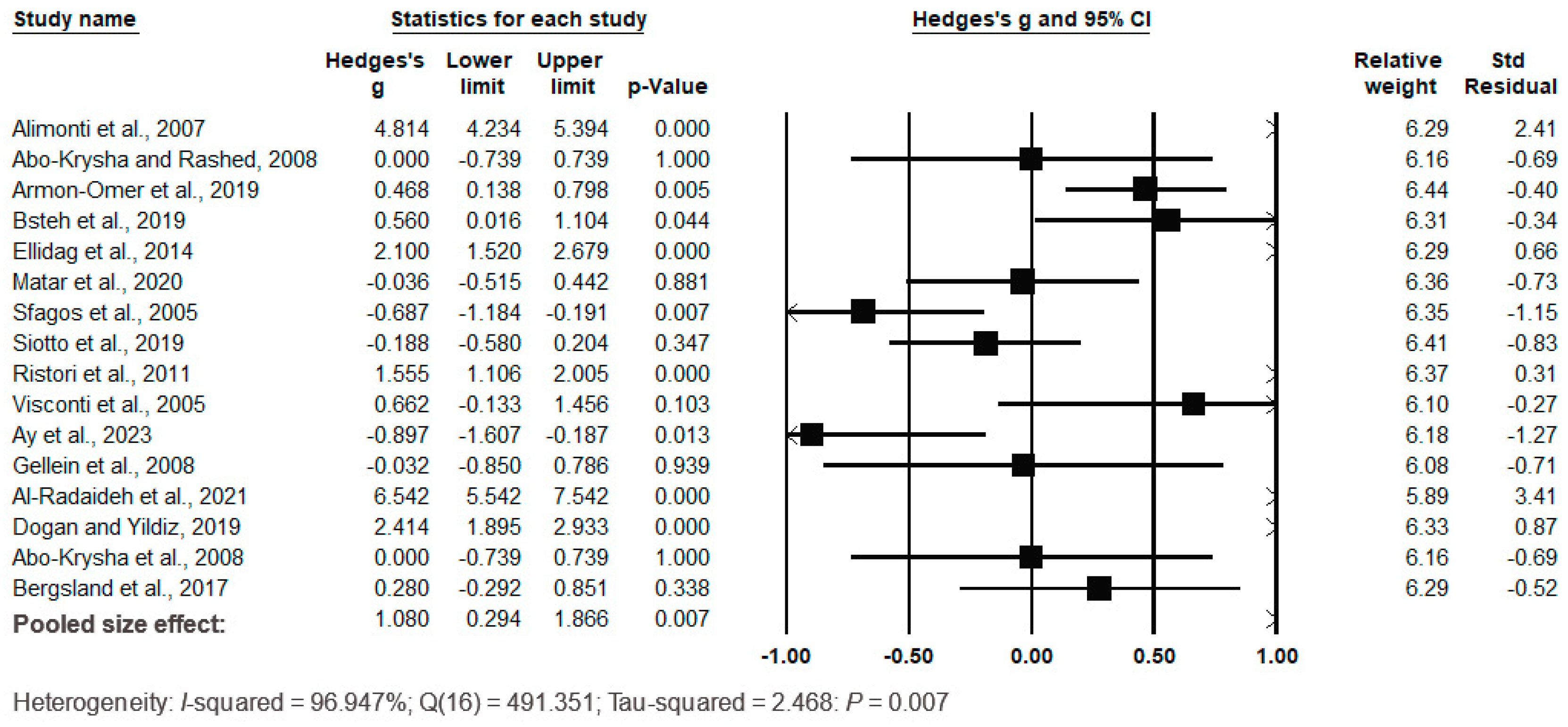
3.5. Meta-Analysis of Serum/Plasma Fe Levels
3.6. Meta-Analysis of the Serum/Plasma Co Levels
3.7. Meta-Analysis of Serum/Plasma Cu Levels
3.8. Meta-Analysis of Mn Serum/Plasma Levels
3.9. Meta-Analysis of Serum/Plasma Se Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filippi, M.; Rocca, M.A. Multiple Sclerosis. In White Matter Diseases; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Xixis, Multiple Sclerosis, StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499849/ (accessed on 15 April 2024.).
- Di Ruscio, A.; Patti, F.; Welner, R.S.; Tenen, D.G.; Amabile, G. Multiple sclerosis: Getting personal with induced pluripotent stem cells. Cell Death Dis. 2015, 6, e1806. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of Neurodegeneration and Axonal Dysfunction in Progressive Multiple Sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Kammeyer, R.; Gluck, L.; Schreiner, T.; Makhani, N. Multiple Sclerosis in Children: Current and Emerging Concepts. Semin. Neurol. 2020, 40, 192–200. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Schaeffer, J.; Cossetti, C.; Mallucci, G.; Pluchino, S. Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders, Chapter 30—Multiple Sclerosis; Elsevier: Cambridge, MA, USA, 2015; pp. 497–520. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- Kaymakamzade, B.; Kilic, A.K.; Kurne, A.T.; Karabudak, R. Progressive Onset Multiple Sclerosis: Demographic, Clinical and Laboratory Characteristics of Patients with and without Relapses in the Course. Noro Psikiyatr. Arsivi 2019, 56, 23–26. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar] [PubMed] [PubMed Central]
- Omerhoca, S.; Akkas, S.Y.; Icen, N.K. Multiple sclerosis: Diagnosis and Differrential Diagnosis. Noro Psikiyatr. Arsivi 2018, 55, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ford, H. Clinical presentation and diagnosis of multiple sclerosis. Clin. Med. 2020, 20, 380–383. [Google Scholar] [CrossRef]
- Alonso, R.; Contentti, E.C.; Imhoff, G.; Lopez, P.A.; Rubstein, A.; Tizio, S. Barriers against a successful MS treatment: The importance of effectiveness beyond efficacy. Mult. Scler. Relat. Disord. 2019, 30, 129–135. [Google Scholar] [CrossRef]
- Eva, L.; Pleș, H.; Covache-Busuioc, R.-A.; Glavan, L.A.; Bratu, B.-G.; Bordeianu, A.; Dumitrascu, D.-I.; Corlatescu, A.D.; Ciurea, A.V. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines 2023, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Hachim, M.Y.; Elemam, N.M.; Maghazachi, A.A. The Beneficial and Debilitating Effects of Environmental and Microbial Toxins, Drugs, Organic Solvents and Heavy Metals on the Onset and Progression of Multiple Sclerosis. Toxins 2019, 11, 147. [Google Scholar] [CrossRef]
- Moradi, A.; Honarjoo, N.; Besalatpour, A.A.; Etemadifar, M. Human exposure to dust and heavy metals in industrial regions and its relationship with the prevalence of multiple sclerosis disease. Environ. Monit. Assess. 2023, 195, 471. [Google Scholar] [CrossRef]
- Sarihi, S.; Niknam, M.; Mahjour, S.; Hosseini-Bensenjan, M.; Moazzen, F.; Soltanabadi, S.; Akbari, H. Toxic heavy metal concentrations in multiple sclerosis patients: A systematic review and meta-analysis. EXCLI J. 2021, 20, 1571–1584. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. NeuroToxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Wei, R.; Yong, V.W.; Xue, M. The Important Role of Zinc in Neurological Diseases. Biomolecules 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, S.; Gu, J.; Yu, L. Selenium and human nervous system. Chin. Chem. Lett. 2023, 34, 108043. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Krukow, P.; Jonak, K.; Maciejewski, M.; Szczepanek, D.; Jonak, K.; Flieger, J.; Maciejewski, R. Analysis of Trace Elements in Human Brain: Its Aim, Methods, and Concentration Levels. Front. Chem. 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.; Nathoo, N.; Mahjoub, Y.; Dunn, J.F.; Yong, V.W. Iron in multiple sclerosis: Roles in neurodegeneration and repair. Nat. Rev. Neurol. 2014, 10, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.W.; Bini, S.A. Neurologic Dysfunction Associated With Mechanically Assisted Crevice Corrosion and Elevated Cobalt Ion Levels After Total Hip Arthroplasty. Arthroplast. Today 2021, 11, 217–221. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Jagodić, J.; Pavlović, S.; Dinčić, E.; Kuveljić, J.; Manojlović, D.; Živković, M. Essential trace element levels in multiple sclerosis: Bridging demographic and clinical gaps, assessing the need for supplementation. J. Trace Elem. Med. Biol. 2024, 83, 127421. [Google Scholar] [CrossRef]
- Palm, R.; Hallmans, G. Zinc and copper in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1982, 45, 691–698. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Catalanotto, F.; Donaldson, J.O.; Ostrom, K.M.; Testa, M.A. Zinc in muliple sclerosis. Ann. Neurol. 1983, 14, 450–454. [Google Scholar] [CrossRef]
- Ho, S.; Catalanotto, F.A.; Lisak, R.P.; Dore-Duffy, P. Zinc in multiple sclerosis. II: Correlation with disease activity and elevated plasma membrane–bound zinc in erythrocytes from patients with multiple sclerosis. Ann. Neurol. 1986, 20, 712–715. [Google Scholar] [CrossRef]
- Kapaki, E.; Segditsa, J.; Papageorgiou, C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol. Scand. 1989, 79, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Feldman, E.B.; Feldman, D.S. Trace element status in multiple sclerosis. Am. J. Clin. Nutr. 1989, 50, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Masoud, S.A.; Fakharian, E. Assessment of serum magnesium, copper, and zinc levels in multiple sclerosis (MS) patients. Psychiatry Behav. Sci. 2007, 1, 38–42. [Google Scholar]
- Gellein, K.; Skogholt, J.H.; Aaseth, J.; Thoresen, G.B.; Lierhagen, S.; Steinnes, E.; Syversen, T.; Flaten, T.P. Trace elements in cerebrospinal fluid and blood from patients with a rare progressive central and peripheral demyelinating disease. J. Neurol. Sci. 2008, 266, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Harbige, L.S.; Pinto, E.; Xiang, M.; Shareif, M.K. Circulating plasma cytokines, zinc, copper, vitamins A and E in multiple sclerosis patients and healthy controls. Proc. Nutr. Soc. 2011, 70, E36. [Google Scholar] [CrossRef]
- Ghazavi, A.; Kianbakht, S.; Ghasami, K.; Mosayebi, G. High copper and low zinc serum levels in Iranian patients with multiple sclerosis: A case control study. Clin. Lab. 2012, 58, 161–164. [Google Scholar] [PubMed]
- Al-Zubaidi, M.A. The effect of interferon beta-1b and methylprednisolone treatment on the serum trace elements in Iraqi patients with multiple sclerosis. J. Clin. Diagn. Res. 2012, 6, 994–998. [Google Scholar]
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy metals and neurodegenerative diseases: An observational study. Biol. Trace Elem. Res. 2014, 161, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, A.; Mohseni, M.; Amraei, R.; Alizadeh, A.; Mazloomzadeh, S. Investigation the amount of copper, lead, zinc and cadmium levels in serum of Iranian multiple sclerosis patients. J. Chem. Pharm. Sci. 2015, 8, 40–45. [Google Scholar]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. NeuroToxicology 2007, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Matar, A.; Jennani, S.; Abdallah, H.; Mohsen, N.; Borjac, J. Serum iron and zinc levels in Lebanese multiple sclerosis patients. Acta Neurol. Taiwanica 2020, 29, 5–11. [Google Scholar] [PubMed]
- Mezzaroba, L.; Simão, A.N.C.; Oliveira, S.R.; Flauzino, T.; Alfieri, D.F.; Pereira, W.L.d.C.J.; Kallaur, A.P.; Lozovoy, M.A.B.; Kaimen-Maciel, D.R.; Maes, M.; et al. Antioxidant and Anti-inflammatory Diagnostic Biomarkers in Multiple Sclerosis: A Machine Learning Study. Mol. Neurobiol. 2020, 57, 2167–2178. [Google Scholar] [CrossRef]
- Oraby, M.I.; Hussein, M.; Elkareem, R.A.; Elfar, E. The emerging role of serum zinc in motor disability and radiological findings in patients with multiple sclerosis. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 60. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Uebelhör, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower serum zinc levels in patients with multiple sclerosis compared to healthy controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Karpińska, E.; Kochanowicz, J.; Soroczyńska, J.; Jakoniuk, M.; Wilkiel, M.; Mariak, Z.D.; Borawska, M.H. Dietary habits; concentration of copper, zinc, and Cu-to-Zn ratio in serum and ability status of patients with relapsing-remitting multiple sclerosis. Nutrition 2017, 39–40, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ristori, G.; Brescianini, S.; Pino, A.; Visconti, A.; Vittori, D.; Coarelli, G.; Cotichini, R.; Bocca, B.; Forte, G.; Pozzilli, C.; et al. Serum elements and oxidative status in clinically isolated syndromes: Imbalance and predictivity. Neurology 2011, 76, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Cotichini, R.; Cannoni, S.; Bocca, B.; Forte, G.; Ghazaryan, A.; Santucci, S.; D’Ippolito, C.; Stazi, M.A.; Salvetti, M.; et al. Concentration of elements in serum of patients affected by multiple sclerosis with first demyelinating episode: A six-month longitudinal follow-up study. Ann. Dell'istituto Super. Sanita 2005, 41, 217–222. [Google Scholar]
- Ay, A.; Alkanli, N.; Atli, E.; Gurkan, H.; Gulyasar, T.; Guler, S.; Sipahi, T.; Sut, N. Investigation of Relationship Between Small Noncoding RNA (sncRNA) Expression Levels and Serum Iron, Copper, and Zinc Levels in Clinical Diagnosed Multiple Sclerosis Patients. Mol. Neurobiol. 2023, 60, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Nashmi, A.D.; Hassan, A.F.; Hammad, M.M. Estimation the Level of Metals (Lead, Cadmium, Copper and Zinc) In Multiple Sclerosis Patients in Basra\Iraq. Indian J. Forensic Med. Toxicol. 2020, 14, 1029–1035. [Google Scholar] [CrossRef]
- Abo-Krysha, N.; Rashed, L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: An Egyptian study. Mult. Scler. J. 2008, 14, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New insights on the nutrition status and antioxidant capacity in Multiple Sclerosis patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Haschka, D.; Tymoszuk, P.; Berek, K.; Petzer, V.; Hegen, H.; Wurth, S.; Auer, M.; Zinganell, A.; Di Pauli, F.; et al. Serum hepcidin levels in multiple sclerosis. Mult. Scler. J.—Exp. Transl. Clin. 2019, 5, 2055217319885984. [Google Scholar] [CrossRef]
- Ellidag, H.Y.; Kurtulus, F.; Yaman, A.; Eren, E.; Yılmaz, N.; Aydin, O.; Bayındır, A. Serum iron metabolism markers including hepcidin in multiple sclerosis patients. Neurochem. J. 2014, 8, 226–230. [Google Scholar] [CrossRef]
- Sfagos, C.; Makis, A.C.; Chaidos, A.; Hatzimichael, E.C.; Dalamaga, A.; Kosma, K.; Bourantas, K.L. Serum ferritin, transferrin and soluble transferrin receptor levels in multiple sclerosis patients. Mult. Scler. J. 2005, 11, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Filippi, M.M.; Simonelli, I.; Landi, D.; Ghazaryan, A.; Vollaro, S.; Ventriglia, M.; Pasqualetti, P.; Rongioletti, M.C.A.; Squitti, R.; et al. Oxidative stress related to iron metabolism in relapsing remitting multiple sclerosis patients with low disability. Front. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- Al-Radaideh, A.; El-Haj, N.; Hijjawi, N. Iron deposition and atrophy in cerebral grey matter and their possible association with serum iron in relapsing-remitting multiple sclerosis. Clin. Imaging 2021, 69, 238–242. [Google Scholar] [CrossRef]
- Doğan, H.O.; Yildiz, Ö.K. Serum NADPH oxidase concentrations and the associations with iron metabolism in relapsing remitting multiple sclerosis. J. Trace Elem. Med. Biol. 2019, 55, 39–43. [Google Scholar] [CrossRef]
- Bergsland, N.; Agostini, S.; Laganà, M.M.; Mancuso, R.; Mendozzi, L.; Tavazzi, E.; Cecconi, P.; Clerici, M.; Baglio, F. Serum iron concentration is associated with subcortical deep gray matter iron levels in multiple sclerosis patients. NeuroReport 2017, 28, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Visconti, A.; Santucci, S.; Ghazaryan, A.; Figà-Talamanca, L.; Cannoni, S.; Bocca, B.; Pino, A.; Violante, N.; Alimonti, A.; et al. Quantification of chemical elements in blood of patients affected by multiple sclerosis. Ann. Dell'istituto Super. Sanita 2005, 41, 213–216. [Google Scholar] [PubMed]
- De Riccardis, L.; Buccolieri, A.; Muci, M.; Pitotti, E.; De Robertis, F.; Trianni, G.; Manno, D.; Maffia, M. Copper and ceruloplasmin dyshomeostasis in serum and cerebrospinal fluid of multiple sclerosis subjects. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, F.; Bakhsgi, H.; Akbaripoor, A. Serum Iron and Ferritin in Patients with Multiple Sclerosis. Zahedan J. Res. Med. Sci. 2013, 15, e93005. [Google Scholar]
- Janghorbani, M.; Shaygannejad, V.; Hakimdavood, M.; Salari, M. Trace Elements in Serum Samples of Patients with Multiple Sclerosis. Athens J. Health 2017, 4, 145–154. [Google Scholar] [CrossRef]
- Rieder, H.; Schoettli, G.; Seiler, H. Trace Elements in Whole Blood of Multiple Sclerosis. Eur. Neurol. 1983, 22, 85–92. [Google Scholar] [CrossRef]
- Jensen, G.E.; Gissel-Nielsen, G.; Clausen, J. Leucocyte glutathione peroxidase activity and selenium level in multiple sclerosis. J. Neurol. Sci. 1980, 48, 61–67. [Google Scholar] [CrossRef]
- Korpela, H.; Kinnunen, E.; Juntunen, J.; Kumpulainen, J.; Koskenvuo, M. Serum selenium concentration, glutathione peroxidase activity and lipid peroxides in a co-twin control study on multiple sclerosis. J. Neurol. Sci. 1989, 91, 79–84. [Google Scholar] [CrossRef]
- Mazzella, G.; Sinforiani, E.; Savoldi, F.; Allegrini, M.; Lanzola, E.; Scelsi, R. Blood cells glutathione peroxidase activity and selenium in multiple sclerosis. Eur. Neurol. 1983, 22, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 2014, 13, 62. [Google Scholar] [CrossRef]
- Goli, Z.; Mashayekhi, S.; Mazdeh, M.; Taheri, M.; Zafari, S.; Safari, M.R. Comparison of Selenium Serum Levels in Patients WithMultiple Sclerosis and Normal Individuals. Avicenna J. Care Health Oper. Room 2023, 1, 7–10. [Google Scholar] [CrossRef]
- Wikström, J.; Westermarcik, T.; Palo, J. Selenium, Vitamin E and Copper in Multiple Sclerosis. Acta Neurol. Scand. 1976, 54, 287–290. [Google Scholar] [CrossRef]
- Nakhaee, S.; Amirabadizadeh, A.; Farnia, V.; Azadi, N.A.; Mansouri, B.; Radmehr, F. Association between biological lead concentrations and autism spectrum disorder (ASD) in children: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2023, 201, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009. [Google Scholar]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration Version 5.1.0. 2011. (Updated March 2011). The Cochrane Collaboration. Available online: www.cochrane-handbook.org (accessed on 13 September 2013).
- Mata, D.A.; Ramos, M.A.; Bansal, N.; Khan, R.; Guille, C.; Di Angelantonio, E.; Sen, S. Prevalence of depression and depressivesymptoms among resident physicians: A systematic review and meta-analysis. JAMA 2015, 314, 2373–2383. [Google Scholar] [CrossRef]
- Peeters, W.; Brande, R.v.D.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I.R. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Lakićević, N.; Pavlović, S. Mercury and Autism Spectrum Disorder: Exploring the Link through Comprehensive Review and Meta-Analysis. Biomedicines 2023, 11, 3344. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Jian, B.; Wu, C.; Jiang, C.-Z.; Kang, Y.; Zhou, J.-X.; Yang, F.; Liang, Y. A comparison of blood metal levels in autism spectrum disorder and unaffected children in Shenzhen of China and factors involved in bioaccumulation of metals. Environ. Sci. Pollut. Res. 2018, 25, 17950–17956. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hong, D.K.; Choi, B.Y.; Suh, S.W. Zinc in the Brain: Friend or Foe? Int. J. Mol. Sci. 2020, 21, 8941. [Google Scholar] [CrossRef] [PubMed]
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, J.R.; Oteiza, P.I. Zinc and the aging brain. Genes Nutr. 2014, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 54272. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liu, M.; Yang, Y.; Wang, R.; Chen, S.; Liu, Z.; Yan, F.; Chen, X.; Bi, J.; et al. Alleviating effects of zinc and 24-epibrassionlide on cadmium accumulation in rice plants under nitrogen application. Chemosphere 2023, 313, 137650. [Google Scholar] [CrossRef]
- Elitt, C.M.; Fahrni, C.J.; Rosenberg, P.A. Zinc homeostasis and zinc signaling in white matter development and injury. Neurosci. Lett. 2019, 707, 134247. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, M.; Frederiksen, J.L. Zinc in Multiple Sclerosis: A Systematic Review and Meta-Analysis. ASN Neuro 2016, 8, 1759091416651511. [Google Scholar] [CrossRef] [PubMed]
- Nirooei, E.; Kashani, S.M.A.; Owrangi, S.; Malekpour, F.; Niknam, M.; Moazzen, F.; Nowrouzi-Sohrabi, P.; Farzinmehr, S.; Akbari, H. Blood Trace Element Status in Multiple Sclerosis: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 200, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, M.M.; Haapasalo, J.; Haapasalo, H.; E Fleming, R.; Britton, R.S.; Bacon, B.R.; Parkkila, S. Expression of iron-related genes in human brain and brain tumors. BMC Neurosci. 2009, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Neves, P.; Gozzelino, R. Multilevel Impacts of Iron in the Brain: The Cross Talk between Neurophysiological Mechanisms, Cognition, and Social Behavior. Pharmaceuticals 2019, 12, 126. [Google Scholar] [CrossRef]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.; Brandenburg, V.; Rocca, H.-P.B.-L. How to diagnose iron deficiency in chronic disease: A review of current methods and potential marker for the outcome. Eur. J. Med. Res. 2013, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, E.; Marley, A.; A Samaan, M.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Nawaz, A.; Khattak, N.N.; Khan, M.S.; Nangyal, H.; Sabri, S.; Shakir, M. Deficiency of vitamin B12 and its relation with neurological disorders: A critical review. J. Basic Appl. Zool. 2020, 81, 10. [Google Scholar] [CrossRef]
- Khosravi-Largani, M.; Pourvali-Talatappeh, P.; Rousta, A.M.; Karimi-Kivi, M.; Noroozi, E.; Mahjoob, A.; Asaadi, Y.; Shahmohammadi, A.; Sadeghi, S.; Shakeri, S.; et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci 2018, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, C.S.; Damulin, I.V.; O Shulpekova, Y.; A Andreev, E. Neurological disorders in vitamin B12 deficiency. Ter. arkhiv 2019, 91, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, Z.-Y.; Liu, H.-N. Meta-analysis of the relationship between homocysteine, vitamin B12, folate, and multiple sclerosis. J. Clin. Neurosci. 2011, 18, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Arseniou, S.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.-F.A.; Michalopoulou, A.; Andravizou, A.; Dastamani, M.; Paterakis, K.; et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2017, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Washington-Hughes, C.; Ralle, M.; Schmidt, K. Copper and the brain noradrenergic system. JBIC J. Biol. Inorg. Chem. 2019, 24, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, S.; Huang, X.; Chen, X.; Shan, H.; Zhang, M. The Role of Copper Homeostasis in Brain Disease. Int. J. Mol. Sci. 2022, 23, 13850. [Google Scholar] [CrossRef] [PubMed]
- Dales, J.-P.; Desplat-Jégo, S. Metal Imbalance in Neurodegenerative Diseases with a Specific Concern to the Brain of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.M.; Larsen, C.; White, L.R.; Aasly, J.; Sjobakk, T.E.; Flaten, T.P.; Sonnewald, U.; Syversen, T. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biol. Trace Elem. Res. 2003, 93, 1–8. [Google Scholar] [CrossRef]
- Sarmadi, M.; Bidel, Z.; Najafi, F.; Ramakrishnan, R.; Teymoori, F.; Zarmehri, H.A.; Nazarzadeh, M. Copper concentration in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 45, 102426. [Google Scholar] [CrossRef]
- Ye, Q.; Park, J.E.; Gugnani, K.; Betharia, S.; Pino-Figueroa, A.; Kim, J. Influence of iron metabolism on manganese transport and toxicity. Metallomics 2017, 9, 1028–1046. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical Regional Differences in Selenium Levels in the Human Brain. Biol. Trace Elem. Res. 2015, 163, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.; Drobyshev, E.; Blume, B.; Michalke, B. Selenium at the Neural Barriers: A Review. Front. Neurosci. 2021, 15, 630016. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2022, 200, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef]
- Shreenath, A.P.; Hashmi, M.F.; Dooley, J. Selenium Deficiency. [Updated 2023 Oct 29]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482260/ (accessed on 10 April 2024).
- Zhou, J.; Zhang, W.; Cao, Z.; Lian, S.; Li, J.; Nie, J.; Huang, Y.; Zhao, K.; He, J.; Liu, C. Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis. Nutrients 2023, 15, 3706. [Google Scholar] [CrossRef]



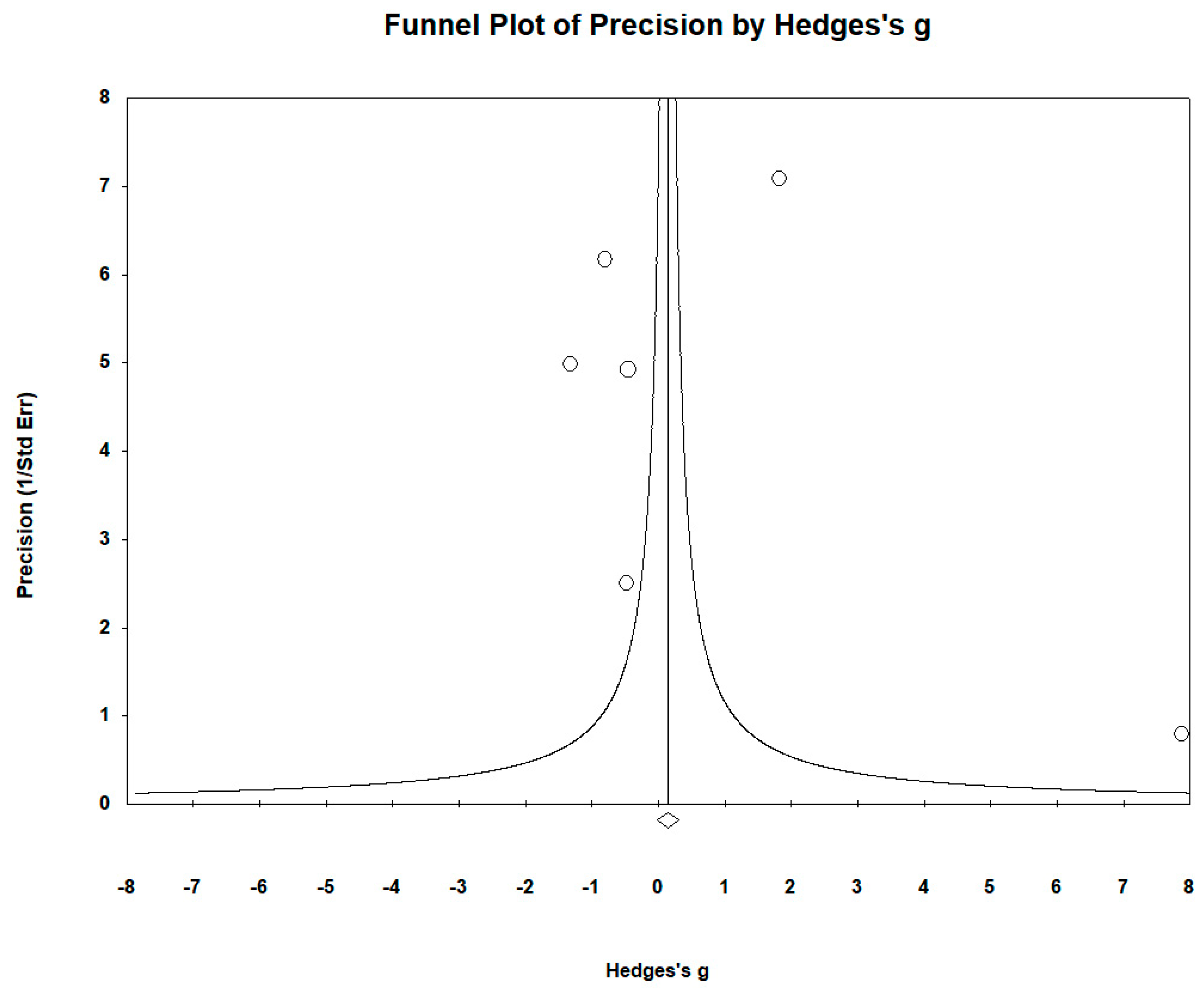

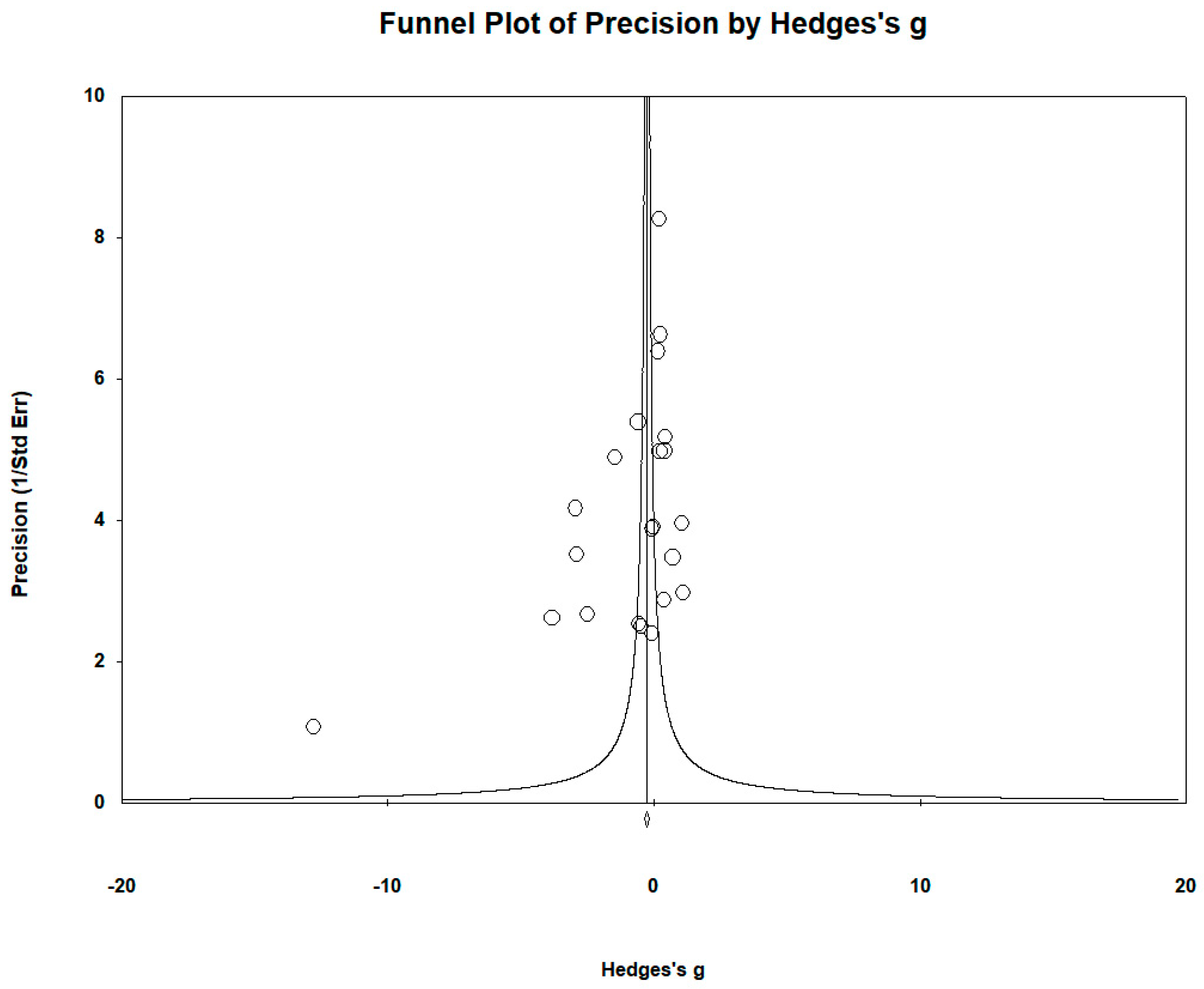




| Σ | Study | Element | Type of Study | Country | Sample Size Cases/Controls | Mean Age Cases/Controls | Sex: Female/Male; Cases/Controls | Biological Matrix | Element Level µg/L (Mean ± SD): Cases/Controls | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 1. | Stojsavljević et al., 2024 [37] | Zn | Case–control | Serbia | 215/100 | 43 ± 10/42 ± 9 | 120/95; 66/34 | Serum | 725 ± 250/1827 ± 1003 |
| 2. | 2. | Palm and Hallmans, 1982 [38] | Zn | Case–control | Sweden | 50/50 | N.A./N.A. | 29/21; 29/21 | Serum | 850 ± 124/863 ± 105 |
| 3. | 3. | Dore-Duffy et al., 1983 [39] | Zn | Case–control | USA | 68/60 | N.A./N.A. | 50/18; 30/30 | Plasma | 843 ± 157/785 ± 118 |
| 4. | 4. | Ho et al., 1986 [40] | Zn | Case–control | USA | 45/23 | N.A./N.A. | N.A./N.A. | Plasma | 889 ± 203/883 ± 98 |
| 5. | 5. | Kapaki et al., 1989 [41] | Zn | Case–control | Greece | 15/28 | 34 ± 10/46 ± 14 | 3/12; 10/18 | Serum | 1033 ± 137/1098 ± 190 |
| 6. | 6. | Smith et al., 1989 [42] | Zn | Cohort | USA | 27/33 | N.A./N.A. | 17/10; 11/22 | Plasma | 1026 ± 196/1000 ± 70 |
| 7. | 7. | Masoud and Fakharian, 2007 [43] | Zn | Cohort | Iran | 35/35 | 32 ± 7/35 ± 5 | 28/7; 28/7 | Serum | 856 ± 137/1098 ± 85 |
| 8. | 8. | Gellein et al., 2008 [44] | Zn | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 333 ± 46/340 ± 59 |
| 9. | 9. | Harbige et al., 2011 [45] | Zn | Case–control | UK | 21/9 | N.A./N.A. | N.A./N.A. | Plasma | 883 ± 163/856 ± 131 |
| 10. | 10. | Ghazavi et al., 2012 [46] | Zn | Case–control | Iran | 60/60 | N.A./N.A. | 43/17; 38/22 | Serum | 399 ± 321/1275 ± 425 |
| 11. | 11. | Al-Zubaidi, 2012 [47] | Zn | Case–control | Iraq | 32/32 | 32 ± 7/N.A. | 24/8; N.A. | Serum | 1066 ± 216/1504 ± 33 |
| 12. | 12. | Giacoppo et al., 2014 [48] | Zn | Case–control | Italy | 41/23 | 41 ± 2/35 ± 2 | 31/10; 14/9 | Plasma | 2615 ± 628/2811 ± 569 |
| 13. | 13. | Ghoreishi et al., 2015 [49] | Zn | Case–control | Iran | 50/50 | 32 ± 3/32 ± 3 | N.A./N.A. | Serum | 720 ± 39/460 ± 46 |
| 14. | 14. | Alimonti et al., 2007 [50] | Zn | Case–control | Italy | 60/124 | 39 ± 10/45 ± 13 | 38/22; 43/81 | Serum | 650 ± 25/795 ± 38 |
| 15. | 15. | Matar et al., 2020 [51] | Zn | Case–control | Lebanon | 27/42 | 43 ± 13/38 ± 16 | 14/13; 29/13 | Serum | 806 ± 152/820 ± 176 |
| 16. | 16. | Mezzaroba et al., 2020 [52] | Zn | Case–control | Brazil | 174/182 | 42 ± 13/40 ± 10 | 121/53; 128/54 | Serum | 1090 ± 254/1540 ± 442 |
| 17. | 17. | Oraby et al., 2019 [53] | Zn | Case–control | Egypt | 25/12 | 31 ± 9/29 ± 7 | 21/4; 14/11 | Serum | 653 ± 169/822 ± 155 |
| 18. | 18. | Pawlitzki et al., 2018 [54] | Zn | Cohort | Germany | 151/50 | 43 ± 12/43 ± 14 | 113/38; 38/12 | Serum | 817 ± 137/955 ± 137 |
| 19. | 19. | Socha et al., 2017 [55] | Zn | Case–control | Poland | 101/68 | 41 ± 10/40 ± 13 | 64/37; 47/21 | Serum | 776 ± 195/992 ± 315 |
| 20. | 20. | Ristori et al., 2011 [56] | Zn | Case–control | Italy | 49/49 | 36 ± 7/33 ± 6 | 29/20; 26/23 | Serum | 655 ± 83/808 ± 142 |
| 21. | 21. | Visconti et al., 2005 [57] | Zn | Case–control | Italy | 12/12 | 28 ± 8/28 ± 9 | 8/4; 7/5 | Serum | 864 ± 160/781 ± 120 |
| 22. | 22. | Ay et al., 2023 [58] | Zn | Case–control | Türkiye | 16/16 | 45 ± 7/44 ± 12 | 9/7; 11/5 | Serum | 1439 ± 568/1292 ± 316 |
| 23. | 23. | Nashmi et al., 2020 [59] | Zn | Case–control | Iraq | 25/25 | 42 ± 9/41 ± 10 | 15/10; 14/11 | Serum | 667 ± 76/933 ± 71 |
| 24. | 1. | Alimonti et al., 2007 [50] | Fe | Case–control | Italy | 60/124 | 39 ± 10/45 ± 13 | 38/22; 43/81 | Serum | 936 ± 117/1610 ± 149 |
| 25. | 2. | Abo-Krysha and Rashed, 2008 [60] | Fe | Case–control | Egypt | 20/10 | 30 ± 9/N.A. | Only females | Serum | 595 ± 31/595 ± 47 |
| 26. | 3. | Armon-Omer et al., 2019 [61] | Fe | Cross-sectional | Israel | 63/83 | 45 ± 14/41 ± 12 | 42/21; 49/34 | Serum | 627 ± 353/787 ± 330 |
| 27. | 4. | Bsteh et al., 2019 [62] | Fe | Case–control | Austria | 71/16 | 46 ± 8/32 ± 12 | N.A./N.A. | Serum | 857 ± 325/1070 ± 560 |
| 28. | 5. | Ellidag et al., 2014 [63] | Fe | Case–control | Türkiye | 35/35 | 38 ± 11/38 ± 10 | 15/20; 22/13 | Serum | 673 ± 85/816 ± 43 |
| 29. | 6. | Matar et al., 2020 [51] | Fe | Case–control | Lebanon | 27/42 | 43 ± 13/38 ± 16 | 14/13; 29/13 | Serum | 847 ± 351/833 ± 397 |
| 30. | 7. | Sfagos et al., 2005 [64] | Fe | Case–control | Greece | 27/40 | 38 ± 6/N.A. | 17/10; N.A. | Serum | 1669 ± 478/1417 ± 258 |
| 31. | 8. | Siotto et al., 2019 [65] | Fe | Case–control | Italy | 60/42 | 37 ± 9/40 ± 11 | 45/15; 22/20 | Serum | 857 ± 430/786 ± 276 |
| 32. | 9. | Ristori et al., 2011 [56] | Fe | Case–control | Italy | 49/49 | 36 ± 7/33 ± 6 | 29/20; 26/23 | Serum | 985 ± 352/1707 ± 548 |
| 33. | 10. | Visconti et al., 2005 [57] | Fe | Case–control | Italy | 12/12 | 28 ± 8/28 ± 9 | 8/4; 7/5 | Serum | 1318 ± 527/1686 ± 547 |
| 34. | 11. | Ay et al., 2023 [58] | Fe | Case–control | Türkiye | 16/16 | 45 ± 7/44 ±12 | 9/7; 11/5 | Serum | 1762 ± 747/1226 ± 347 |
| 35. | 12. | Gellein et al., 2008 [44] | Fe | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 1058 ± 196/1053 ± 111 |
| 36. | 13. | Al-Radaideh et al., 2021 [66] | Fe | Case–control | Jordan | 65/34 | 18–58/20–60 | 41/24; 18/16 | Serum | 560 ± 40/833 ± 84 |
| 37. | 14 | Doğan and Yildiz, 2019 [67] | Fe | Case–control | Türkiye | 53/45 | 37 ± 10/35 ± 10 | 11/42; 10/35 | Serum | 538 ± 110/794 ± 101 |
| 38. | 15. | Abo-Krysha et al., 2008 [60] | Fe | Case–control | Egypt | 20/10 | 29.94 ± 8.84 | 20/0; 10/0 | Serum | 595 ± 305/595 ± 466 |
| 39. | 16. | Bergsland et al., 2017 [68] | Fe | Case–control | Italy | 22/24 | 46.3/50.1 | 13/9; 17/7 | Serum | 1120 ± 660/1320 ± 740 |
| 40. | 1. | Stojsavljević et al., 2024 [37] | Co | Case–control | Sebia | 215/100 | 43 ± 10/42 ± 9 | 120/95; 66/34 | Serum | 0.574 ± 0.238/1.316 ± 0.630 |
| 41. | 2. | Alimonti et al., 2007 [50] | Co | Case–control | Italy | 60/124 | 39 ± 10/45 ± 13 | 38/22; 43/81 | Serum | 0.14 ± 0.03/0.l16 ± 0.03 |
| 42. | 3. | Forte et al., 2005 [69] | Co | Case–control | Italy | 60/60 | 39 ± 10/38 ± 10 | 40/20; N.A. | Plasma | 0.22 ± 0.10/0.11 ± 0.06 |
| 43. | 4. | Ristori et al., 2011 [56] | Co | Case–control | Italy | 49/49 | 36 ± 7/33 ± 6 | 29/20; 26/23 | Serum | 0.17 ± 0.10/0.12 ± 0.12 |
| 44. | 5. | Visconti et al., 2005 [57] | Co | Case–control | Italy | 12/12 | 28 ± 8/28 ± 9 | 8/4; 7/5 | Serum | 0.21 ± 0.11/0.16 ± 0.09 |
| 45. | 6. | Gellein et al., 2008 [44] | Co | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 0.075 ± 0.014/0.066 ± 0.007 |
| 46. | 1. | Stojsavljević et al., 2024 [37] | Cu | Case–control | Serbia | 215/100 | 43 ± 10/42 ± 9 | 120/95; 66/34 | Serum | 873 ± 205/908 ± 131 |
| 47. | 2. | Alimonti et al. 2007 [50] | Cu | Case–control | Italy | 60/164 | 39 ± 10/45 ± 13 | 38/22; 43/81 | Serum | 938 ± 46/950 ± 51 |
| 48. | 3. | De Riccardis et al., 2018 [70] | Cu | Case–control | Italy | 38/39 | N.A./N.A. | 29/9; 18/21 | Serum | 1045 ± 45/820 ± 69 |
| 49. | 4. | Forte et al., 2005 [69] | Cu | Case–control | Italy | 60/60 | 39 ± 10/38 ± 10 | 40/20; N.A. | Plasma | 1445 ± 481/926 ± 144 |
| 50. | 5. | Ghoreishi et al., 2015 [49] | Cu | Case–control | Iran | 50/50 | 32 ± 3/32 ± 3 | N.A./N.A. | Serum | 1882 ± 82/1031± 45 |
| 51. | 6. | Kapaki et al., 1989 [41] | Cu | Case–control | Greece | 15/28 | 34 ± 10/46 ± 14 | 3/12; 10/18 | Serum | 870 ± 150/1030 ± 140 |
| 52. | 7. | Masoud and Fakharian, 2007 [43] | Cu | Cohort | Iran | 35/35 | 32 ± 7/35 ± 5 | 28/7; 28/7 | Serum | 1160 ± 195/1337 ± 134 |
| 53. | 8. | Siotto et al., 2019 [65] | Cu | Case–control | Italy | 60/42 | 37 ± 9/40 ± 11 | 45/15; 22/20 | Serum | 900 ± 19/841 ± 22 |
| 54. | 9. | Smith et al., 1989 [42] | Cu | Cohort | USA | 27/33 | N.A./N.A. | 17/10; 11/22 | Plasma | 1736 ± 92/1735 ± 46 |
| 55. | 10. | Socha et al., 2017 [55] | Cu | Case–control | Poland | 101/68 | 41 ± 10/40 ± 13 | 64/37; 47/21 | Serum | 928 ± 398/988 ± 309 |
| 56. | 11. | Ristori et al., 2011 [56] | Cu | Case–control | Italy | 49/49 | 36 ± 7/33 ± 6 | 29/20; 26/23 | Serum | 916 ± 160/956 ± 193 |
| 57. | 12. | Visconti et al., 2005 [57] | Cu | Case–control | Italy | 12/12 | 28 ± 8/28 ± 9 | 8/4; 7/5 | Serum | 1034 ± 228/953 ± 75.2 |
| 58. | 13. | Ay et al., 2023 [58] | Cu | Case–control | Türkiye | 16/16 | 45 ± 7/44 ±12 | 9/7; 11/5 | Serum | 1179 ± 227/1268 ± 244 |
| 59. | 14. | Nashmi et al., 2020 [59] | Cu | Case–control | Iraq | 25/25 | 42 ± 9/41 ± 10 | 15/10; 14/11 | Serum | 1616 ± 159/1164 ± 195 |
| 60. | 15. | Iranmanesh et al., 2012 [71] | Cu | Case–control | Iran | 25/25 | 28 ± 3/N.A. | 16/9; N.A. | Serum | 886 ± 196/1104 ± 371 |
| 61. | 16. | Palm and Hallmans, 1982 [38] | Cu | Case–control | Sweden | 50/50 | N.A./N.A. | 29/21; 29/21 | Serum | 1000 ± 210/1071 ± 134 |
| 62. | 17. | Ghazavi et al., 2012 [46] | Cu | Case–control | Iran | 60/60 | N.A./N.A. | 43/17; 38/22 | Serum | 1152 ± 412/939 ± 296 |
| 63. | 18. | Janghorbani et al., 2017 [72] | Cu | Case–control | Iran | 55/95 | 32 ± 2/45 ± 2 | 47/8; 67/28 | Plasma | 1670 ± 223/1060 ± 195 |
| 64. | 19. | Harbige et al., 2011 [45] | Cu | Case–control | UK | 21/9 | N.A./N.A. | N.A./N.A. | Plasma | 1119 ± 309/957 ± 189 |
| 65. | 20. | Gellein et al., 2008 [44] | Cu | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 487 ± 117/478 ± 133 |
| 66. | 21. | Rieder et al.,1983 [73] | Cu | Case–control | Switzerland | 119/35 | N.A./N.A. | 63/53; 21/14 | Plasma | 888 ± 187/968 ± 198 |
| 67. | 22. | Giacoppo et al., 2014 [48] | Cu | Case–control | Italy | 41/23 | 41 ± 2/35 ± 2 | 31/10; 14/9 | Plasma | 623 ± 177/610 ± 199 |
| 68. | 1. | Stojsavljević et al., 2024 [37] | Mn | Case–control | Serbia | 215/100 | 43 ± 10/42 ± 9 | 120/95; 66/34 | Serum | 0.299 ± 0.284/4.390 ± 2.672 |
| 69. | 2. | Alimonti et al., 2007 [50] | Mn | Case–control | Italy | 60/124 | 39 ± 10/45 ± 13 | 38/22; 43/81 | Serum | 0.66 ± 0.08/0.60 ± 0.05 |
| 70. | 3. | Forte et al., 2005 [69] | Mn | Case–control | Italy | 60/60 | 39 ± 10/38 ± 10 | 40/20; N.A. | Plasma | 8.39 ± 3.93/7.80 ± 3.00 |
| 71. | 4. | Ristori et al., 2011 [56] | Mn | Case–control | Italy | 49/49 | 36 ± 7/33 ± 6 | 29/20; 26/23 | Serum | 0.74 ± 0.30/0.57 ± 0.17 |
| 72. | 5. | Visconti et al., 2005 [57] | Mn | Case–control | Italy | 12/12 | 28 ± 8/28 ± 9 | 8/4; 7/5 | Serum | 1.13 ± 0.33/0.63 ± 0.16 |
| 73. | 6. | Janghorbani et al., 2017 [72] | Mn | Case–control | Iran | 55/95 | 32 ± 2/45 ± 2 | 47/8; 67/28 | Plasma | 0.94 ± 0.009/0.96 ± 0.007 |
| 74. | 7. | Gellein et al., 2008 [44] | Mn | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 2.64 ± 0.58/3.09 ± 0.35 |
| 75. | 1. | Stojsavljević et al., 2024 [37] | Se | Case–control | Serbia | 215/100 | 43 ± 10/42 ± 9 | 120/95; 66/34 | Serum | 72.230 ± 18.792/86.706 ± 11.684 |
| 76. | 2. | Jensen et al., 1980 [74] | Se | Case–control | Denmark | 14/12 | N.A./N.A. | N.A./N.A. | Serum | 85 ± 10/97 ± 14 |
| 77. | 3. | Korpela et al., 1989 [75] | Se | Cohort | Finland | 12/7 | 46 ± 9/N.A. | N.A./N.A. | Serum | 123 ± 17/120 ± 18 |
| 78. | 4. | Mazzella et al., 1983 [76] | Se | Case–control | Italy | 20/16 | 37 ± 9/41 ± 6 | 14/6; N.A. | Plasma | 86.4 ± 16.02/60.6 ±17.5 |
| 79. | 5. | Smith et al., 1989 [42] | Se | Cohort | USA | 27/33 | N.A./N.A. | 17/10; 11/22 | Plasma | 99 ± 3.95/104 ± 0.79 |
| 80. | 6. | Socha et al.,2014 [77] | Se | Case–control | Poland | 101/63 | 41 ± 10/41 ± 14 | 64/37; 43/20 | Serum | 55.2 ± 16.2/79.2 ± 20.6 |
| 81. | 7. | Gellein et al., 2008 [44] | Se | Case–control | Norway | 9/13 | 44 ± 3/49 ± 4 | 9/0; 8/5 | Plasma | 45.4 ± 2.1/41.1 ± 2.2 |
| 82. | 8. | Giacoppo et al., 2014 [48] | Se | Case–control | Italy | 41/23 | 41 ± 2/35 ± 2 | 31/10; 14/9 | Plasma | 68.60 ± 19.02/71.10 ± 18.09 |
| 83. | 9. | Goli et al., 2023 [78] | Se | Case–control | Iran | 70/70 | N.A. | N.A. | Serum | 85 ± 40/123 ± 60 |
| 84. | 10. | Wilkström et al., 1976 [79] | Se | Case–control | Finland | 27/18 | N.A. | N.A. | Serum | 46.4 ± 12.9/43.6 ± 13.0 |
| Study | Selection | Comparability | Outcome | Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness | Size | Non-Respondents | Determination of Outcome | Statistical Test | For Biological Matrix | Average per Group | ||
| Zn | ||||||||
| Stojsavljević et al., 2024 [37] | a | a | a | a | a | a | 6 | |
| Palm and Hallmans, 1982 [38] | a | a | b | a | a | a | 5 | |
| Dore-Duffy et al., 1983 [39] | a | a | b | a | a | a | 5 | |
| Ho et al., 1986 [40] | a | a | c | a | a | a | 4 | |
| Kapaki et al., 1989 [41] | a | a | a | a | a | a | 6 | |
| Smith et al., 1989 [42] | b | a | b | a | a | a | 4 | |
| Masoud and Fakharian, 2007 [43] | b | a | a | a | a | a | 5 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | |
| Harbige et al., 2011 [45] | a | a | c | a | a | a | 4 | |
| Ghazavi et al., 2012 [46] | a | a | b | a | a | a | 5 | |
| Al-Zubaidi, 2012 [47] | a | a | c | a | a | a | 4 | |
| Giacoppo et al., 2014 [48] | a | a | a | a | a | a | 6 | |
| Ghoreishi et al., 2015 [49] | a | a | b | a | a | a | 5 | |
| Alimonti et al., 2007 [50] | a | a | a | a | a | a | 6 | |
| Matar et al., 2020 [51] | a | a | a | a | a | a | 6 | |
| Mezzaroba et al., 2020 [52] | a | a | a | a | a | a | 6 | |
| Oraby et al., 2019 [53] | a | a | a | a | a | a | 6 | |
| Pawlitzki et al., 2018 [54] | b | a | a | a | a | a | 5 | |
| Socha et al., 2017 [55] | a | a | a | a | a | a | 6 | |
| Ristori et al., 2011 [56] | a | a | a | a | a | a | 6 | |
| Visconti et al., 2005 [57] | a | a | a | a | a | a | 6 | |
| Ay et al., 2023 [58] | a | a | a | a | a | a | 6 | |
| Nashmi et al., 2020 [59] | a | a | a | a | a | a | 6 | 5.39 |
| Fe | ||||||||
| Alimonti et al., 2007 [50] | a | a | a | a | a | a | 6 | |
| Abo-Krysha and Rashed, 2008 [60] | a | a | b | a | a | a | 5 | |
| Armon-Omer et al., 2019 [61] | b | a | a | a | a | a | 5 | |
| Bsteh et al., 2019 [62] | a | a | b | a | a | a | 5 | |
| Ellidag et al., 2014 [63] | a | a | a | a | a | a | 6 | |
| Matar et al., 2020 [51] | a | a | a | a | a | a | 6 | |
| Sfagos et al., 2005 [64] | a | a | c | a | a | a | 4 | |
| Siotto et al., 2019 [65] | a | a | a | a | a | a | 6 | |
| Ristori et al., 2011 [56] | a | a | a | a | a | a | 6 | |
| Visconti et al., 2005 [57] | a | a | a | a | a | a | 6 | |
| Ay et al., 2023 [58] | a | a | a | a | a | a | 6 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | |
| Al-Radaideh et al., 2021 [66] | a | a | b | a | a | a | 5 | |
| Doğan and Yildiz, 2019 [67] | a | a | a | a | a | a | 6 | |
| Abo-Krysha et al., 2008 [60] | a | a | a | a | a | a | 6 | |
| Bergsland et al., 2017 [68] | a | a | a | a | a | a | 6 | 5.62 |
| Co | ||||||||
| Stojsavljević et al., 2024 [37] | a | a | a | a | a | a | 6 | |
| Alimonti et al., 2007 [50] | a | a | a | a | a | a | 6 | |
| Forte et al., 2005 [69] | a | a | b | a | a | a | 5 | |
| Ristori et al., 2011 [56] | a | a | a | a | a | a | 6 | |
| Visconti et al., 2005 [57] | a | a | a | a | a | a | 6 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | 5.83 |
| Cu | ||||||||
| Stojsavljević et al., 2024 [37] | a | a | a | a | a | a | 6 | |
| Alimonti et al. 2007 [50] | a | a | a | a | a | a | 6 | |
| De Riccardis et al., 2018 [70] | a | a | b | a | a | a | 5 | |
| Forte et al., 2005 [69] | a | a | b | a | a | a | 5 | |
| Ghoreishi et al., 2015 [49] | a | a | b | a | a | a | 5 | |
| Kapaki et al., 1989 [41] | a | a | a | a | a | a | 6 | |
| Masoud and Fakharian, 2007 [43] | b | a | a | a | a | a | 5 | |
| Siotto et al., 2019 [65] | a | a | a | a | a | a | 6 | |
| Smith et al., 1989 [42] | b | a | b | a | a | a | 4 | |
| Socha et al., 2017 [55] | a | a | a | a | a | a | 6 | |
| Ristori et al., 2011 [56] | a | a | a | a | a | a | 6 | |
| Visconti et al., 2005 [57] | a | a | a | a | a | a | 6 | |
| Ay et al., 2023 [58] | a | a | a | a | a | a | 6 | |
| Nashmi et al., 2020 [59] | a | a | a | a | a | a | 6 | |
| Iranmanesh et al., 2012 [71] | a | a | c | a | a | a | 4 | |
| Palm and Hallmans, 1982 [38] | a | a | b | a | a | a | 5 | |
| Ghazavi et al., 2012 [46] | a | a | b | a | a | a | 5 | |
| Janghorbani et al., 2017 | a | a | a | a | a | a | 6 | |
| Harbige et al., 2011 [45] | a | a | c | a | a | a | 4 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | |
| Rieder et al.,1983 [73] | a | a | b | a | a | a | 5 | |
| Giacoppo et al., 2014 [48] | a | a | a | a | a | a | 6 | 5.41 |
| Mn | ||||||||
| Stojsavljević et al., 2024 [37] | a | a | a | a | a | a | 6 | |
| Alimonti et al., 2007 [50] | a | a | a | a | a | a | 6 | |
| Forte et al., 2005 [69] | a | a | b | a | a | a | 5 | |
| Ristori et al., 2011 [56] | a | a | a | a | a | a | 6 | |
| Visconti et al., 2005 [57] | a | a | a | a | a | a | 6 | |
| Janghorbani et al., 2017 [72] | a | a | a | a | a | a | 6 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | 5.86 |
| Se | ||||||||
| Stojsavljević et al., 2024 [37] | a | a | a | a | a | a | 6 | |
| Jensen et al., 1980. [74] | a | a | c | a | a | a | 4 | |
| Korpela et al., 1989 [75] | b | a | c | a | a | a | 3 | |
| Mazzella et al., 1983 [76] | a | a | b | a | a | a | 5 | |
| Smith et al., 1989 [42] | b | a | b | a | a | a | 4 | |
| Socha et al.,2014 [77] | a | a | a | a | a | a | 6 | |
| Gellein et al., 2008 [44] | a | a | a | a | a | a | 6 | |
| Giacoppo et al., 2014 [48] | a | a | a | a | a | a | 6 | |
| Goli et al., 2023 [78] | a | a | c | a | a | a | 4 | |
| Wilkström et al., 1976 [79] | a | a | c | a | a | a | 4 | 4.80 |
| Average of the study: | 5.48 | |||||||
| Total Number of Analyzed Studies | Higher in Cases Than in Controls | Lower in Cases Than in Controls | No Statistically Significant Difference between Cases and Controls | Pooled Size Effects | |
|---|---|---|---|---|---|
| Zn | 23 | 3 | 10 | 10 | Significantly lower; p = 0.012 * |
| Fe | 16 | 2 | 7 | 7 | Significantly lower; p = 0.007 * |
| Co | 6 | 1 | 4 | 1 | No significant changes; p = 0.326 |
| Cu | 22 | 8 | 4 | 10 | Significantly higher; p = 0.002 * |
| Mn | 7 | 3 | 3 | 1 | No significant changes; p = 0.590 |
| Se | 10 | 2 | 4 | 2 | No significant changes; p = 0.351 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojsavljević, A.; Jagodić, J.; Perović, T.; Manojlović, D.; Pavlović, S. Changes of Target Essential Trace Elements in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Biomedicines 2024, 12, 1589. https://doi.org/10.3390/biomedicines12071589
Stojsavljević A, Jagodić J, Perović T, Manojlović D, Pavlović S. Changes of Target Essential Trace Elements in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Biomedicines. 2024; 12(7):1589. https://doi.org/10.3390/biomedicines12071589
Chicago/Turabian StyleStojsavljević, Aleksandar, Jovana Jagodić, Tatjana Perović, Dragan Manojlović, and Slađan Pavlović. 2024. "Changes of Target Essential Trace Elements in Multiple Sclerosis: A Systematic Review and Meta-Analysis" Biomedicines 12, no. 7: 1589. https://doi.org/10.3390/biomedicines12071589
APA StyleStojsavljević, A., Jagodić, J., Perović, T., Manojlović, D., & Pavlović, S. (2024). Changes of Target Essential Trace Elements in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Biomedicines, 12(7), 1589. https://doi.org/10.3390/biomedicines12071589









