Nocturnal Glucose Profile According to Timing of Dinner Rapid Insulin and Basal and Rapid Insulin Type: An Insulclock® Connected Insulin Cap-Based Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Population and Database
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Population
3.2. Nocturnal Glucose Dynamics Depending on the Rapid Insulin Injection Timing
3.3. Analysis According to Dinner Ultrarapid Insulin Use
3.4. Analysis According to the Second-Generation Basal Insulin Type
3.5. Analysis According to the Use of a Second Insulin Injection (Correction Dose)
3.6. Multivariable Analysis
- The use of a URI was independently associated with a reduced risk (−52%) of glucose events < 70 mg/dL (3.9 mmol/L) (adjusted R-squared: 0.017; p = 0.003), and with a lower (−64%) TBR70 (adjusted R-squared: 0.016; p = 0.003);
- Not adding a second (correction) injection after dinner was independently associated with:
- ○
- A reduction in overnight hypoglycemia: −47% glucose events < 70 mg/dL), adjusted R-squared: 0.017, p = 0.003, and −61% TBR70 (adjusted R-squared: 0.016; p = 0.003);
- ○
- A reduction in overnight hyperglycemia: −21% glucose AUC of over 180 mg/dL (adjusted R-squared: 0.017; p < 0.001);
- ○
- More time in the recommended glucose range 70–180 mg/dL (TIR): +6.7% (62.7 ± 29.6 mg/dL vs. 56.0 ± 27.4) (adjusted R-squared: 0.048; p < 0.001).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin Therapy and Dietary Adjustments to Normalize Glycemia and Prevent Nocturnal Hypoglycemia after Evening Exercise in Type 1 Diabetes: A Randomized Controlled Trial. BMJ Open Diabetes Res. Care 2015, 3, e000085. [Google Scholar] [CrossRef] [PubMed]
- Jennum, P.; Stender-Petersen, K.; Rabøl, R.; Jørgensen, N.R.; Chu, P.-L.; Madsbad, S. The Impact of Nocturnal Hypoglycemia on Sleep in Subjects With Type 2 Diabetes. Diabetes Care 2015, 38, 2151–2157. [Google Scholar] [CrossRef]
- Lane, W.; Lambert, E.; George, J.; Rathor, N.; Thalange, N. Exploring the Burden of Mealtime Insulin Dosing in Adults and Children With Type 1 Diabetes. Clin. Diabetes 2021, 39, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mozzillo, E.; Franceschi, R.; Di Candia, F.; Ricci, A.; Leonardi, L.; Girardi, M.; Rosanio, F.M.; Marcovecchio, M.L. Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. J. Pers. Med. 2022, 12, 2058. [Google Scholar] [CrossRef]
- Slattery, D.; Amiel, S.A.; Choudhary, P. Optimal Prandial Timing of Bolus Insulin in Diabetes Management: A Review. Diabet. Med. 2018, 35, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Peralta, F.; Valledor, X.; López-Picado, A.; Abreu, C.; Fernández-Rubio, E.; Cotovad, L.; Pujante, P.; García-Fernández, E.; Azriel, S.; Corcoy, R.; et al. Ultrarapid Insulin Use Can Reduce Postprandial Hyperglycemia and Late Hypoglycemia, Even in Delayed Insulin Injections: A Connected Insulin Cap-Based Real-World Study. Diabetes Technol. Ther. 2024, 26, 1–10. [Google Scholar] [CrossRef]
- EMA Humalog. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/humalog (accessed on 16 January 2023).
- EMA Fiasp. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fiasp (accessed on 1 April 2023).
- Tejera-Pérez, C.; Chico, A.; Azriel-Mira, S.; Lardiés-Sánchez, B.; Gomez-Peralta, F. Área de Diabetes-SEEN Connected Insulin Pens and Caps: An Expert’s Recommendation from the Area of Diabetes of the Spanish Endocrinology and Nutrition Society (SEEN). Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2023, 14, 1077–1091. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Fernández-Rubio, E.; Cotovad, L.; Pujante, P.; Gaztambide, S.; Bellido, D.; Menéndez Torre, E.; Ruiz-Valdepeñas, S.; Bello, H.; et al. Efficacy of a Connected Insulin Pen Cap in People With Noncontrolled Type 1 Diabetes: A Multicenter Randomized Clinical Trial. Diabetes Care 2023, 46, 206–208. [Google Scholar] [CrossRef]
- Martyn-Nemeth, P.; Schwarz Farabi, S.; Mihailescu, D.; Nemeth, J.; Quinn, L. Fear of Hypoglycemia in Adults with Type 1 Diabetes: Impact of Therapeutic Advances and Strategies for Prevention—A Review. J. Diabetes Complicat. 2016, 30, 167–177. [Google Scholar] [CrossRef]
- Datye, K.A.; Boyle, C.T.; Simmons, J.; Moore, D.J.; Jaser, S.S.; Sheanon, N.; Kittelsrud, J.M.; Woerner, S.E.; Miller, K.M. Timing of Meal Insulin and Its Relation to Adherence to Therapy in Type 1 Diabetes. J. Diabetes Sci. Technol. 2018, 12, 349–355. [Google Scholar] [CrossRef]
- Peyrot, M.; Rubin, R.R.; Kruger, D.F.; Travis, L.B. Correlates of Insulin Injection Omission. Diabetes Care 2010, 33, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Newson, R.S.; Liao, B.; Kennedy-Martin, T.; Battelino, T. Missed and Mistimed Insulin Doses in People with Diabetes: A Systematic Literature Review. Diabetes Technol. Ther. 2021, 23, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Cobry, E.; McFann, K.; Messer, L.; Gage, V.; VanderWel, B.; Horton, L.; Chase, H.P. Timing of Meal Insulin Boluses to Achieve Optimal Postprandial Glycemic Control in Patients with Type 1 Diabetes. Diabetes Technol. Ther. 2010, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Bolli, G.B.; DeVries, J.H. New Long-Acting Insulin Analogs: From Clamp Studies to Clinical Practice. Diabetes Care 2015, 38, 541–543. [Google Scholar] [CrossRef]
- Battelino, T.; Edelman, S.V.; Nishimura, R.; Bergenstal, R.M. Comparison of Second-Generation Basal Insulin Analogs: A Review of the Evidence from Continuous Glucose Monitoring. Diabetes Technol. Ther. 2021, 23, 20–30. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Chico Ballesteros, A.; Marco Martínez, A.; Pérez Corral, B.; Conget Donlo, I.; Fuentealba Melo, P.; Zaragozá Arnáez, F.; Matabuena Rodríguez, M. Insulin Glargine 300 U/Ml versus Insulin Degludec 100 U/Ml Improves Nocturnal Glycaemic Control and Variability in Type 1 Diabetes under Routine Clinical Practice: A Glucodensities-Based Post Hoc Analysis of the OneCare Study. Diabetes Obes. Metab. 2024, 26, 1993–1997. [Google Scholar] [CrossRef]
- Pettus, J. Time to get serious about insulin timing. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2017, 23, 503–505. [Google Scholar] [CrossRef]
- McCall, A.L. Insulin Therapy and Hypoglycemia. Endocrinol. Metab. Clin. North Am. 2012, 41, 57–87. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Gomez-Rodriguez, S.; Ruiz, L. Insulclock: A Novel Insulin Delivery Optimization and Tracking System. Diabetes Technol. Ther. 2019, 21, 209–214. [Google Scholar] [CrossRef]
- Harvey, R.A.; Dassau, E.; Zisser, H.; Seborg, D.E.; Doyle, F.J. Design of the Glucose Rate Increase Detector: A Meal Detection Module for the Health Monitoring System. J. Diabetes Sci. Technol. 2014, 8, 307–320. [Google Scholar] [CrossRef]
- Danne, T.P.A.; Joubert, M.; Hartvig, N.V.; Kaas, A.; Knudsen, N.N.; Mader, J.K. Association Between Treatment Adherence and Continuous Glucose Monitoring Outcomes in People With Diabetes Using Smart Insulin Pens in a Real-World Setting. Diabetes Care 2024, 47, 995–1003. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous Glucose Monitoring and Metrics for Clinical Trials: An International Consensus Statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Danne, T.; Axel Schweitzer, M.; Keuthage, W.; Kipper, S.; Kretzschmar, Y.; Simon, J.; Wiedenmann, T.; Ziegler, R. Impact of Fast-Acting Insulin Aspart on Glycemic Control in Patients with Type 1 Diabetes Using Intermittent-Scanning Continuous Glucose Monitoring Within a Real-World Setting: The GoBolus Study. Diabetes Technol. Ther. 2021, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Klaff, L.; Cao, D.; Dellva, M.A.; Tobian, J.; Miura, J.; Dahl, D.; Lucas, J.; Bue-Valleskey, J. Ultra Rapid Lispro Improves Postprandial Glucose Control Compared with Lispro in Patients with Type 1 Diabetes: Results from the 26-Week PRONTO-T1D Study. Diabetes Obes. Metab. 2020, 22, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Chan, J.C.N. Targeting Postprandial Glucose Control Using Ultra-Rapid Insulins: Is Faster Better? Sci. Bull. 2022, 67, 2392–2394. [Google Scholar] [CrossRef]
- Heise, T.; Piras de Oliveira, C.; Juneja, R.; Ribeiro, A.; Chigutsa, F.; Blevins, T. What Is the Value of Faster Acting Prandial Insulin? Focus on Ultra Rapid Lispro. Diabetes Obes. Metab. 2022, 24, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Bhadada, S.K. Glycaemic Efficacy and Safety of Mealtime Faster-Acting Insulin Aspart Administered by Injection as Compared to Insulin Aspart in People with Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Diabet. Med. J. Br. Diabet. Assoc. 2021, 38, e14515. [Google Scholar] [CrossRef]
- Owens, D.R.; S Bailey, T.; Fanelli, C.G.; Yale, J.-F.; Bolli, G.B. Clinical Relevance of Pharmacokinetic and Pharmacodynamic Profiles of Insulin Degludec (100, 200 U/mL) and Insulin Glargine (100, 300 U/mL)—A Review of Evidence and Clinical Interpretation. Diabetes Metab. 2019, 45, 330–340. [Google Scholar] [CrossRef]
- Schmelzeisen-Redeker, G.; Schoemaker, M.; Kirchsteiger, H.; Freckmann, G.; Heinemann, L.; Del Re, L. Time Delay of CGM Sensors: Relevance, Causes, and Countermeasures. J. Diabetes Sci. Technol. 2015, 9, 1006–1015. [Google Scholar] [CrossRef]
- Rossetti, P.; Quirós, C.; Moscardó, V.; Comas, A.; Giménez, M.; Ampudia-Blasco, F.J.; León, F.; Montaser, E.; Conget, I.; Bondia, J.; et al. Closed-Loop Control of Postprandial Glycemia Using an Insulin-on-Board Limitation Through Continuous Action on Glucose Target. Diabetes Technol. Ther. 2017, 19, 355–362. [Google Scholar] [CrossRef]

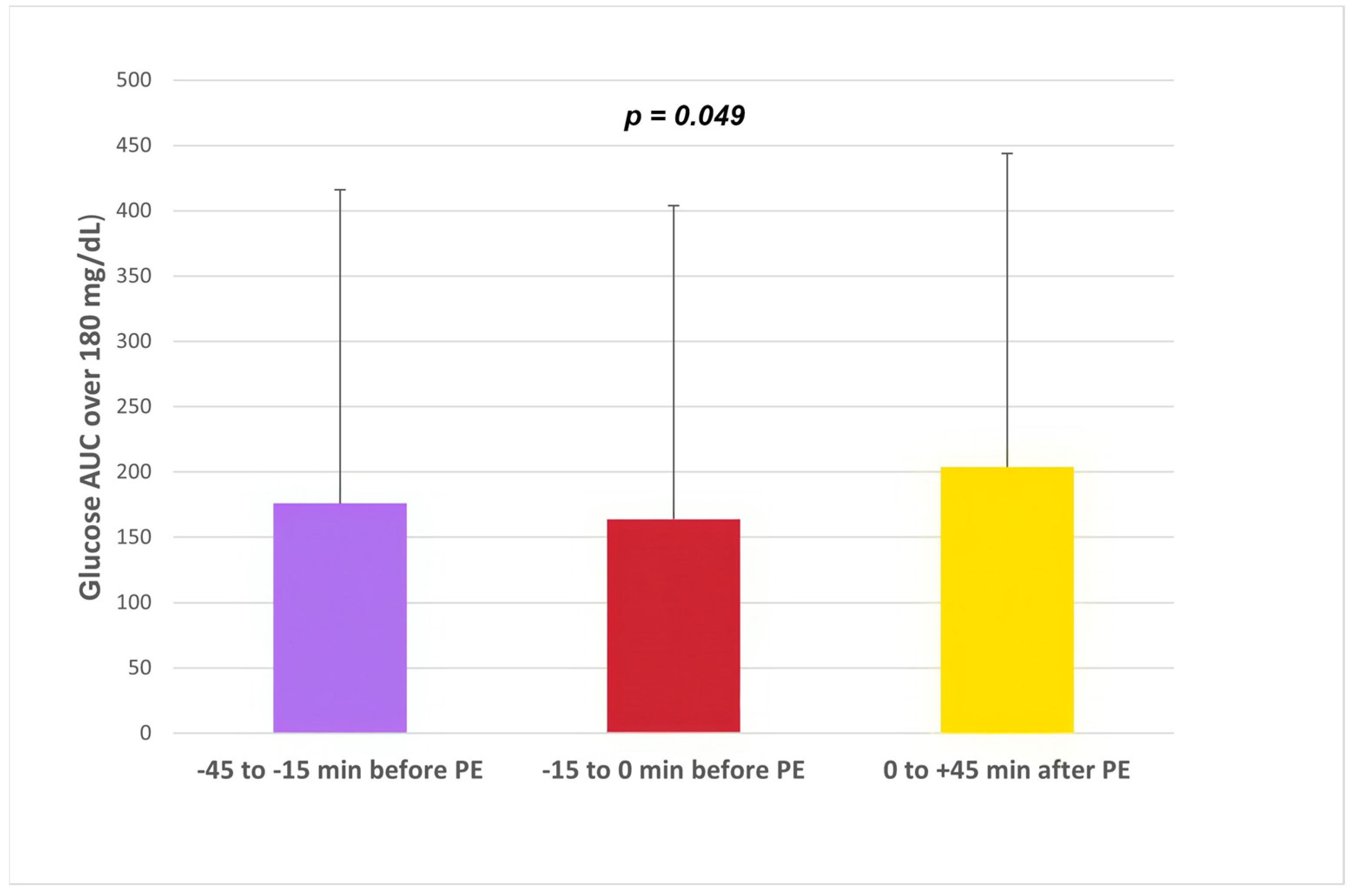

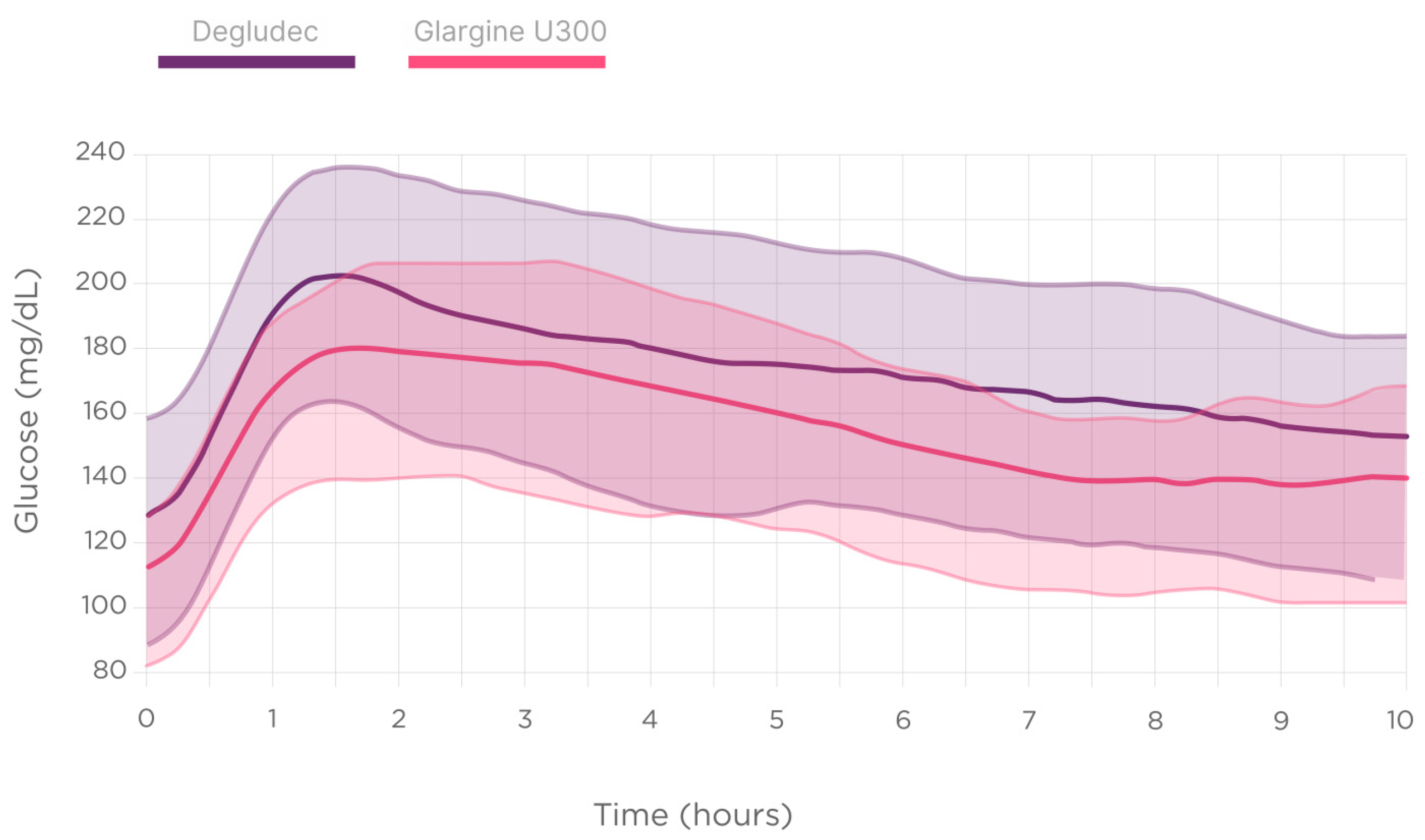
| All | Glargine U300 | Degludec | p | URI | RI | p | −45/−15 min | −15/−0 min | 0/+45 min | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 49 | 16 | 33% | 33 | 67% | 22 | 45% | 27 | 55% | |||||||||
| Events | 775 | 273 | 35.2% | 502 | 64.8% | 306 | 39.5% | 469 | 60.5% | 136 | 17.5% | 231 | 29.8% | 408 | 52.6% | |||
| TIR (%) | 63.4 ± 28 | 70.7 ± 25 | 58.5 ± 30 | <0.001 | 61.1 ± 30 | 61.7 ± 28 | 0.96 | 61.9 ± 29 | 64.2 ± 27 | 59.7 ± 30 | 0.26 | |||||||
| TBR70 (%) | 3.3 ± 11.8 | 3.55 ± 12 | 3.38 ± 12 | 0.81 | 1.7 ± 7 | 4.6 ± 14 | 0.003 | 4.2 ± 14 | 3.0 ± 11 | 3.4 ± 11 | 0.67 | |||||||
| Low glucose events (%) | 11.1 | 10.62 | 11.35 | 0.75 | 7.1 | 13.6 | 0.005 | 11.0 | 9.5 | 12.0 | 0.63 | |||||||
| AUC70 (mg/dL × h) | 119 ± 496 | 134 ± 526 | 112 ± 486 | 0.49 | 41 ± 200 | 171 ± 640 | 0.04 | 148 ± 540 | 96 ± 435 | 124 ± 552 | 0.34 | |||||||
| AUC180 (mg/dL × h) | 186 ± 256 | 134 ± 218 | 215 ± 270 | <0.001 | 204 ± 266 | 175 ± 248 | 0.36 | 176 ± 271 | 164 ± 237 | 204 ± 260 | 0.049 | |||||||
| Average glucose value | 165 ± 42 | 154 ± 38 | 159 ± 44 | <0.001 | 170 ± 42 | 162 ± 43 | 0.01 | 163 ± 44 | 162 ± 40 | 167 ± 43 | 0.38 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Peralta, F.; Valledor, X.; Abreu, C.; Fernández-Rubio, E.; Cotovad, L.; Pujante, P.; Azriel, S.; Pérez-González, J.; Vallejo, A.; Ruiz-Valdepeñas, L.; et al. Nocturnal Glucose Profile According to Timing of Dinner Rapid Insulin and Basal and Rapid Insulin Type: An Insulclock® Connected Insulin Cap-Based Real-World Study. Biomedicines 2024, 12, 1600. https://doi.org/10.3390/biomedicines12071600
Gómez-Peralta F, Valledor X, Abreu C, Fernández-Rubio E, Cotovad L, Pujante P, Azriel S, Pérez-González J, Vallejo A, Ruiz-Valdepeñas L, et al. Nocturnal Glucose Profile According to Timing of Dinner Rapid Insulin and Basal and Rapid Insulin Type: An Insulclock® Connected Insulin Cap-Based Real-World Study. Biomedicines. 2024; 12(7):1600. https://doi.org/10.3390/biomedicines12071600
Chicago/Turabian StyleGómez-Peralta, Fernando, Xoan Valledor, Cristina Abreu, Elsa Fernández-Rubio, Laura Cotovad, Pedro Pujante, Sharona Azriel, Jesús Pérez-González, Alba Vallejo, Luis Ruiz-Valdepeñas, and et al. 2024. "Nocturnal Glucose Profile According to Timing of Dinner Rapid Insulin and Basal and Rapid Insulin Type: An Insulclock® Connected Insulin Cap-Based Real-World Study" Biomedicines 12, no. 7: 1600. https://doi.org/10.3390/biomedicines12071600
APA StyleGómez-Peralta, F., Valledor, X., Abreu, C., Fernández-Rubio, E., Cotovad, L., Pujante, P., Azriel, S., Pérez-González, J., Vallejo, A., Ruiz-Valdepeñas, L., & Corcoy, R. (2024). Nocturnal Glucose Profile According to Timing of Dinner Rapid Insulin and Basal and Rapid Insulin Type: An Insulclock® Connected Insulin Cap-Based Real-World Study. Biomedicines, 12(7), 1600. https://doi.org/10.3390/biomedicines12071600







