Enhancing Neoadjuvant Virotherapy’s Effectiveness by Targeting Stroma to Improve Resectability in Pancreatic Cancer
Abstract
:1. Introduction
2. Current Clinically Approved Therapies for Treating BR/LA PDAC
3. Oncolytic Virotherapy—An Emerging Cancer Immunotherapy for BR/LA PDAC
4. Intricate Interplay of Acellular Components of the TME in PDAC Development and Treatment Resistance
5. Targeting Stromal Acellular Components in PDAC by Stroma-Depleting Enzymes
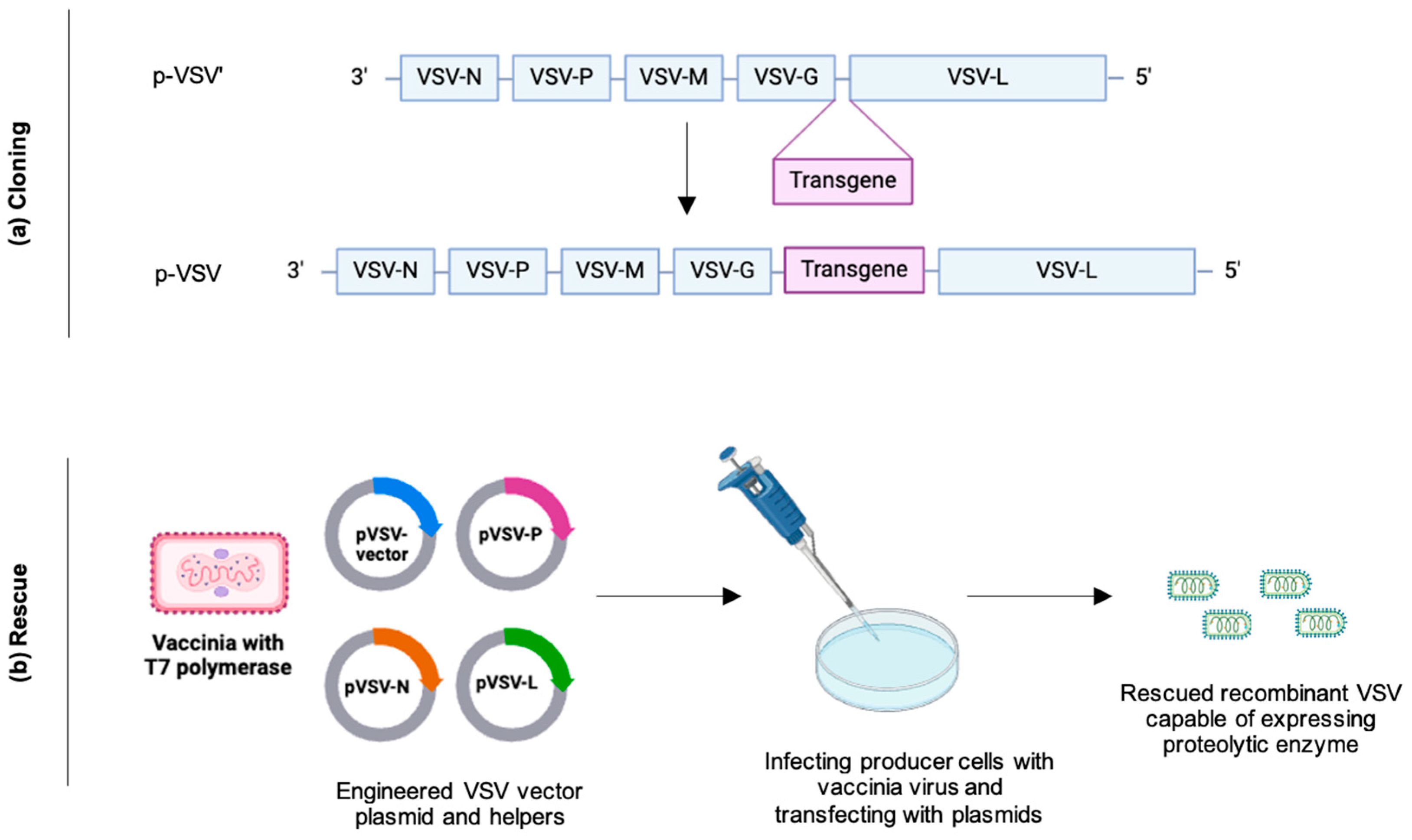
6. Are Oncolytic Viruses Expressing Stroma-Degrading Proteolytic Enzymes the Next Game-Changer?
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute Surveillance, Epidemiology and End Results Program. Cancer Stat Facts: Pancreatic Cancer. 2023. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 19 December 2023).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, J.K.; Stenzinger, A. Allelic Ratio of KRAS Mutations in Pancreatic Cancer. Oncologist 2015, 20, e8–e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.; Wang, Z.; Wu, X.; Zhou, C.; Yan, J.; Hu, B.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.N.C.; Andini, K.D.; Peters, G.J.; Kazemier, G.; Giovannetti, E. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin. Cancer Biol. 2022, 82, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Tsang, E.S.; Tempero, M.A. Therapeutic targets in the pancreatic adenocarcinoma microenvironment: Past challenges and opportunities for the future. J. Cancer Metastasis Treat. 2021, 7, 33. [Google Scholar] [CrossRef]
- Hartupee, C.; Nagalo, B.M.; Chabu, C.Y.; Tesfay, M.Z.; Coleman-Barnett, J.; West, J.T.; Moaven, O. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target. Front. Immunol. 2024, 15, 1287459. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Beatty, G.L. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu. Rev. Pathol. 2023, 18, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Goulart, M.R.; Stasinos, K.; Fincham, R.E.A.; Delvecchio, F.R.; Kocher, H.M. T cells in pancreatic cancer stroma. World J. Gastroenterol. WJG 2021, 27, 7956–7968. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, M.; Chen, H.; Shang, D.; Das, J.K.; Song, J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer 2020, 19, 32. [Google Scholar] [CrossRef]
- Weaver, V.M.; Butcher, D.T.; Alliston, T. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar]
- Neesse, A.; Michl, P.; Frese, K.K.; Feig, C.; Cook, N.; Jacobetz, M.A.; Lolkema, M.P.; Buccholz, M.; Olive, K.P.; Gress, T.M.; et al. Stromal biology and therapy in pancreatic cancer. Gut 2011, 60, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Soweid, A.M. The borderline resectable and locally advanced pancreatic ductal adenocarcinoma: Definition. Endosc. Ultrasound 2017, 6, S76–S78. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 27 December 2023).
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Evans, D.B.; Wolff, R.A. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.D.; Ray, P.; Heilbrun, L.K.; Vaitkevicius, V.K.; Saiki, J.H.; Rivkin, S.E.; Rossof, A.H.; Moore, T.N. 5-fluorouracil, methyl-CCNU, and radiotherapy with or without testolactone for localized adenocarcinoma of the exocrine pancreas. A southwest oncology group study. Cancer 1980, 46, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B. What Makes a Pancreatic Cancer Resectable? In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2018; Volume 38, pp. 300–305. [Google Scholar]
- Jain, A.J.; Maxwell, J.E.; Katz, M.H.G.; Snyder, R.A. Surgical Considerations for Neoadjuvant Therapy for Pancreatic Adenocarcinoma. Cancers 2023, 15, 4174. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Akita, H.; Tomokuni, A.; Kobayashi, S.; Ohigashi, H.; Fijiwara, Y.; Yano, M.; Sakon, M.; Ishikawa, O. Preoperative Gemcitabine-based Chemoradiation Therapy for Borderline Resectable Pancreatic Cancer: Impact of Venous and Arterial Involvement Status on Surgical Outcome and Pattern of Recurrence. Ann. Surg. 2016, 264, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Andrianello, S.; Malleo, G.; De Gregorio, L.; Scarpa, A.; Mino-Kenudson, M.; Maginno, L.; Ferrone, C.R.; Lillemoe, K.D.; Bassi, C.; et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann. Surg. 2017, 266, 142–148. [Google Scholar] [CrossRef]
- Saka, B.; Balci, S.; Basturk, O.; Bagci, P.; Postlewait, L.M.; Maithel, S.; Knight, J.; El-Rayes, B.; Kooby, D.; Sarmiento, J.; et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2–≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann. Surg. Oncol. 2016, 23, 2010–2018. [Google Scholar]
- Merza, N.; Farooqui, S.K.; Dar, S.H.; Varughese, T.; Awan, R.U.; Qureshi, L.; Ansari, S.A.; Qureshi, H.; Mcilvaine, J.; Vohra, I.; et al. Folfirinox vs. Gemcitabine + Nab-Paclitaxel as the First-Line Treatment for Pancreatic Cancer: A Systematic Review and Meta-Analysis. World J. Oncol. 2023, 14, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Santucci, J.; Tacey, M.; Thomson, B.; Michael, M.; Wong, R.; Shapiro, J.; Jennens, R.; Clarke, K.; Pattison, S.; Burge, M.; et al. Impact of first-line FOLFIRINOX versus Gemcitabine/Nab-Paclitaxel chemotherapy on survival in advanced pancreatic cancer: Evidence from the prospective international multicentre PURPLE pancreatic cancer registry. Eur. J. Cancer 2022, 174, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Citterio, C.; Anselmi, E.; Cavanna, L.; Vecchia, S. FOLFIRINOX or Gemcitabine Plus Nab-paclitaxel as First Line Treatment in Pancreatic Cancer: A Real-World Comparison. Cancer Diagn. Progn. 2024, 4, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Sudo, K.; Nakamura, K.; Kita, E.; Hara, R.; Takayama, W.; Ishii, H.; Yamaguchi, T. Gemcitabine plus nab-paclitaxel for locally advanced or borderline resectable pancreatic cancer. Sci. Rep. 2019, 9, 16187–16188. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy with FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma. JAMA Oncol. 2018, 4, 963. [Google Scholar] [CrossRef] [PubMed]
- Bratlie, S.O.; Wennerblom, J.; Vilhav, C.; Persson, J.; Rangelova, E. Resectable, borderline, and locally advanced pancreatic cancer—“The good, the bad, and the ugly” candidates for surgery? J. Gastrointest. Oncol. 2021, 12, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Rangelova, E.; Wefer, A.; Persson, S.; Valente, R.; Tanaka, K.; Orsini, N.; Segersvard, R.; Arnelo, U.; Chiaro, M.D. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann. Surg. 2021, 273, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Gemenetzis, G.; Blair, A.B.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Borel Rinkes, I.H.M.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Khachfe, H.H.; Habib, J.R.; Nassour, I.; Al Harthi, S.; Jamali, F.R. Borderline Resectable and Locally Advanced Pancreatic Cancers: A Review of Definitions, Diagnostics, Strategies for Treatment, and Future Directions. Pancreas 2021, 50, 1243–1249. [Google Scholar] [CrossRef]
- Amato, B.; Rispoli, C.; Iannone, L.; Testa, S.; Compagna, R.; Rocco, N. Surgical margins of resection for breast cancer: Current evidence. Minerva Chir. 2012, 67, 445–452. [Google Scholar]
- Maggino, L.; Malleo, G.; Marchegiani, G.; Viviani, E.; Nessi, C.; Ciprani, D.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. Arch. Surg. 2019, 154, 932–942. [Google Scholar] [CrossRef]
- Sultana, A.; Smith, C.T.; Cunningham, D.; Starling, N.; Neoptolemos, J.P.; Ghaneh, P. Meta-Analyses of Chemotherapy for Locally Advanced and Metastatic Pancreatic Cancer. J. Clin. Oncol. 2007, 25, 2607–2615. [Google Scholar] [CrossRef]
- Kurahara, H.; Maemura, K.; Mataki, Y.; Sakoda, M.; Iino, S.; Kawasaki, Y.; Arigami, T.; Mori, S.; Kijima, Y.; Ueno, S.; et al. A Therapeutic Strategy for Resectable Pancreatic Cancer Based on Risk Factors of Early Recurrence. Pancreas 2018, 47, 753–758. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O'Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Sugimoto, H.; Nomoto, S.; Takeda, S.; Kodera, Y.; Nakao, A. Aggressive surgery for borderline resectable pancreatic cancer: Evaluation of National Comprehensive Cancer Network guidelines. Pancreas 2013, 42, 1004–1010. [Google Scholar] [CrossRef]
- Xia, T.; Xu, P.; Mou, Y.; Zhang, X.; Song, S.; Zhou, Y.; Lu, C.; Zhu, Q.; Xu, Y.; Jin, W.; et al. Factors predicting recurrence after left-sided pancreatectomy for pancreatic ductal adenocarcinoma. World J. Surg. Oncol. 2023, 21, 191. [Google Scholar] [CrossRef]
- Schorn, S.; Demir, I.E.; Samm, N.; Scheufele, F.; Calavrezos, L.; Sargut, M.; Schirren, R.M.; Friess, H.; Ceyhan, G.O. Meta-analysis of the impact of neoadjuvant therapy on patterns of recurrence in pancreatic ductal adenocarcinoma. BJS Open 2018, 2, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Swayden, M.; Iovanna, J.; Soubeyran, P. Pancreatic cancer chemo-resistance is driven by tumor phenotype rather than tumor genotype. Heliyon 2018, 4, e01055. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Ou, F.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chung, M.; Schwartz, L.H.; et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017, 17, 505. [Google Scholar] [CrossRef]
- Jang, J.; Han, Y.; Lee, H.; Kim, S.; Kwon, W.; Lee, K.; Oh, D.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Proswithive, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Yoo, C.; Shin, S.H.; Kim, K.; Jeong, J.H.; Chang, H.; Kang, J.H.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers 2019, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, H.; Tsuruga, Y.; Orimo, T.; Wakayama, K.; Shimada, S.; Nagatsu, A.; Yokoo, H.; Kamiyama, T.; Katoh, N.; Taketomi, A. R0 Resection for Locally Advanced Pancreatic Cancer with Low-dose Gemcitabine with Wide Irradiation Area as Neoadjuvant Chemoradiotherapy. In Vivo 2018, 32, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Sastri, S.C.; Arya, S.; Mehta, S.; Patil, P.; Shrivastava, S.; Phurailatpam, R.; Shrikhande, S.V.; Engineer, R. Dose escalated concurrent chemo-radiation in borderline resectable and locally advanced pancreatic cancers with tomotherapy based intensity modulated radiotherapy: A phase II study. J. Gastrointest. Oncol. 2019, 10, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Coinu, A.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Lonati, V.; Aitini, E.; Barni, S. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: A meta-analytical review of published studies. Pancreas 2015, 44, 515–521. [Google Scholar] [CrossRef]
- Peterson, S.; Loaiza-Bonilla, A.; Ben-Josef, E.; Drebin, J.A.; Westendorf-Overley, C.; Anthony, L.B.; DeSimone, P.A.; Goel, G.; Kudrimoti, M.R.; Dineen, S.P.; et al. Neoadjuvant nab -paclitaxel and gemcitabine (AG) in borderline resectable (BR) or unresectable (UR) locally advanced pancreatic adenocarcinoma (LAPC) in patients ineligible for FOLFIRINOX. J. Clin. Oncol. 2016, 34, 328. [Google Scholar] [CrossRef]
- Yamada, S.; Fujii, T.; Yokoyama, Y.; Kawashima, H.; Maeda, O.; Suzuki, K.; Okada, T.; Ono, E.; Yamaguchi, J.; Takano, N.; et al. Phase I study of chemoradiotherapy using gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 815–821. [Google Scholar] [CrossRef]
- Hazel, J.J.; Thirlwell, M.P.; Huggins, M.; Maksymiuk, A.; MacFarlane, J.K. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: A prospective randomized trial. J. Can. Assoc. Radiol. 1981, 32, 164–165. [Google Scholar]
- Klaassen, D.J.; MacIntyre, J.M.; Catton, G.E.; Engstrom, P.F.; Moertel, C.G. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1985, 3, 373–378. [Google Scholar] [CrossRef]
- Loehrer, P.J.; Feng, Y.; Benson, A.B.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; et al. Gemcitabine Alone Versus Gemcitabine Plus Radiotherapy in Patients with Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef]
- Sajjad, M.; Batra, S.; Hoffe, S.; Kim, R.; Springett, G.; Mahipal, A. Use of Radiation Therapy in Locally Advanced Pancreatic Cancer Improves Survival: A SEER Database Analysis. Am. J. Clin. Oncol. 2018, 41, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Kharofa, J.; Mierzwa, M.; Olowokure, O.; Sussman, J.; Latif, T.; Gupta, A.; Xie, C.; Patel, S.; Esslinger, H.; Mcgill, B.; et al. Pattern of Marginal Local Failure in a Phase II Trial of Neoadjuvant Chemotherapy and Stereotactic Body Radiation Therapy for Resectable and Borderline Resectable Pancreas Cancer. Am. J. Clin. Oncol. 2019, 42, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Comito, T.; Cozzi, L.; Clerici, E.; Franzese, C.; Tozzi, A.; Iftode, C.; Navarria, P.D.; Agostino, G.; Rimassa, L.; Carnaghi, C.; et al. Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol. Cancer Res. Treat. 2017, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, B.; Zhou, Y.; Wan, T.; Si, X. Impact of tumor size on survival of patients with resected pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. BMC Cancer 2018, 18, 985. [Google Scholar] [CrossRef]
- Sekigami, Y.; Michelakos, T.; Fernandez-del Castillo, C.; Kontos, F.; Qadan, M.; Wo, J.Y.; Harrison, J.; Deshpande, V.; Catalano, O.; Lillemoe, K.D.; et al. Intraoperative Radiation Mitigates the Effect of Microscopically Positive Tumor Margins on Survival Among Pancreatic Adenocarcinoma Patients Treated with Neoadjuvant FOLFIRINOX and Chemoradiation. Ann. Surg. Oncol. 2021, 28, 4592–4601. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Ushida, Y.; Oba, A.; Ono, Y.; Sato, T.; Inoue, Y.; Takahashi, Y.; Saiura, A.; Ito, H. Impact of Tumor Size on the Outcomes of Patients with Resectable Distal Pancreatic Cancer: Lessons Learned from a Series of 158 Radical Resections. Ann. Surg. Oncol. 2022, 29, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Michelakos, T.; Pergolini, I.; Castillo, C.; Honselmann, K.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of Resectability and Survival in Patients with Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment with FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yamamoto, T.; Yamaki, S.; Sakaguchi, T.; Sekimoto, M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann. Gastroenterol. Surg. 2019, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Maggino, L.; Malleo, G.; Crippa, S.; Belfiori, G.; Nobile, S.; Gasparini, G.; Lionetto, G.; Luchini, C.; Mattiolo, P.; Schiavo-Lena, M.; et al. CA19.9 Response and Tumor Size Predict Recurrence Following Post-neoadjuvant Pancreatectomy in Initially Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2023, 30, 207–219. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; Rafael de Souza, W.; Loureiro, M.S.; Freire de Malo, F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef]
- Geoffroy, K.; Bourgeois-Daigneault, M. The pros and cons of interferons for oncolytic virotherapy. Cytokine Growth Factor Rev. 2020, 56, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, D.F.; Lyles, D.S. Oncolytic Vesicular Stomatitis Virus Induces Apoptosis via Signaling through PKR, Fas, and Daxx. J. Virol. 2007, 81, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, T.; Chen, M.; Munir, M.; Liu, H. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front. Cell. Infect. Microbiol. 2023, 13, 1142172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, X.; Cheng, P. Remodeling of Tumor Immune Microenvironment by Oncolytic Viruses. Front. Oncol. 2021, 10, 561372. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Rooney, C.M.; Dispenzieri, A.; Peng, K.; Russel, S.J. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 2020, 34, 3310–3322. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Robinson, M.; Love, C.A.; Coffin, R.S.; Han, Z.; Branston, R.H.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Toda, M.; Martuza, R.L.; Rabkin, S.D. Tumor Growth Inhibition by Intratumoral Inoculation of Defective Herpes Simplex Virus Vectors Expressing Granulocyte–Macrophage Colony-Stimulating Factor. Mol. Ther. 2000, 2, 324–329. [Google Scholar] [CrossRef]
- Patel, M.R.; Jacobson, B.A.; Ji, Y.; Drees, J.; Tang, S.; Xiong, K.; Wang, H.; Prigge, J.E.; Dash, A.S.; Kratzke, A.K.; et al. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget 2015, 6, 33165–33177. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, J.; Gao, H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 6765–6781. [Google Scholar] [CrossRef]
- Amakye, D.; Jagani, Z.; Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 2013, 19, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. CR 2019, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.M.; Kearney, J.F.; Yeh, J.J. The PDAC Extracellular Matrix: A Review of the ECM Protein Composition, Tumor Cell Interaction, and Therapeutic Strategies. Front. Oncol. 2021, 11, 751311. [Google Scholar] [CrossRef] [PubMed]
- Maneshi, P.; Mason, J.; Dongre, M.; Öhlund, D. Targeting Tumor-Stromal Interactions in Pancreatic Cancer: Impact of Collagens and Mechanical Traits. Front. Cell Dev. Biol. 2021, 9, 787485. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. (Regul. Ed.) 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.M.; Willumsen, N.; Dehlendorff, C.; Johansen, A.Z.; Jensen, B.V.; Hansen, C.P.; Hasselby, J.P.; Bojesen, S.E.; Pfeiffer, P.; Nielsen, S.E.; et al. Clinical value of serum hyaluronan and propeptide of type III collagen in patients with pancreatic cancer. Int. J. Cancer 2020, 146, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Rychlíková, J.; Vecka, M.; Jáchymová, M.; Macášek, J.; Hrabák, P.; Zeman, M.; Vavrova, L.; Roupal, J.; Krechler, T.; Ak, A. Osteopontin as a discriminating marker for pancreatic cancer and chronic pancreatitis. Cancer Biomark. Sect. A Dis. Markers 2016, 17, 55–65. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189–196. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef]
- Cheng, X.; Sato, N.; Kohi, S.; Yamaguchi, K. Prognostic Impact of Hyaluronan and Its Regulators in Pancreatic Ductal Adenocarcinoma. PLoS ONE 2013, 8, e80765. [Google Scholar] [CrossRef]
- Shields, M.A.; Dangi-Garimella, S.; Redig, A.J.; Munshi, H.G. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem. J. 2012, 441, 541–552. [Google Scholar] [CrossRef]
- Armstrong, T.; Packham, G.; Murphy, L.B.; Bateman, A.C.; Conti, J.A.; Fine, D.R.; Johnson, C.D.; Benyon, R.C.; Iredale, J.P. Type I Collagen Promotes the Malignant Phenotype of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2004, 10, 7427–7437. [Google Scholar] [CrossRef]
- Tavares-Valente, D.; Cannone, S.; Greco, M.R.; Carvalho, T.M.A.; Baltazar, F.; Queirós, O.; Agrimi, G.; Reshkin, S.J.; Cardone, R.A. Extracellular Matrix Collagen I Differentially Regulates the Metabolic Plasticity of Pancreatic Ductal Adenocarcinoma Parenchymal Cell and Cancer Stem Cell. Cancers 2023, 15, 3868. [Google Scholar] [CrossRef]
- Dangi-Garimella, S.; Sahai, V.; Ebine, K.; Kumar, K.; Munshi, H.G. Three-Dimensional Collagen I Promotes Gemcitabine Resistance In Vitro in Pancreatic Cancer Cells through HMGA2-Dependent Histone Acetyltransferase Expression. PLoS ONE 2013, 8, e64566. [Google Scholar] [CrossRef]
- Tian, C.; Clauser, K.R.; Öhlund, D.; Rickelt, S.; Huang, Y.; Gupta, M.; Mani, D.R.; Carr, S.S.; Tuveson, D.A.; Hynes, R.O.; et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19609–19618. [Google Scholar] [CrossRef]
- Öhlund, D.; Lundin, C.; Ardnor, B.; Öman, M.; Naredi, P.; Sund, M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br. J. Cancer 2009, 101, 91–97. [Google Scholar] [CrossRef]
- Owusu-Ansah, K.G.; Song, G.; Chen, R.; Edoo, M.I.A.; Li, J.; Chen, B.; Wu, J.; Zhou, L.; Xie, H.; Jiang, D.; et al. COL6A1 promotes metastasis and predicts poor prognosis in patients with pancreatic cancer. Int. J. Oncol. 2019, 55, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, J. Laminin-332 mediates proliferation, apoptosis, invasion, migration and epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, Y.; Cheung, M.; Cao, M.; Yu, C.; Chen, L.; Zhan, L.; He, Z.; Sun, C. LAMB3 mediates apoptotic, proliferative, invasive, and metastatic behaviors in pancreatic cancer by regulating the PI3K/Akt signaling pathway. Cell Death Dis. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Z.; Zeng, X.; Wu, Q.; Liao, X.; Wang, X.; Han, C.; Yu, T.; Zhu, G.; Qin, W.; et al. Evaluation of the diagnostic ability of laminin gene family for pancreatic ductal adenocarcinoma. Aging 2019, 11, 3679–3703. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Frémillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Chambers, C.R.; Herrmann, D.; Timpson, P.; Pereira, B.A. Dynamic Stromal Alterations Influence Tumor-Stroma Crosstalk to Promote Pancreatic Cancer and Treatment Resistance. Cancers 2021, 13, 3481. [Google Scholar] [CrossRef] [PubMed]
- Reader, C.S.; Vallath, S.; Steele, C.W.; Haider, S.; Brentnall, A.; Desai, A.; Moore, K.M.; Jamieson, N.B.; Chang, D.; Bailey, P.; et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 2019, 249, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Oyanagi, J.; Hoshino, D.; Togo, S.; Kumagai, H.; Miyagi, Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci. Rep. 2019, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Munshi, H.G.; Stack, M.S. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006, 25, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-F.; Gotwals, P.J.; Koteliansky, V.E.; Sheppard, D.; Van De Water, L. The EIIIA Segment of Fibronectin Is a Ligand for Integrins α9β1 and α4β1 Providing a Novel Mechanism for Regulating Cell Adhesion by Alternative Splicing. J. Biol. Chem. 2002, 277, 14467. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ansari, D.; Zhou, Q.; Sasor, A.; Said Hilmersson, K.; Andersson, R. Stromal fibronectin expression in patients with resected pancreatic ductal adenocarcinoma. World J. Surg. Oncol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Topalovski, M.; Brekken, R.A. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2016, 381, 252–258. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ferdous, K.U.; Roy, S.; Nitul, I.A.; Mamun, F.; Hossain, M.H.; Subhan, N.; Alam, M.A.; Haque, M.A. Polyphenolic compounds of amla prevent oxidative stress and fibrosis in the kidney and heart of 2K1C rats. Food Sci. Nutr. 2020, 8, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol.—Gastrointest. Liver Physiol. 2005, 289, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xu, C.; Dong, J.; Wei, J. An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy. J. Immunother. Cancer 2024, 12, e008431. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillen-Ponce, C.; Farrera-Sal, M.; Moreno, F.; et al. Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Korn, R.L.; Rosen, L.S.; LoRusso, P.; Dychter, S.S.; Zhu, J.; Maneval, D.C.; Jiang, P.; Shepard, H.M.; Frost, G.; et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br. J. Cancer 2018, 118, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients with Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Van Cutsem, E.; Sigal, D.; Oh, D.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifer, A.E.; Bates, S.E.; et al. HALO 109-301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20) + nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA)itle. HALO 109-301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20) nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA). J. Clin. Oncol. 2020, 38, 4. [Google Scholar]
- Muñoz, N.M.; Williams, M.; Dixon, K.; Dupuis, C.; McWatters, A.; Avritscher, R.; Manrique, S.Z.; McHugh, K.; Murthy, R.; Tam, A.; et al. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J. Immunother. Cancer 2021, 9, e001800. [Google Scholar] [CrossRef]
- Sheth, R.A.; Hesketh, R.; Kong, D.S.; Wicky, S.; Oklu, R. Barriers to Drug Delivery in Interventional Oncology. J. Vasc. Interv. Radiol. 2013, 24, 1201–1207. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.B.; Shields, A.F.; Behl, D.; et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients with Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol. 2019, 37, 1062–1069. [Google Scholar] [CrossRef]
- Seki, T.; Saida, Y.; Kishimoto, S.; Lee, J.; Otowa, Y.; Yamamoto, K.; Chandramouli, G.V.; Devasahayam, N.; Mitchell, J.B.; Kirshna, M.C.; et al. PEGPH20, a PEGylated human hyaluronidase, induces radiosensitization by reoxygenation in pancreatic cancer xenografts. A molecular imaging study. Neoplasia 2022, 30, 100793. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zamloot, V.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Young, C.A.; Blazar, B.R.; Manuel, E.R. Collagenase-Expressing Salmonella Targets Major Collagens in Pancreatic Cancer Leading to Reductions in Immunosuppressive Subsets and Tumor Growth. Cancers 2021, 13, 3565. [Google Scholar] [CrossRef]
- Luo, J.; Cao, J.; Ma, G.; Wang, X.; Sun, Y.; Zhang, C.; Shi, Z.; Zeng, Y.; Zhang, T.; Huang, P. Collagenase-Loaded H-TiO2 Nanoparticles Enhance Ultrasound Imaging-Guided Sonodynamic Therapy in a Pancreatic Carcinoma Xenograft Model via Digesting Stromal Barriers. ACS Appl. Mater. Interfaces 2022, 14, 40535–40545. [Google Scholar] [CrossRef]
- Daniel Bonfil, R.; Medina, P.A.; Gomez, D.E.; Farias, E.; Lazarowski, A.; Gritti, M.F.L.; Meiss, R.P.; Bustuoabad, O.D. Expression of gelatinise/type IV collagenase in tumor necrosis correlates with cell detachment and tumor invasion. Clin. Exp. Metastasis 1992, 10, 211–220. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Prado, W.A.; Lavayen, V.; Büttenbender, S.L.; Beckenkamp, A.; Martins, B.S.; Ludtke, D.S.; Campo, L.F.; Rodembusch, F.S.; Buffon, A.; et al. Bromelain-Functionalized Multiple-Wall Lipid-Core Nanocapsules: Formulation, Chemical Structure and Antiproliferative Effect Against Human Breast Cancer Cells (MCF-7). Pharm. Res. 2017, 34, 438–452. [Google Scholar] [CrossRef]
- Romano, B.; Fasolino, I.; Pagano, E.; Capasso, R.; Pace, S.; De Rosa, G.; Milic, N.; Orlando, P.; Izzo, A.A.; Borrelli, F. chemopreventive action of bromelain, from pineapple stem (Ananas comosus L.), on colon carcinogenesis is related to antiproliferative and proapoptotic effects. Mol. Nutr. Food Res. 2014, 58, 457–465. [Google Scholar] [PubMed]
- Wang, X.; He, L.; Wei, B.; Yan, G.; Wang, J.; Tang, R. Bromelain-immobilized and lactobionic acid-modified chitosan nanoparticles for enhanced drug penetration in tumor tissues. Int. J. Biol. Macromol. 2018, 115, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, J.; Xu, X.; Fang, Q.; Tang, R. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumor. Mater. Sci. Eng. C 2020, 113, 111004. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Haddix, S.G.; Taghipour, N.; Scaria, S.; Taraballi, F.; Cevenini, A.; Yazdi, I.K.; Corbo, C.; Palomba, R.; Khaled, S.Z.; et al. Bromelain Surface Modification Increases the Diffusion of Silica Nanoparticles in the Tumor Extracellular Matrix. ACS Nano 2014, 8, 9874–9883. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, C.; Huang, J.; Jiang, Y.; Miao, Q.; Pu, K. Semiconducting Polymer Nanoenzymes with Photothermic Activity for Enhanced Cancer Therapy. Angew. Chem. (Int. Ed.) 2018, 57, 3995–3998. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Shen, M.; Samuel, C.S.; Schlossmann, J.; Bennett, R.G. Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways. Mol. Cell. Endocrinol. 2019, 487, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.G.; Kharbanda, K.K.; Tuma, D.J. Inhibition of markers of hepatic stellate cell activation by the hormone relaxin. Biochem. Pharmacol. 2003, 66, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E.N.; Pickford, L.B.; Salles, A.L.; Piercy, C.E.; Grove, B.H.; Erikson, M.E.; Amento, E.P. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Investig. 1996, 98, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Choi, I.; Lee, H.; Yan, H.H.; Son, M.K.; Ahn, H.M.; Hong, J.; Yun, C.; Hong, S. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017, 396, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, X.; Cai, J.J.; Chen, L.Z.; Gong, Y.S.; Wang, L.X.; Gao, Z.; Zhang, H.Q.; Huang, W.J.; Zhou, H. Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway. Drug Des. Dev. Ther. 2015, 9, 4599–4611. [Google Scholar] [CrossRef]
- Wihastyoko, H.Y.L.; Soeharto, S.; Widjajanto, E.; Kusworini, K.; Pardjianto, B. Biological Activity of Papain and Papain-like (Cathepsin-K and Cathepsin-B) Enzymes as Therapeutical Modality Candidates in Degrading Collagen in Abnormal Scar. Res. J. Pharm. Technol. 2021, 14, 4957–4962. [Google Scholar] [CrossRef]
- Korenč, M.; Lenarčič, B.; Novinec, M. Human cathepsin L, a papain-like collagenase without proline specificity. FEBS J. 2015, 282, 4328–4340. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Marshall, G.W. Papain-gel degrades intact nonmineralized type I collagen fibrils. Scanning 2009, 31, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kafienah, W.; Buttle, D.J.; Burnett, D.; Hollander, A.P. Cleavage of native type I collagen by human neutrophil elastase. Biochem. J. 1998, 330 Pt 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, C.L.; Hasty, D.L.; Seyer, J.M.; Kang, A.H. Specific cleavage of human type III collagen by human polymorphonuclear leukocyte elastase. J. Biol. Chem. 1980, 255, 12006–12010. [Google Scholar] [CrossRef] [PubMed]
- Gadek, J.E.; Fells, G.A.; Wright, D.G.; Crystal, R.G. Human neutrophil elastase functions as a type III collagen “Collagenase”. Biochem. Biophys. Res. Commun. 1980, 95, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Gadher, S.J.; Schmid, T.M.; Heck, L.W.; Woolley, D.E. Cleavage of Collagen Type X by Human Synovial Collagenase and Neutrophil Elastase. Matrix 1989, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yonejima, Y.; Tsujimoto, Y.; Suzuki, Y.; Watanabe, K. thermostable collagenolytic protease with a very large molecular mass produced by thermophilic Bacillus sp. strain MO-1. Appl. Microbiol. Biotechnol. 2001, 57, 103–108. [Google Scholar] [PubMed]
- Itoi, Y.; Horinaka, M.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Characteristic Features in the Structure and Collagen-Binding Ability of a Thermophilic Collagenolytic Protease from the Thermophile Geobacillus collagenovorans MO-1. J. Bacteriol. 2006, 188, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Su, H.; Zhao, G.; Gao, X.; Zhou, M.; Wang, P.; Zhao, H.; Xie, P.; Zhang, X.; Chen, X.; et al. Structural and mechanistic insights into collagen degradation by a bacterial collagenolytic serine protease in the subtilisin family. Mol. Microbiol. 2013, 90, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, Q.; Li, Y.; Liu, L.; Tang, X.; Tang, B. Maturation Process and Characterization of a Novel Thermostable and Halotolerant Subtilisin-Like Protease with High Collagenolytic Activity but Low Gelatinolytic Activity. Appl. Environ. Microbiol. 2022, 88, e0218421. [Google Scholar] [CrossRef]
- Blair, A.B.; Sorber, R.; Rozich, N.S.; Burkhart, R.A. A Qualitative Review of Neoadjuvant Chemotherapy in Resectable Pancreatic Adenocarcinoma. Pancreas 2019, 48, 973–984. [Google Scholar] [CrossRef]
- Klinkenbijl, J.H.; Jeekel, J.; Sahmoud, T.; van Pel, R.; Couvreur, M.L.; Veenhof, C.H.; Arnaud, J.P.; Gonzalez, D.G.; T de Wit, L.; Hennipman, A.; et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 1999, 230, 776. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Rojas, J.J.; Gros, A.; Mercade, E.; Cascallo, M.; Alemany, R. Hyaluronidase Expression by an Oncolytic Adenovirus Enhances Its Intratumoral Spread and Suppresses Tumor Growth. Mol. Ther. 2010, 18, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Garcia-Carbonero, R.; Laquente, B.; Álvarez, R.; Mato-Berciano, A.; Gimenez-Alejandre, M.; Morgado, S.; Rodriguez-Garcia, A.; Maliandi, M.V.; Riesco, M.C.; et al. VCN-01 disrupts pancreatic cancer stroma and exerts antitumor effects. J. Immunother. Cancer 2021, 9, e003254. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Porosnicu, M.; Markovic, D.; Barber, G.N. Genetically Engineered Vesicular Stomatitis Virus in Gene Therapy: Application for Treatment of Malignant Disease. J. Virol. 2002, 76, 895–904. [Google Scholar] [CrossRef]
- Bickenbach, K.A.; Gonen, M.; Tang, L.H.; O’Reilly, E.; Goodman, K.; Brennan, M.F.; D'Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Jarnagin, W.R.; et al. Downstaging in Pancreatic Cancer: A Matched Analysis of Patients Resected Following Systemic Treatment of Initially Locally Unresectable Disease. Ann. Surg. Oncol. 2012, 19, 1663–1669. [Google Scholar] [CrossRef]



| PDAC Type | Cohort | Therapy | R0 Resectability (%) | MS/OS (Months) | Source |
|---|---|---|---|---|---|
| BR | 134 | FOLFIRINOX or modified FOLFIRINOX followed by SBRT, then surgery | 64% | 22 | [42] |
| BR | 27 | Gemcitabine and external beam radiation followed by surgery | 51.8% | 21 | [43] |
| 23 | Upfront surgery followed by chemoradiation | 26.1% | 12 | ||
| R and BR | 119 | Gemcitabine followed by radiotherapy, then surgery | 72% | 16.0 | [44] |
| 127 | Immediate surgery followed by adjuvant gemcitabine | 40% | 14.3 | ||
| BR and LA | 135 | Gemcitabine monotherapy, gemcitabine-capecitabine, and gemcitabine-erlotinib or FOLFIRINOX followed by surgery | NR | 17.1 | [45] |
| 359 | Upfront surgery | NR | 7.1 | ||
| BR and LA | 34 | Low-dose gemcitabine and wide irradiation area | 47.1% | 39.0 | [46] |
| BR and LA | 30 | Dose escalated radiotherapy (helical tomotherapy) with concurrent chemotherapy (gemcitabine) | 39.0% (BR) | 11.8 (LA), 17.3 (BR) | [47] |
| BR and LA | 253 | FOLFIRINOX with or without radiotherapy | 39.4% | NR | [48] |
| BR and LA | 20 | Gemcitabine and nab-paclitaxel followed by RT, then surgery | 20% | NR | [49] |
| LA | 12 | chemoradiotherapy using gemcitabine plus nab-paclitaxel | 50% | NR | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdous, K.U.; Tesfay, M.Z.; Cios, A.; Shelton, R.S.; Hartupee, C.; Urbaniak, A.; Chamcheu, J.C.; Mavros, M.N.; Giorgakis, E.; Mustafa, B.; et al. Enhancing Neoadjuvant Virotherapy’s Effectiveness by Targeting Stroma to Improve Resectability in Pancreatic Cancer. Biomedicines 2024, 12, 1596. https://doi.org/10.3390/biomedicines12071596
Ferdous KU, Tesfay MZ, Cios A, Shelton RS, Hartupee C, Urbaniak A, Chamcheu JC, Mavros MN, Giorgakis E, Mustafa B, et al. Enhancing Neoadjuvant Virotherapy’s Effectiveness by Targeting Stroma to Improve Resectability in Pancreatic Cancer. Biomedicines. 2024; 12(7):1596. https://doi.org/10.3390/biomedicines12071596
Chicago/Turabian StyleFerdous, Khandoker Usran, Mulu Z. Tesfay, Aleksandra Cios, Randal S. Shelton, Conner Hartupee, Alicja Urbaniak, Jean Christopher Chamcheu, Michail N. Mavros, Emmanouil Giorgakis, Bahaa Mustafa, and et al. 2024. "Enhancing Neoadjuvant Virotherapy’s Effectiveness by Targeting Stroma to Improve Resectability in Pancreatic Cancer" Biomedicines 12, no. 7: 1596. https://doi.org/10.3390/biomedicines12071596






