Abstract
The approved anthelmintic salicylanilide drug niclosamide has shown promising anticancer and antimicrobial activities. In this study, new niclosamide derivatives with trifluoromethyl, trifluoromethylsulfanyl, and pentafluorosulfanyl substituents replacing the nitro group of niclosamide were prepared (including the ethanolamine salts of two promising salicylanilides) and tested for their anticancer activities against esophageal adenocarcinoma (EAC) cells. In addition, antifungal activity against a panel of Madurella mycetomatis strains, the most abundant causative agent of the neglected tropical disease eumycetoma, was evaluated. The new compounds revealed higher activities against EAC and fungal cells than the parent compound niclosamide. The ethanolamine salt 3a was the most active compound against EAC cells (IC50 = 0.8–1.0 µM), and its anticancer effects were mediated by the downregulation of anti-apoptotic proteins (BCL2 and MCL1) and by decreasing levels of β-catenin and the phosphorylation of STAT3. The plausibility of binding to the latter factors was confirmed by molecular docking. The compounds 2a and 2b showed high in vitro antifungal activity against M. mycetomatis (IC50 = 0.2–0.3 µM) and were not toxic to Galleria mellonella larvae. Slight improvements in the survival rate of G. mellonella larvae infected with M. mycetomatis were observed. Thus, salicylanilides such as 2a and 3a can become new anticancer and antifungal drugs.
1. Introduction
Salicylic acid and its prominent natural and synthetic derivatives possess considerable biological activities [1]. Niclosamide is a salient salicylanilide-based antiparasitic drug developed by Bayer which has been widely applied for the therapy of tapeworm infections since the 1960s [2]. Repurposing studies of this drug revealed promising anticancer and antimicrobial activities [3,4]. The anticancer effects of niclosamide were associated with its function as a mitochondrial uncoupler and its ability to form toxic reactive oxygen and nitrogen species (RONS). Interference with vital cellular signaling pathways (e.g., Wnt/β-catenin, Notch, STAT3, mTOR, or NF-κB) was also documented for niclosamide and structurally related salicylanilides in various cancers [5,6].
Esophageal cancer (EC) is characterized by high global annual incidences (604,100 new cases) and mortality rates (544,076 deaths) with a rising tendency (an increase of 35–37% until 2030). Hot spots for EC are in East, Central, and South Asia, South and East Africa, and Northwest Europe [7,8]. Thus, EC is a growing health problem since a late diagnosis of rapidly growing EC is the common case leading to a poor prognosis with 5-year survival rates below 20% [8,9,10]. The aggressive clinical features render EC a highly challenging cancer disease [11,12]. There are two basic EC subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). While ESCC is predominant globally, EAC incidence is rising especially in Western countries (two-thirds of EC cases) due to obesity and associated diseases such as gastroesophageal reflux disease and Barrett’s esophagus [13,14].
Therapy of EC is confined to endoscopy, surgery, and platinum-based chemoradiotherapy [15,16]. Niclosamide has also been studied in EC models and showed promising results in paclitaxel-resistant EC by the suppression of Wnt/β-catenin [17]. In addition, niclosamide suppressed STAT3 (signal transducer and activator of transcription 3) signaling in EC cells and sensitized them to commonly applied anticancer drugs such as cisplatin and paclitaxel [18]. Of note, increased mitochondrial respiration and glycolytic activity were associated with an enhanced aggressive EAC phenotype, while mitochondrial uncoupling has generally become a suitable target to overcome cancer resistance [19,20,21].
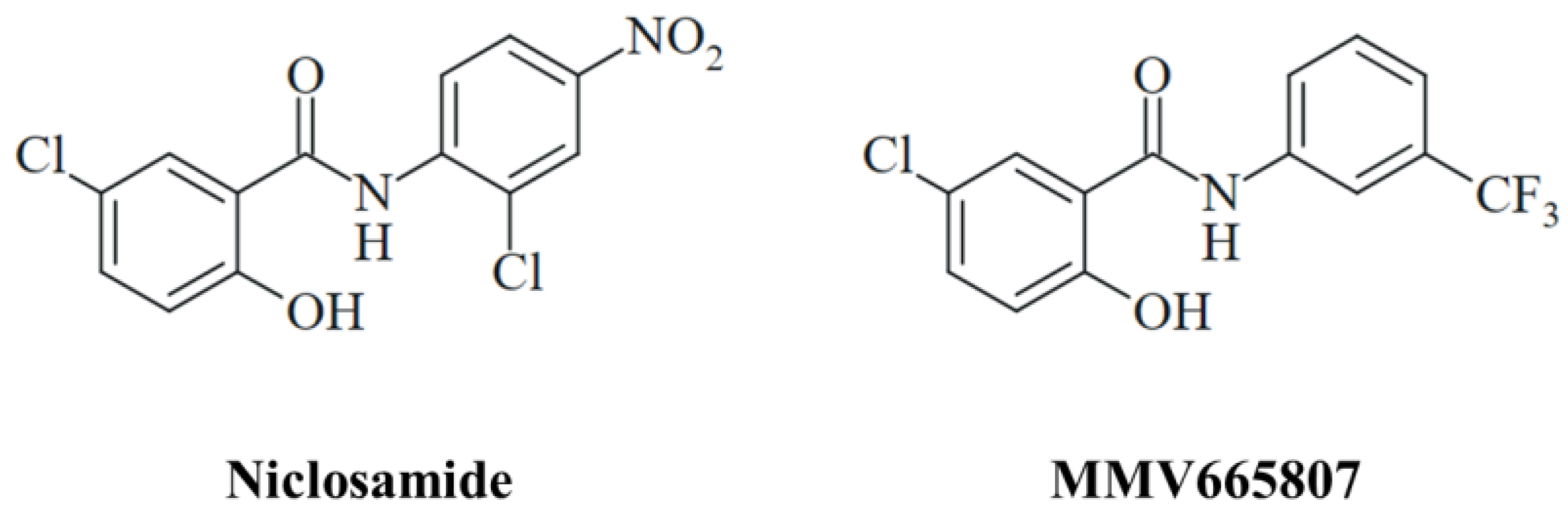
The antimicrobial activities of niclosamide are broad in scope following the paradigm of a “magic blanket”, and the drug exerted especially promising results as an antiviral agent [22,23]. The antifungal properties of niclosamide and related salicylanilides are likewise sound and diverse affecting several human pathogenic fungi including Candida spp., Trichophyton spp., Cryptococcus neoformans, Sporothrix brasiliensis, and Madurella mycetomatis [24]. The latter pathogen, M. mycetomatis, is the causative agent of fungal mycetoma (eumycetoma), which is endemic in the vast regions of the “mycetoma belt” in Asia and Africa and which is classified as a neglected tropical disease (NTD) since current treatment options are inefficient and/or unavailable for the patients [25]. Surgery and the antifungal itraconazole are currently applied for the treatment of eumycetoma [26]. Fosravuconazole displayed promising results in a recent clinical trial with mycetoma patients and might replace itraconazole in the future [27]. Niclosamide, its ethanolamine salt, and the trifluoromethyl substituted salicylanilide derivative MMV665807 showed high activities against two East African M. mycetomatis isolates (Figure 1) [28]. Notably, niclosamide was also active against S. brasiliensis, a fungal pathogen responsible for the formation of sporotrichosis which is another fungal NTD endemic in Brazil [29].
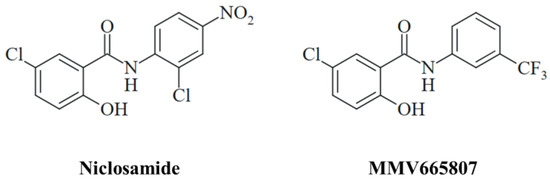
Figure 1.
The structures of the antifungal salicylanilides niclosamide and MMV665807.
Niclosamide has some drawbacks such as genotoxicity based on its chemical structure. It is noteworthy that niclosamide analogs lacking the mutagenic nitro-substituent retained biological activity, and trifluoromethyl substituents (CF3) appear to be especially promising in this regard [28,30,31,32]. The CF3 group is widely applied in drug design because of its substantial pharmacological properties and can be found in several FDA-approved drugs now [33]. Variations such as trifluoromethoxy (OCF3) and trifluoromethylsulfanyl (SCF3) substituents revealed pronounced antigiardial and antibiotic activities [34]. The pentafluorosulfanyl (SF5) group is known as the “super trifluoromethyl group” and was successfully applied for the synthesis of an improved antimalarial 8-SF5 mefloquine analog with higher in vivo activity and a longer half-life than the parent compound [35,36]. In addition, curcuminoids with SF5 substituents showed distinctly increased anticancer activities [37,38]. In the present report, we disclose the synthesis of new CF3- and SF5-substituted niclosamide derivatives and the ethanolamine salts of the two most promising analogs together with their activities against EAC and M. mycetomatis.
2. Materials and Methods
2.1. General Procedures
Column chromatography: silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany). Melting points (uncorrected), Electrothermal 9100 (Thermo Fisher Scientific, Geel, Belgium); IR spectra, Perkin Elmer Spectrum One FT-IR spectrophotometer with ATR sampling unit (Perkin Elmer, Rodgau, Germany; NMR spectra, Bruker Avance 300 spectrometer (Bruker, Billerica, MA, USA); chemical shifts are given in parts per million (δ) downfield from tetramethylsilane as the internal standard; mass spectra, UPLC/Orbitrap (ESI-HRMS, Thermo Fisher Scientific, Geel, Belgium).
2.2. Materials
Starting compounds and reagents were obtained from abcr (Karlsruhe, Germany), Alfa Aesar (Karlsruhe, Germany), Sigma-Aldrich (Darmstadt, Germany), and TCI (Zwijndrecht, Belgium). Niclosamide was purchased from Cayman Chemical (Ann Arbor, MI, USA) for anticancer tests or taken from the COVID-19 response box of MMV/Medicines for Malaria Venture (compound MMV003461) for antifungal tests. The known compounds 2d and 2e were prepared and analyzed according to the literature [39,40].
2.3. Synthesis
2.3.1. Synthesis of New Niclosamide Derivatives—Typical Procedure
Around 0.345 g of 5-chloro-2-hydroxybenzoic acid (2.0 mmol), substituted aniline (1.0 mmol), and 0.385 g of EDCI × HCl (2.0 mmol) were dissolved in CH2Cl2 (10 mL), and the reaction mixture was stirred at room temperature for 24 h. The solution was concentrated under reduced pressure and purified by column chromatography (silica gel 60).
5-Chloro-2-hydroxy-N-(4-pentafluorosulfanylphenyl)benzamide (2a)
Yield: 122 mg (0.33 mmol, 33%); colorless solid of m.p. 222–223 °C; Rf = 0.47 (ethyl acetate/n-hexane, 1:3); υmax(ATR)/cm−1 3006, 1614, 1558, 1506, 1481, 1418, 1350, 1334, 1286, 1221, 1192, 1105, 871, 849, 839, 825, 814, 711, 674, 655; 1H NMR (300 MHz, acetone-d6) δ 7.03 (1 H, d, J = 8.9 Hz), 7.48 (1 H, d, J = 8.9 Hz), 7.8–8.0 (2 H, m), 8.0–8.1 (3 H, m), 10.21 (1 H, s), 11.7–11.8 (1 H, br s); 13C NMR (75.5 MHz, acetone-d6) δ 117.7, 120.6, 121.6, 124.4, 127.7, 128.5, 130.2, 130.6, 135.3, 142.1, 149.8–150.3 (m), 160.3, 168.2; HRMS for C13H10O2NClF5S [M+ + H] calcd. 374.00354, found 374.00250.
5-Chloro-2-hydroxy-N-(4-trifluoromethylsulfanylphenyl)benzamide (2b)
Yield: 172 mg (0.50 mmol, 50%); colorless solid of m.p. 200–201 °C; Rf = 0.56 (ethyl acetate/n-hexane, 1:3); υmax(ATR)/cm−1 3322, 3073, 2943, 2777, 2719, 2568, 1628, 1605, 1589, 1537, 1495, 1420, 1398, 1364, 1322, 1292, 1245, 1220, 1175, 1157, 1142, 1107, 1084, 1013, 968, 894, 855, 827, 818, 773, 756, 724, 703, 683, 654; 1H NMR (300 MHz, acetone-d6) δ 7.04 (1 H, d, J = 8.7 Hz), 7.50 (1 H, d, J = 8.7 Hz), 7.75 (2 H, d, J = 8.9 Hz), 7.97 (2 H, d, J = 8.9 Hz), 8.04 (1 H, s); 13C NMR (75.5 MHz, acetone-d6) δ 117.8, 120.6, 122.8, 124.4, 128.4, 130.2, 130.6, 133.0, 135.2, 138.3, 141.9, 160.3, 168.1; HRMS for C14H10O2NClF3S [M+ + H] calcd. 348.00674, found 348.00589.
5-Chloro-2-hydroxy-N-(3-pentafluorosulfanylphenyl)benzamide (2c)
Yield: 121 mg (0.33 mmol, 33%); colorless solid of m.p. 197 °C; Rf = 0.54 (ethyl acetate/n-hexane, 1:3); υmax(ATR)/cm−1 3329, 3136, 1638, 1617, 1603, 1580, 1562, 1480, 1443, 1435, 1416, 1364, 1332, 1305, 1282, 1216, 1193, 1183, 1150, 1104, 1094, 933, 911, 883, 859, 826, 815, 805, 781, 766, 705, 680, 655; 1H NMR (300 MHz, acetone-d6) δ 7.03 (1 H, d, J = 8.9 Hz), 7.50 (1 H, d, J = 8.9 Hz), 7.6–7.7 (2 H, m), 8.0–8.1 (2 H, m), 8.40 (1 H, s), 10.15 (1 H, s), 11.82 (1 H, s); 13C NMR (75.5 MHz, acetone-d6) δ 117.5, 119.5, 120.7, 122.8, 124.3, 125.6, 128.3, 130.5, 135.3, 139.5, 154.4–154.9 (m), 160.6, 168.4; HRMS for C13H10O2NClF5S [M+ + H] calcd. 374.00354, found 374.00262.
2.3.2. Synthesis of Ethanolamine Salts—Typical Procedure
The niclosamide derivative (0.29 mmol) was dissolved in EtOH (3 mL), and ethanolamine (19 µL, 0.31 mmol) was added. The reaction mixture was stirred for 1 h and then diluted with n-hexane. The formed precipitate was dried in a vacuum.
5-Chloro-2-hydroxy-N-(4-pentafluorosulfanylphenyl)benzamide × ethanolamine (3a)
Yield: 81 mg (0.19 mmol, 66%); colorless gum; 1H NMR (300 MHz, DMSO-d6) δ 2.7–2.8 (2 H, m), 3.5–3.6 (2 H, m), 6.42 (1 H, d, J = 8.9 Hz), 6.96 (1 H, d, J = 8.9 Hz), 7.62 (1 H, s), 7.7–7.9 (4 H, m); 13C NMR (75.5 MHz, DMSO-d6) δ 41.9, 58.9, 112.8, 118.4, 118.7, 120.6, 123.6, 126.7, 128.1, 132.1, 144.0, 145.8–146.2 (m), 166.4, 170.1; HRMS for C13H10O2NClF5S [M+ + H] calcd. 374.00354, found 374.00259; C13H8O2NClF5S [M− − H] calcd. 371.98789, found 371.98883.
5-Chloro-2-hydroxy-N-(4-trifluoromethylsulfanylphenyl)benzamide × ethanolamine (3b)
Yield: 110 mg (0.27 mmol, 93%); colorless gum; 1H NMR (300 MHz, DMSO-d6) δ 2.6–2.7 (2 H, m), 3.4–3.5 (2 H, m), 6.37 (1 H, d, J = 8.9 Hz), 6.92 (1 H, d, J = 8.9 Hz), 7.5–7.6 (3 H, m), 7.78 (2 H, d, J = 8.7 Hz); 13C NMR (75.5 MHz, DMSO-d6) δ 40.1, 61.3, 112.1, 113.6, 118.4, 120.2, 123.7, 128.0, 131.8, 137.4, 143.9, 166.4, 170.6; HRMS for C14H10O2NClF3S [M+ + H] calcd. 348.00674, found 348.00594; C14H8O2NClF3S [M− − H] calcd. 345.99109, found 345.99202.
2.4. Anticancer Activity
2.4.1. Cell Lines and Culture Conditions
The FLO-1, SK-GT-4, and THP-1 cell lines were a gift from Dr. Shrikant Anant’s laboratory. Cells were grown in 10% fetal bovine serum (Fisher Scientific#FB12999102, Pittsburgh, PA, USA) and 1% antibiotic/antimycotic agent (Corning, Tewksbury, MA, USA) in RPMI 1640 media mixed with 25 µM HEPES, L-glutamine (Corning, MA, USA). The cell lines were grown in a 5% CO2 incubator at 37 °C and used below 15 passages for the experiments.
2.4.2. Proliferation Assay
The proliferation assay used a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [41]. Briefly, 5000 FLO-1 and SK-GT-4 cells were counted and seeded in each well in 96-well plates. Post 24 h of incubation, EAC cells were treated with multiple concentrations (0–10 μM) of niclosamide (Cayman chemical#10649, Ann Arbor, MI, USA) and its analogs. At respective time points, 10 μL of stock MTT reagent (Millipore Sigma, Bedford, MA, USA) (3 mg/mL) was added to each well and incubated for 1–2 h under the dark at 37 °C. After incubation, the purple formazan crystals were dissolved in equal volumes of DMSO, and the absorbance of the resulting clear solutions was recorded at 570 nm using a microplate reader [42]. A CCK-8 (Abcam, Cat no. ab228554, Burlingame, CA, USA) assay was used for the THP-1 cells. Briefly, 5000 cells were plated in every well of a 96-well plate. After 24 h of plating, THP-1 cells were treated with niclosamide and compound 3a. After the respective time point, an equal amount of CCK-8 reagent was pipetted to the wells and incubated for one hour in an incubator, and finally, the absorbance was recorded at 450 nm [43]. The percent change in the proliferation was calculated by comparing the cell viability of the treated groups to the untreated control cells.
2.4.3. Colony Formation Assay
In total, 500 SK-GT-4 and FLO-1 cells were seeded in each well of 6-well plates before 24 h treatment of niclosamide analog 3a IC50 concentration and an equivalent dose of niclosamide. The media was changed after 72 h with drug-free media to remove drug exposure and allow them to grow and form colonies for 10–12 days. At the end of the experiment, the colonies were washed using PBS three times, fixed with 10% formalin solution (Fisher Scientific#23-305510, Pittsburgh, PA, USA), and stained using a 1% crystal violet (Sigma-Aldrich#C6158, St. Louis, MO, USA) solution in 10% ethanol for 15 min. The colonies were then washed with water, dried at room temperature, counted, and imaged. The number of colonies in the treated cells was compared with untreated cells [44].
2.4.4. Spheroid Formation Assay
In the ultra-low attachment plates (Corning, Lowell, MA, USA), 500 SK-GT-4 cells were seeded in each well with the spheroid media [DMEM medium supplemented with EGF (20 ng/mL), FGF (20 ng/mL), heparin (4 µg/mL), B27 (10 mL in 500 mL of 50×) (Invitrogen, Carlsbad, CA, USA), and antibiotic pen/strep (1%)]. After 24 h, spheroids were treated with 3a (IC50 dose) and an equivalent dose of niclosamide. Spheroids were counted after 7 days, and images were taken [44].
2.4.5. Western Blot Analysis [45]
A total of 500,000 FLO-1 and SK-GT-4 cells were seeded in 10 cm2 dishes. After 24 h, cells were incubated with a vehicle, niclosamide analog 3a (IC50 concentration), and niclosamide (equivalent dose of 3a IC50 concentration). Then, 72 h post-treatment with the compounds, the media were removed to eliminate the dead/floating cells and washed with PBS. The cells were then scraped using a cell scraper in the lysis buffer containing a protease/phosphatase inhibitor cocktail (Thermo Scientific# A32959, Rockford, IL, USA). Further cells were lysed and sonicated using a probe sonicator (Fisher Scientific Model no FB120). The resulting lysates were centrifuged at 6500 rpm and 4 °C for 10 min. The BSA (Thermo Scientific, Rockford, IL, USA) method was used to estimate total protein levels in the prepared cell lysates, which were diluted with lysis buffer mixed with 2× Laemmli buffer (Biorad#1610737, Hercules, CA, USA) and boiled for 5 min at 37 °C. Equal volumes of protein (50 μg) were used on the SDS-PAGE for separation using gel electrophoresis. Then, the gel was transferred to a PVDF membrane (Immobilon, Millipore, Bedford, MA, USA) using a Biorad’s transfer assembly for 2 h at 90 V. The membranes were removed, incubated in 5% milk powder dissolved in TBST for one hour, washed three times with TBST for 5 min each, and incubated with respective primary antibodies overnight at 4 °C on a shaker. The following day, the blots were washed with TBST three times for 5 min each and incubated with the respective anti-rabbit (Invitrogen#31460) and anti-mouse HRP (Invitrogen#31430) secondary antibodies for 1 h. The membranes were washed with TBST thrice for 7 min each and visualized by Amersham ECL Western Blotting Detection Reagent (Cytiva#RPN2106, Marlborough, MA, USA). The blots were developed using a chemiluminescence system ChemiDoc-XRS+ instrument (Biorad), and Biorad’s image lab program was used to prepare images. All primary antibodies were purchased from cell signaling technology (CST, Beverly, MA, USA), including BCL2 (CST cat no. 4223), BCL-XL (CST cat no. 2762), Bax (CST cat no. 2772), PARP (CST cat no. 9532), p-STAT3 (CST cat no. 4113s), and non-phospho-(Active)-β-Catenin (CST cat no. 8814S) antibodies, while GAPDH (G-9) (sc-365062) was obtained from Santa Cruz Biotech, Inc. (Santa Cruz, CA, USA). All antibodies were diluted in 5% BSA (Sigma-Aldrich#1003561707) in TBST at a 1:1000 dilution.
2.4.6. Molecular Docking
AutoDock Vina 1.1.2 software from the Molecular Graphics Lab, Scripps Research Institute, http://vina.scripps.edu/ (accessed on 20 April 2024), was used for molecular docking of compounds 2a and niclosamide with STAT3 (PDB: 6NJS) and β-catenin (PDB: 1JDH) [46,47,48]. Chain A was selected by removing ligands (SD36 from 6NJS) and other protein chains (hTCF4 from 1JDH) before the molecular docking. Default parameters were applied to STAT3 and β-catenin proteins, niclosamide, and its analog for docking using Autodock tools 1.5.7. Polar hydrogens and Kollman and Gasteiger charges were applied to proteins and ligands prior to the docking. A grid box was designed around the SH2 domain in the case of STAT3 and the TCF-4-binding site in the case of β-catenin. The best protein–ligand conformations (approximately 10) were determined with Lamarckian GA. The most stable ligand–protein complex was selected based on the interaction with key hydrogen bonds and the lowest binding energy. The drug–protein complex was visualized using Pymol, https://pymol.org/2/ (accessed on 20 April 2024) [49].
2.5. Antifungal Activity
2.5.1. Compound Screening
The antifungal activity of the compounds was tested against a geographically and genetically diverse set of M. mycetomatis isolates. These included the MM55 strain from Sudan, strains I1, I3, and I11 from India, strain P1 from Mali, strain SO1 from Somalia, strain Peru72012 from Peru, and strain CBS247.48 whose country of origin was unknown. All isolates were originally obtained from patients and identified and maintained in the Erasmus Medical Centre, Rotterdam, The Netherlands. In vitro susceptibility testing was performed as reported previously [50,51]. The only difference was that tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) was used as a viability dye in endpoint reading instead of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT).
2.5.2. Toxicity and Activity in Galleria mellonella
In vivo efficacy was determined in larvae of the greater wax moth Galleria mellonella. Since G. mellonella is an invertebrate, it is not subject to directive 2010/63/EU of the European law on animal testing. Larvae were housed in the dark in Petri dishes with Whatman paper. To determine the toxicity in G. mellonella larvae, test compounds were injected on three consecutive days at 24 h intervals in the last pro-leg of healthy larvae followed by monitoring of the survival for ten days. If no significant difference between the control and treated larvae was observed, the compound was considered nontoxic. Larvae were further used in infection studies to determine the in vivo activity of test compounds against M. mycetomatis according to our previously published protocol [52].
2.6. DMPK Analysis
Kinetic solubility was tested using 10 mM stock solutions of test compounds in DMSO followed by dilution with an aqueous buffer to a final concentration of 250 µM and vortexed at 900 rpm for 24 h. Then, the samples were filtered and analyzed by UHPLC (Shimadzu Nexera X2, Milton Keynes, UK).
CHI (chromatographic hydrophobicity index) logD was determined according to previously published methods [53,54]. Test compounds were prepared as 0.5 mM solutions in 50:50 acetonitrile/water and analyzed by reversed-phase UHPLC (wavelength 254 nm). By plotting the retention time of a set of reference compounds against known CHI values, the CHI value of test compounds was calculated according to their retention time, and this was then further converted to CHI logD.
Liver microsomes were applied to study the microsomal clearance of test compounds. The test compound (0.5 µM) was incubated with female CD1 mouse liver microsomes (0.5 mg/mL, 50 mM potassium phosphate buffer, pH 7.4), and the reaction started with the addition of excess NADPH (8 mg/mL, 50 mM potassium phosphate buffer, and pH 7.4). Immediately, at time 0, then at 3, 6, 9, 15, and 30 min, an aliquot of the incubation mixture was removed and mixed with acetonitrile to stop the reaction. An internal standard (IS) was added to all samples, the samples were centrifuged to sediment precipitated protein, and the plates were then sealed prior to UPLC-MSMS analysis (Xevo TQ-S Micro, WatersTM, Milford, MA, USA). An exponential decay fit was created from the response ratio (compound peak area/IS peak area) and incubation time, and subsequently, Clint (mL/min/mg protein) was calculated by multiplying the rate constant (k) of the exponential decay curve and incubation conditions (incubation volume/mg protein added).
HepG2 hepatoma cells were used for hepatotoxicity studies. Cells were provided by ECACC with a certificate of analysis confirming STR profiling and were mycoplasma tested every time new stabilates were made. Cells were screened utilizing a resazurin-based fluorescence assay (λex = 540 nm, λem = 590 nm) in a 384-well plate format. HepG2 cells (1 × 105 cells/mL) were incubated with the compound in a 10-point, 1 in 3 dilution series from a top concentration of 100 µM, for 72 h. After the 72 h incubation period, resazurin (0.27 mM in a cell culture medium) was added and the plates were incubated for a further 3–4 h before the fluorescent signal was measured using PHERAstar (BMG Labtech, Ortenberg, Germany). IC50 values were calculated from two independent experiments.
2.7. Statistical Analysis
GraphPad Prism 7 (GraphPad Inc., Boston, MA, USA) was applied for the log-rank test, one-way ANOVA tests, and IC50 calculations. A p-value smaller than 0.05 was considered to be significant. * p < 0.05 and ** p < 0.01.
3. Results
3.1. Chemistry
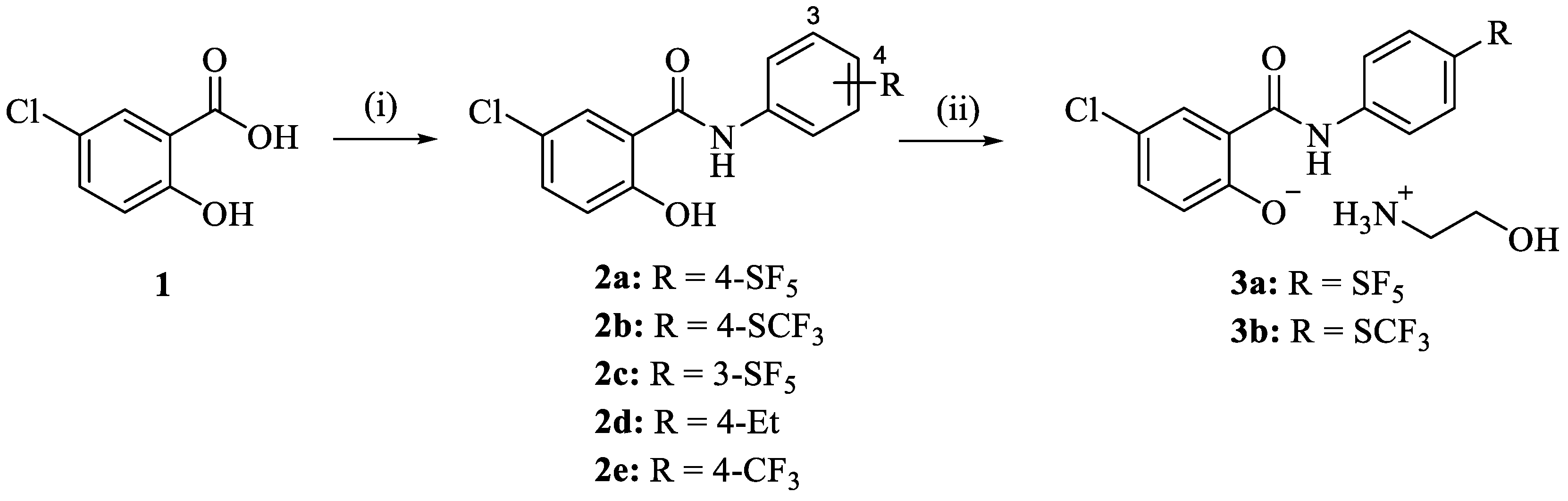
The niclosamide derivatives 2a–e were obtained from the reaction of 5-chlorosalicylic acid (1) with the respective substituted aniline. Compounds 2a and 2b were converted to their corresponding ethanolammonium salts 3a and 3b by reaction with ethanolamine in EtOH (Scheme 1). While 2d and 2e are known compounds prepared for comparison purposes in the following biological activity experiments, analogs 2a–c and the phenolate salts 3a and 3b are new [39,40]. Compounds 2a–e were obtained as solids, while the salts 3a and 3b were amorphous solids or gums. The 1H NMR spectra of 2a–e displayed the aryl protons between 7.0 and 8.1 ppm. The 13C NMR spectra of 2a–c showed the amide carbonyl and phenolic hydroxyl-carbon signals at 168.1–168.4 and 160.3–160.6 ppm, respectively. Some differences were observed in the NMR spectra of the ethanolamine salts 3a and 3b. Aryl protons of the salicyl ring showed highfield shifts in the 1H NMR spectra (e.g., 6.4 ppm for the most highfield aryl doublet signal), while the phenolate carbon (166.4 ppm) and the carbonyl signal (170.1–170.6 ppm) exhibited downfield shifts in the 13C NMR spectra when compared with the corresponding signals of their phenolic precursors 2a and 2b. In addition, proton (2.6–2.8, 3.4–3.6 ppm) and carbon signals (40.1–41.9 ppm, 58.9–61.3 ppm) of the ethanolamine component were observed in the NMR spectra of 3a and 3b. HR-MS detected the molecular ions of the new compounds 2a–c, 3a, and 3b. Original spectra (NMR and ESI-MS) of the target compounds can be found in the Supplementary Materials (Figures S1–S19).
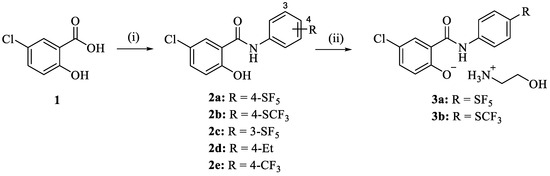
Scheme 1.
Reagents and conditions: (i) subst. aniline, EDCI, CH2Cl2, r.t., 24 h, 33–50%; (ii) ethanolamine, EtOH, r.t., 1 h, 66–93%.
3.2. Anticancer Activity
Compounds 2a–d, 3a, 3b, and the parent drug niclosamide were initially tested for antiproliferative activity against SK-GT-4 and FLO-1 EAC cell lines (Table 1). 4-Pentafluorosulfanylanilide salt 3a was the most active compound against both tested cell lines (IC50 = 1.03 µM for SK-GT-4 and 0.81 µM for FLO-1 cells) and slightly more active than niclosamide (IC50 = 1.77 µM for SK-GT-4 and 1.05 µM for FLO-1 cells) and cisplatin (IC50 = 2.28 µM for SK-GT-4 and 1.70 µM for FLO-1 cells) after 72 h. Compound 3a exhibited promising sub-micromolar activity against FLO-1 cells. Compounds 2a (4-pentafluorosulfanylanilide), 2b (4-trifluoromethylanilide), and 2c (3-pentafluorosulfanylanilide) as well as the salt 3b conserved the anticancer activity of niclosamide, but they were slightly less active against FLO-1 cells than niclosamide. 4-Ethylanilide 2d was the least active compound of this compound series. All compounds showed antiproliferative activities against EAC cell lines in the low micromolar concentration range.

Table 1.
Antiproliferative activity (IC50 values in µM) 1 of test compounds 2a–d, 3a, and 3b against SK-GT-4 and FLO-1 EAC cells after 72 h. Niclosamide and cisplatin served as positive controls.
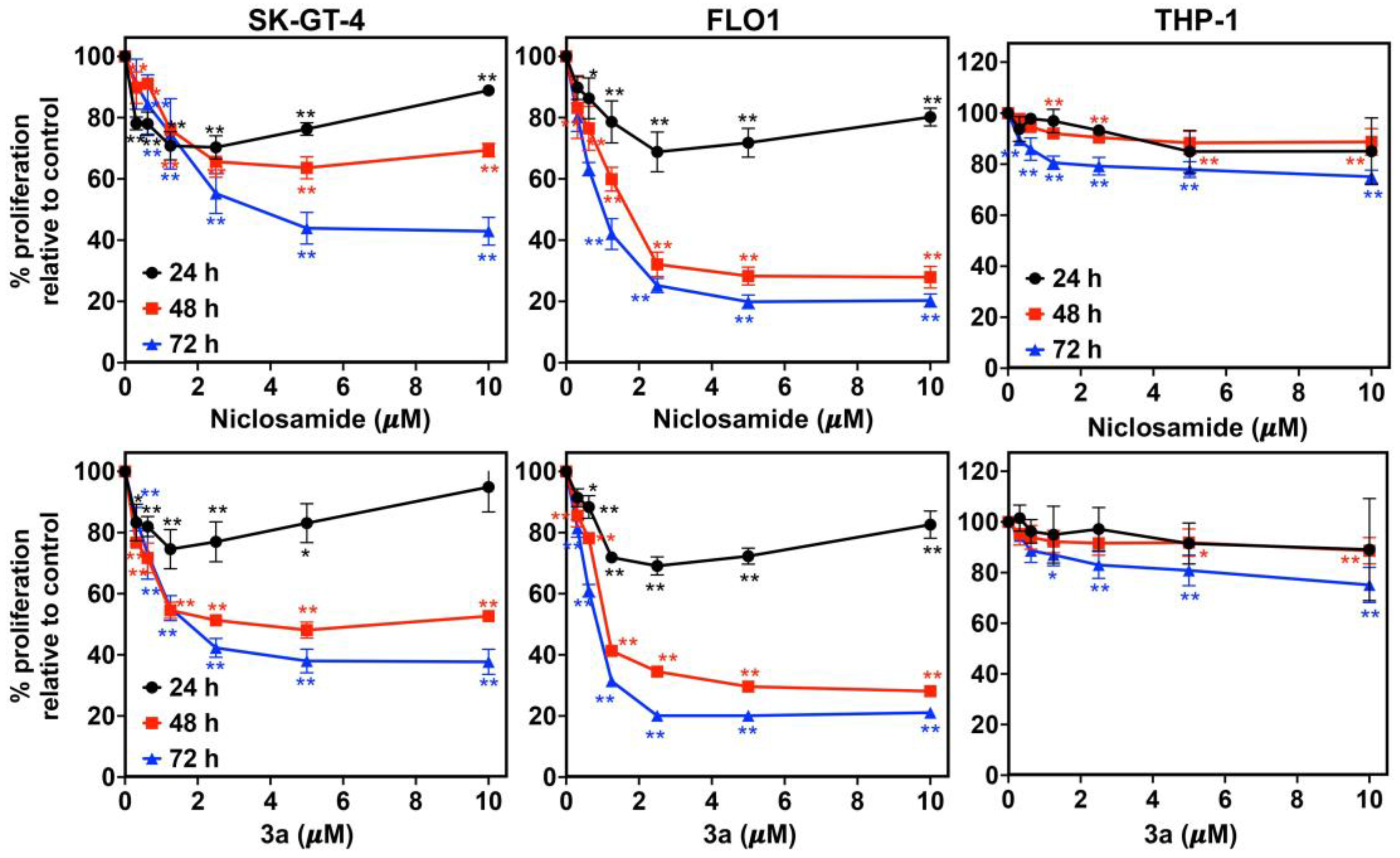
Compound 3a exhibited dose- and time-dependent antiproliferative activities against SK-GT-4 and FLO-1 cells, analogously to niclosamide (Figure 2). While their activity was low after 24 h, it increased significantly after 48 h to reach a maximum activity after 72 h. In addition, 3a did not affect the proliferation of non-malignant THP-1 monocyte cells (IC50 > 10 µM), which indicates a distinct tumor selectivity for this compound.
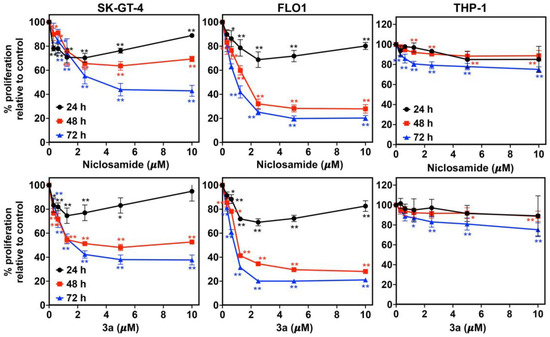
Figure 2.
Time- and dose-dependent activity of niclosamide (upper row) and 3a (bottom row) against EAC cell lines (SK-GT-4 and FLO-1) and THP-1 monocytes. * p < 0.05 and ** p < 0.01 as analyzed by one-way ANOVA test.
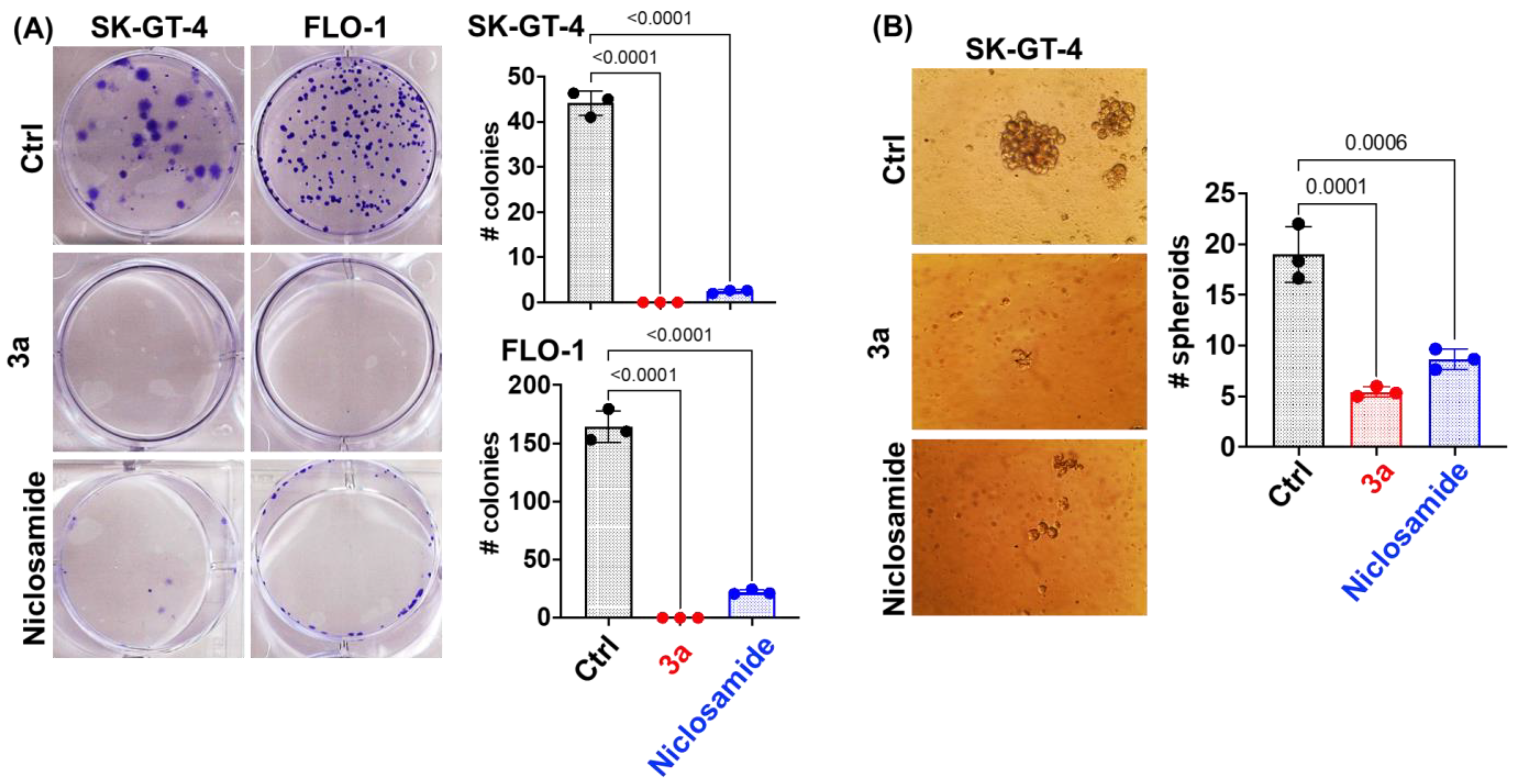
We used an IC50 concentration of 3a at 48 h (~1.5 and ~0.8 µM for SK-GT-4 and FLO-1, respectively) and an equivalent concentration of niclosamide for further studies. Compound 3a and niclosamide were tested for their long-term effect on the clonogenic potential of EAC cells (SK-GT-4 and FLO-1) using the colony formation assay (Figure 3A). Compound 3a exhibited a complete inhibition of colony formation (size and number) in both EAC cell lines indicating an irreversible anticancer effect. Niclosamide was slightly less efficient than 3a. The eradication of cancer stem cells (CSCs) is an attractive therapeutic strategy since they play a crucial role in EAC aggressiveness and resistance [55]. A spheroid formation assay was applied to evaluate the effects of 3a and niclosamide on CSCs. Compound 3a strongly inhibited spheroid formation by SK-GT-4 cells and outperformed niclosamide again (Figure 3B).
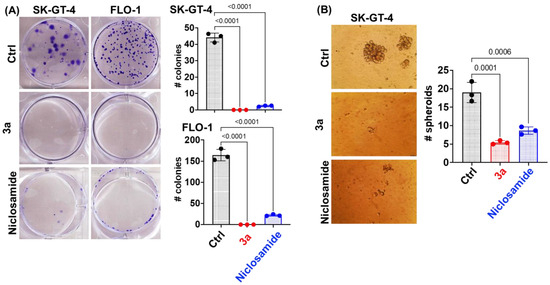
Figure 3.
(A) Niclosamide and 3a (1.5 and 0.8 µM for SK-GT-4 and FLO-1, respectively) inhibit colony formation by EAC cells at 48 h time point. (B) Niclosamide and 3a (1.5 µM) inhibit spheroid formation by SK-GT-4 cells.
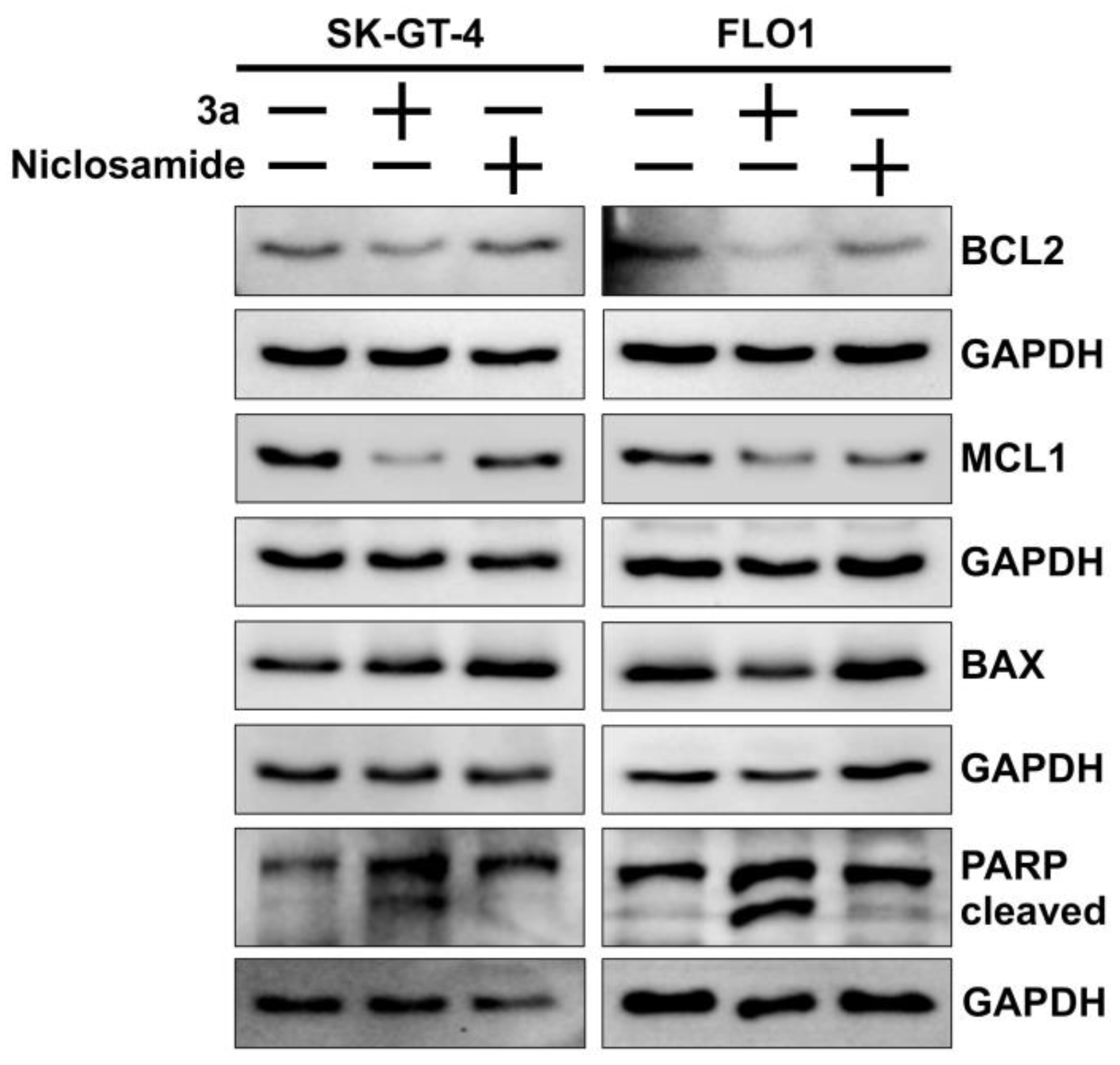
The colony formation and spheroid inhibition assays indicate a considerable induction of cancer cell death such as apoptosis by 3a. Apoptosis is a form of programmed cell death, and anti-apoptotic mechanisms are a hallmark of resistant cancers [56]. Western blot analyses showed that the anti-apoptotic proteins BCL2 (B-cell lymphoma 2) and MCL1 (myeloid cell leukemia 1) were downregulated in EAC cells treated with 3a while the expression of pro-apoptotic BAX (BCL2-associated X) did not differ from untreated cells (Figure 4). In addition, 3a exhibited stronger PARP cleavage as a sign of apoptosis and a higher suppression of BCL2 and MCL1 than niclosamide in both EAC cell lines.
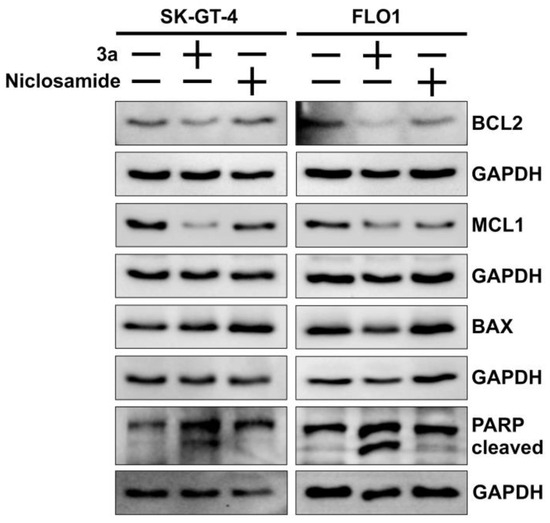
Figure 4.
Effects of niclosamide and 3a (1.5 and 0.8 µM for SK-GT-4 and FLO-1, respectively) on PARP cleavage, and expression of pro-apoptotic BAX and anti-apoptotic BCL2 and MCL1 in EAC cell lines. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) served as control.
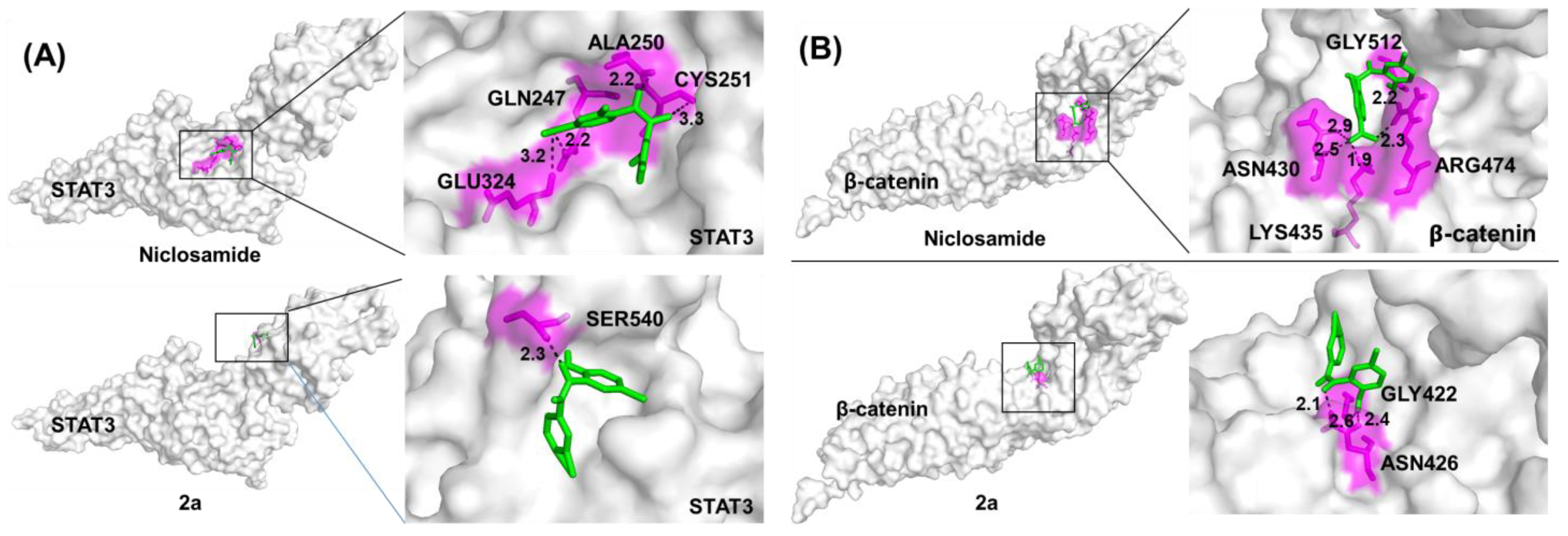
To identify possible protein targets, we carried out Autodock vina molecular docking calculations of 2a (the phenolic precursor of the salt 3a) and niclosamide bound to the protein structures of STAT3 and β-catenin, both of which are known niclosamide targets in cancer cells (Figure 5). To understand the interaction of compound 2a and niclosamide in STAT3 and the β-catenin protein, we selected the SH2 domain and the TCF4-binding site, respectively. Compound 2a bound to STAT3 and β-catenin with improved binding energies (7.2–7.3 kcal/mol) when compared with niclosamide (6.1–6.6 kcal/mol) indicating higher affinities of 2a for both targets when compared with niclosamide (Table 2). Notably, the binding modes of niclosamide and 2a differed considerably in both investigated protein targets. Both compounds were differently oriented in the binding cavities and formed H bonds with different amino acids of the binding sites of STAT3 and β-catenin (Figure 5, Table 2). In addition, 2a required fewer H bonds than niclosamide to generate higher affinities for the binding sites.
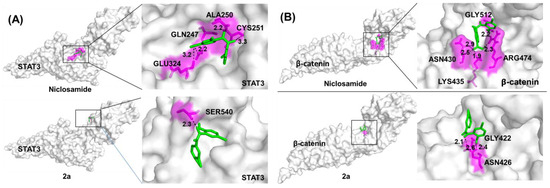
Figure 5.
Molecular docking of niclosamide and 2a (A) in the protein cavity of STAT3 and (B) in the protein cavity of β-catenin at the TCF4 binding site using the Autodock vina software program. Surface view of the binding mode. White: protein; pink: interacting amino acid; green: compound; black dotted line: H bond.

Table 2.
Docking scores for the binding of niclosamide and 2a to STAT3 and β-catenin determined with Autodock vina.
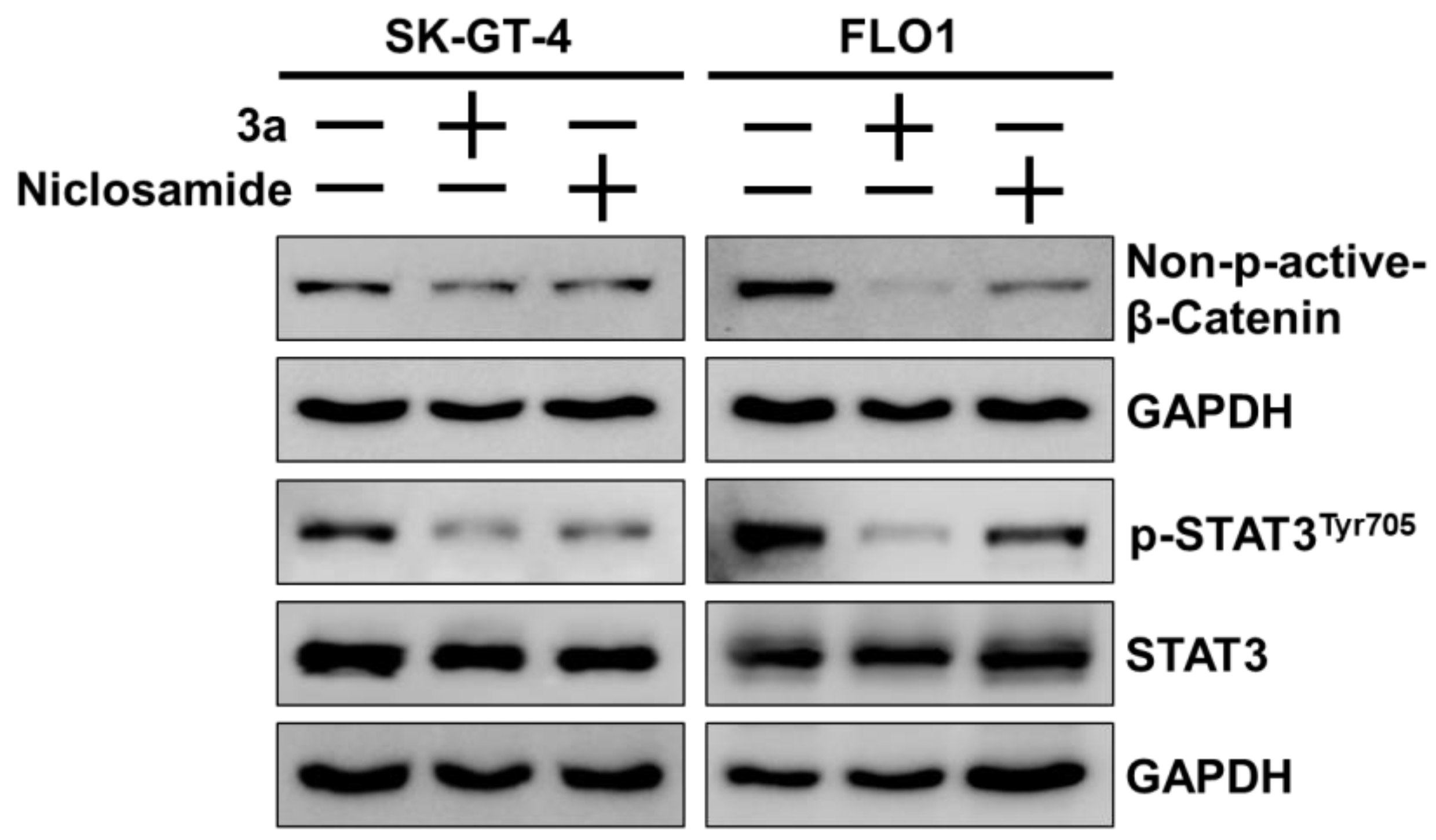
The STAT3 and β-catenin docking results were experimentally validated by Western blots in SK-GT-4 and FLO-1 cells treated with 3a or niclosamide (Figure 6). Compound 3a strongly suppressed activating the phosphorylation of STAT3 at TYR705 in both EAC cell lines without visible effects on the expression of STAT3. Niclosamide also inhibited activating STAT3 phosphorylation but not as strongly as 3a. Likewise, niclosamide and, to a greater extent, 3a suppressed active (non-phosphorylated) β-catenin in SK-GT-1 and FLO-1 cells, while these effects were more pronounced in FLO-1 cells than in SK-GT-4 cells.
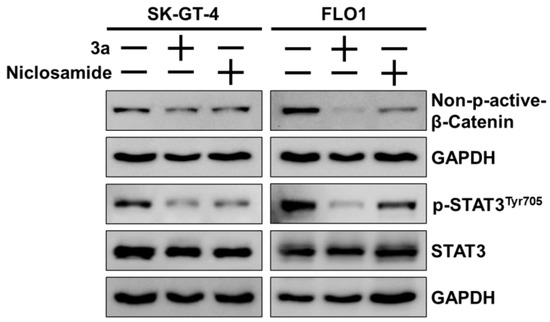
Figure 6.
Effects of niclosamide and 3a (1.5 and 0.8 µM for SK-GT-4 and FLO-1, respectively) on levels of p-STAT3Tyr705, STAT3, and (active) non-phospho-β-catenin in EAC cell lines. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) served as control.
3.3. Antifungal Activity
Initially, compounds 2a–e, 3a, and 3b were tested for their growth inhibitory activity against the M. mycetomatis MM55 strain from Sudan (Table 3). Compounds 2a and 2b exhibited the highest activities against M. mycetomatis in the low nanomolar concentration range (IC50 = 0.3 µM and 0.2 µM, respectively). The corresponding ethanolamine salts 3a and 3b were slightly less active (IC50 = 0.9 µM each) than their phenol precursors. Compounds 2c and 2e showed similar activities (IC50 = 1.3 µM and 0.9 µM, respectively) while 4-ethylanilide 2d was the least active compound of this series (IC50 = 6.6 µM). However, all tested niclosamide derivatives were distinctly more active than the parent drug niclosamide (IC50 = 15.0 µM, IC90 = 23.8 µM).

Table 3.
Antifungal activities (IC50 and IC90 values in µM) 1 of niclosamide and test compounds 2a–e, 3a, and 3b against M. mycetomatis MM55 strain.
Compounds 2a–c and 2e were also tested for their activity (visualized as MIC values) against a panel of eight geographically and genetically diverse M. mycetomatis strains, and MIC50 values (medium inhibitory concentration, the concentration at which 50% of the isolates were completely inhibited) were determined thereof (Table 4). Again, 2a and 2b turned out to be the most active compounds with excellent MIC50 values of 0.5 µM. The Peru72012 strain turned out to be the most sensitive strain (IC50 = 0.3 µM for 2a and 2b). Of note, niclosamide (IC50 = 2.38 µM) and the 3-trifluoromethylanilide analog MMV665807 (IC50 = 9.93 µM) were reportedly less active than 2a and 2b (IC50 = 0.5 µM) against the Somalian SO1 isolate [28]. Compounds 2c and 2e were slightly less active (MIC = 1.0 µM) than 2a and 2b. However, 2c was distinctly active against M. mycetomatis P1 (IC50 = 0.5 µM) and performed within the activity range of 2a and 2b in this strain. The lowest activities (i.e., IC50 = 2.0 µM for 2c and 2e) were found in I11, MM55, and CBS-247.48 strains.

Table 4.
Antifungal activities (MIC values in µM) of 2a–c and 2e against eight M. mycetomatis strains and corresponding MIC50 values.
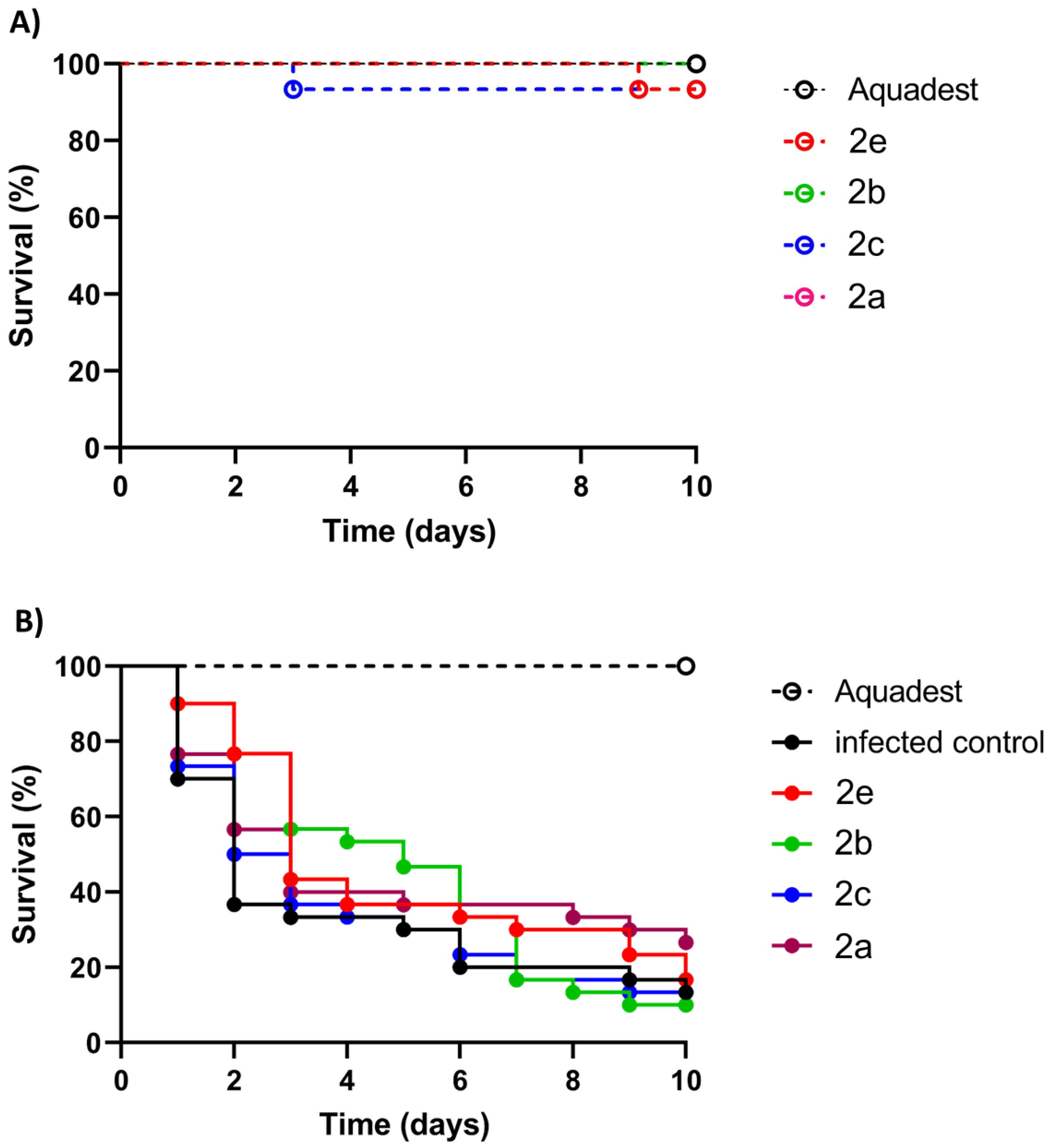
The highly active compounds 2a–c and 2e were selected for in vivo activity testing using larvae of the wax moth Galleria mellonella, which is a well-established invertebrate animal model for the evaluation of fungal infections [57]. At first, the general toxicity of 2a–c and 2e to non-infected G. mellonella larvae at a high concentration of 20 µM was evaluated (Figure 7A). All tested compounds (2a–c and 2e) were non-toxic and showed a promising toxicity profile because the survival curves were above 80% after compound injection. Thus, the compounds were suitable for in vivo experiments with G. mellonella larvae infected with the M. mycetomatis MM55 strain (Figure 7B, Table 5). Although a higher survival percentage was obtained in larvae treated with 2a (26.7% vs. 13.33%), this difference was not statistically significant. Compounds 2b, 2c, and 2e showed no increased survival after ten days. Although 2b showed no improvement after ten days, a considerable temporary effect was observed between days three and six before its efficacy diminished. In terms of the AUC (area under the curve) values, 2a, 2b, and 2e showed comparable effects; however, the increases were statistically not significant. Compound 2c was the least effective compound in the in vivo assay with infected G. mellonella larvae.
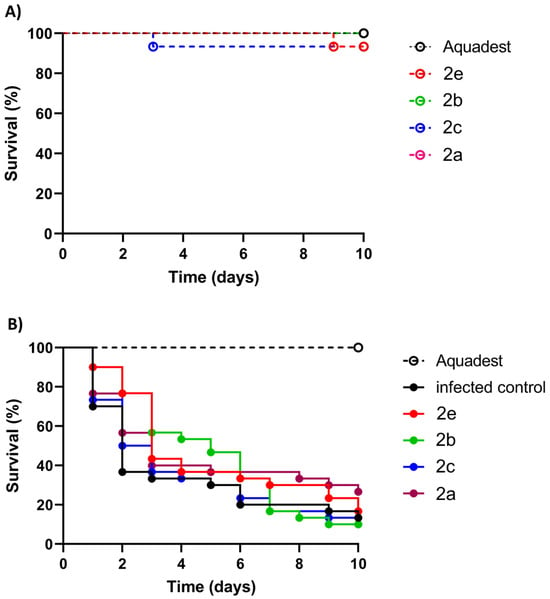
Figure 7.
Evaluation of compounds 2a–c and 2e (20 µM) in Galleria mellonella larvae. (A) Toxicity evaluation of test compounds for 10 days indicated by larval survival (in %). (B) Activity of test compounds in M. mycetomatis MM55-infected G. mellonella larvae indicated by larval survival (in %).

Table 5.
G. mellonella larval survival data after 10 days (d10) for M. mycetomatis-infected larvae treated with 2a–c and 2e (20 µM). AUC, the area under the curve (associated graphs, see Figure 2). Control, untreated infected G. mellonella larvae.
3.4. DMPK Analysis
To evaluate the drug-like properties of the most promising antifungal compounds 2a and 2b, DMPK (drug metabolism and pharmacokinetics) analyses were carried out (Table 6). The experimental logD values of 2a and 2b were between 3 and 5 indicating poor solubility and increased metabolic clearance, which was confirmed for 2b by the microsomal clearance assays. Compound 2a, however, showed a reasonable clearance and half-life (>30 min) in liver microsomes. HepG2 hepatoma cells of low tumorigenicity were used as a liver cell model for drug toxicity studies. Both 2a and 2b were toxic to HepG2 albeit less toxic than to EAC cells. Kinetic solubility experiments revealed low solubility for 2a (0.67 µM) and stability issues for 2b.

Table 6.
Drug metabolism and pharmacokinetics (DMPK) study of 2a and 2b including logD values, microsomal clearance and half-life, and cytotoxic activity against HepG2 hepatoma cells (IC50 values from two independent experiments) using resazurin assay.
4. Discussion
The new compounds 2a and 2b are potential drug candidates obtained by a simple synthetic procedure from commercially available starting materials. This enables large-scale syntheses and the mass production of these drug candidates which is of importance for future clinical studies and global applications [24,58]. Compounds 2a and 2b displayed improved activities against the fungus M. mycetomatis when compared with the activity of the parent compound niclosamide, an approved anthelminthic. In addition, the ethanolammonium salt 3a revealed higher anticancer activities against EAC cell models when compared with the activities of niclosamide and cisplatin, and it was not toxic to non-malignant THP-1 cells. Thus, the replacement of the problematic nitro group of niclosamide by SF5 and SCF3 moieties conserved and even enhanced the anticancer and antifungal activities of niclosamide. Notably, a SF5 and SCF3 substitution of the anilide ring appeared superior to the ethyl and CF3 substitution of compounds 2d and 2e. The 3-pentafluorosulfanylanilide 2c was less antifungal than 2a and 2b except for one strain (P1), but 2c exhibited better anticancer activities against EAC than 2a and 2b. The described positive effects of SF5 and SCF3 groups on biological activities are in line with previously disclosed antimicrobial, antiparasitic, and anticancer compounds [34,35,36,37,38].
Given the limited arsenal of anticancer drugs available for the therapy of EAC, the superior activities of the new SF5-substituted salicylanilides in comparison with the performance of the standard drug cisplatin are promising [15,16]. A deeper investigation of the anticancer activity of salt 3a against EAC cells revealed persistent long-term effects by blocking EAC cell colony formation and inhibiting EAC spheroid growth. The strong suppression of the anti-apoptotic proteins BCL2 and MCL1 indicates apoptosis induction as a mode of cell death in EAC cells treated with 3a. Of note, BCL2 expression was correlated with EAC development, and the downregulation of MCL1 was associated with apoptosis induction in EAC cells [59,60]. Since cancer resistance to the BCL2 inhibitor venetoclax is based on the upregulation of alternative anti-apoptotic factors such as MCL1, the suppression of both BCL2 and MCL1 by 3a appears especially promising [61]. The reason why salt 3a is more anticancer-active than 2a is unclear; however, in a study with liver cancer cells, niclosamide ethanolamine also showed slightly higher cytotoxicities than niclosamide [62].
Mechanistically, niclosamide is an inhibitor of Wnt/β-catenin and STAT3 signaling [17,18]. Molecular docking of salicylanilide 2a revealed STAT3 and β-catenin as possible drug targets of its phenolate 3a, which was confirmed in EAC cells by Western blotting experiments. Notably, 3a turned out to be a stronger inhibitor of STAT3 and β-catenin than niclosamide. STAT3 signaling plays a vital role in EAC including FLO-1 cells and was connected with anti-apoptotic BCL2 and MCL1 expression levels [63,64]. In addition, β-catenin was found to be a crucial factor in FLO-1 cells and a valuable drug target in resistant EAC cells [65,66]. Oncogenic Wnt/β-catenin signaling is closely associated with drug resistance, cancer stemness, and tumor initiation [67]. Hence, small-molecule inhibitors of the Wnt/β-catenin pathway can potentially become valuable anticancer drugs [68].
Compound 3a revealed a low toxicity to THP-1 monocytes. In vivo experiments with G. mellonella larvae exhibited generally low or even absent toxicities of compounds 2a–c and 2e. Despite the low toxicity, none of the compounds were able to prolong the survival of M. mycetomatis-infected larvae at the applied doses of 20 µM. Because of the low toxicity, higher doses than 20 µM and/or prolonged treatment might be applied in future studies to achieve better responses by infected larvae. A combination with other antifungals might also be a reasonable strategy to improve the therapeutic outcome.
An important mode of action of niclosamide is based on its uncoupling properties accompanied by ATP depletion. Several azole drugs also decrease ATP levels via fungal cell membrane damage [69]. However, suppressed ATP levels as a sign of toxicity were documented in hepatocytes treated with itraconazole and fluconazole [70]. While no toxic effects on G. mellonella larvae and THP-1 cells were found, toxicity to HepG2 hepatoma cells was observed for 2a and 2b (IC50 = 2.3–3.0 µM), which was slightly lower than their toxic effects on EAC cells. However, the activity of 2a and 2b against HepG2 cells was distinctly lower than their activity against M. mycetomatis cells. It might indicate activity against liver cancer, though, since they were only slightly less active against HepG2 cells than a published SF5-substituted vorinostat analog (IC50 = 1.8 µM) which was designed as an anticancer agent [71]. Certain azoles such as ketoconazole and posaconazole were also toxic to HepG2 cells at higher concentrations, which was associated with reduced cellular ATP levels [72]. In liver microsomes, 2a exhibited a reasonable stability and half-life which reminds us of the improved pharmacokinetics of a SF5-substituted mefloquine analog [35,36]. However, caution is advised for these data because of the low solubility of 2a, which can cause issues with PK properties and analysis (e.g., a low MS response was found for 2a in the clearance assay). The solubility problem can be solved by salt formulations such as 3a. Compound 2b was found to be rather unstable which might be attributed to the SCF3 substituent being sensitive to oxidation to sulfoxides [73]. How far such sulfoxides contribute to the biological activity of 2b remains to be shown.
5. Conclusions
The synthesis of SF5- and SCF3-substituted niclosamide-type salicylanilides is straightforward, and the new compounds 2a–c and 3a revealed distinct anticancer and antifungal activities. Mechanistic studies in EAC cells showed considerable interference with BCL2- and MCL1-associated anti-apoptotic mechanisms and suppression of crucial oncogenic signaling pathways by inhibiting STAT3 and β-catenin. Unique binding modes of 3a in STAT3 and β-catenin, which differed distinctly from niclosamide binding to these targets, were visualized by docking calculations. Together with low toxicities found in non-malignant monocytes and invertebrate animals, compounds such as 2a and 3a have great potential to become new anticancer and antifungal drugs in the future. Only a closer look at possible hepatotoxic effects seems to be necessary in advanced animal studies of these compounds with vertebrate models.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12071621/s1, Figures S1–S12: 1H and 13C NMR spectra; Figures S13–S19: ESI-HRMS spectra.
Author Contributions
Conceptualization, L.W., W.v.d.S., P.D. and B.B.; methodology, J.M., D.V., N.M., L.W., W.v.d.S., P.D. and B.B.; software, P.D.; validation, J.M., D.V., N.M., G.B. and B.B.; formal analysis, J.M., D.V., H.G., N.M., G.B. and B.B.; investigation, J.M., D.V., H.G., N.M., G.B. and B.B.; resources, L.W., R.S., W.v.d.S. and P.D.; writing—original draft preparation, B.B.; writing—review and editing, N.M., R.S., W.v.d.S. and P.D.; supervision, L.W., W.v.d.S., P.D. and B.B.; project administration, B.B.; funding acquisition, L.W., R.S., W.v.d.S. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
J.M. was supported by the China Scholarship Council (CSC), number 202008520048. W.v.d.S. was supported by several fundings, like the Stichting Erasmus Trustfonds grant 97030.2022.101.718, the Aspasia grant 015.013.033 from the Dutch Research Council, and an EUR fellowship of the Erasmus University.
Institutional Review Board Statement
No ethical approval was required for the in vivo experiments since Galleria mellonella is an invertebrate animal model.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data are available from the authors upon request. Antifungal activities of test compounds against M. mycetomatis can also be found on the Mycetoma Open Source/MycetOS website, https://github.com/OpenSourceMycetoma (accessed on 30 April 2024).
Acknowledgments
The authors thank Luiza Cruz (Drugs for Neglected Diseases initiative, DNDi) for networking and assistance with the mycetoma project. We thank Matthew Todd (University College London), Anthony Sama, and all contributors to MycetOS not named here or in the author’s line for fruitful discussions. We are grateful to Ahmed Fahal for providing the M. mycetomatis strain MM55, Dipak Thampke and Jagdish Chander for providing strains I1, I3, and I11, and Beatriz Bustamante for providing strain Peru72012 used for the in vitro antifungal susceptibility testing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yeasmin, F.; Choi, H.W. Natural salicylates and their roles in human health. Int. J. Mol. Sci. 2020, 21, 9049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, J.; Du, J.; Wang, H.; Liu, J.; Wang, G. Niclosamide as a promising therapeutic player in human cancer and other diseases. Int. J. Mol. Sci. 2022, 23, 16116. [Google Scholar] [CrossRef]
- Kadri, H.; Lambourne, O.A.; Mehellou, Y. Niclosamide, a drug with many (re)purposes. ChemMedChem 2018, 13, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mook Jr, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an anthelmintic drug. Cell Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, A.M.; Ye, J. The magic bullet: Niclosamide. Front. Oncol. 2022, 12, 1004978. [Google Scholar] [CrossRef] [PubMed]
- Kauerová, T.; Pérez-Pérez, M.-J.; Kollar, P. Salicylanilides and their anticancer properties. Int. J. Mol. Sci. 2023, 24, 1728. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Njeij, B.; McCarty, T.R.; Birk, J.W. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J. Gastroenterol. Hepatol. 2016, 31, 1141–1146. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Ratajczak, T.; Kumar, G.; Albandar, H.; Redlien, P.; Yacyshyn, S.; Markert, R. Metabolic characteristics of esophageal carcinoma in a veteran population. J. Clin. Oncol. 2016, 34, 4023. [Google Scholar] [CrossRef]
- Xu, Q.L.; Li, H.; Zhu, Y.J.; Xu, G. The treatments and postoperative complications of esophageal cancer: A review. J. Cardiothorac. Surg. 2020, 15, 163. [Google Scholar] [CrossRef]
- Patel, N.; Benipal, B. Incidence of esophageal cancer in the United States from 2001–2015: A United States cancer statistics analysis of 50 states. Cureus 2018, 10, e3709. [Google Scholar] [CrossRef]
- Blot, W.J.; Tarone, R.E. Esophageal Cancer. In Cancer Epidemiology and Prevention, 4th ed.; Thun, M., Linet, M.S., Cerhan, J.R., Haiman, C.A., Schottenfeld, D., Eds.; Oxford University Press: New York, NY, USA, 2017; pp. 579–592. [Google Scholar]
- Wang, H.; Li, S.; Liu, T.; Chen, J.; Dang, J. Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 998620. [Google Scholar]
- Shaw, C.F., III. Gold-based therapeutic agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, H.; Yuan, J.; Yao, Y. Targeting Wnt/β-catenin by anthelmintic drug niclosamide overcomes paclitaxel resistance in esophageal cancer. Fundam. Clin. Pharmacol. 2021, 35, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Chen, Y.-K.; Hsu, Y.-J.; Lin, B.-R. Niclosamide inhibits cell proliferation and enhances the responsiveness of esophageal cancer cells to chemotherapeutic agents. Oncol. Rep. 2020, 43, 549–561. [Google Scholar] [CrossRef]
- Xu, Y.; Feingold, P.L.; Surman, D.R.; Brown, K.; Xi, S.; Davis, J.L.; Hernandez, J.; Schrump, D.S.; Ripley, R.T. Bile acid and cigarette smoke enhance the aggressive phenotype of esophageal adenocarcinoma cells by downregulation of the mitochondrial uncoupling protein-2. Oncotarget 2017, 8, 101057–101071. [Google Scholar] [CrossRef] [PubMed]
- Bokil, A.; Sancho, P. Mitochondrial determinants of chemoresistance. Cancer Drug Resist. 2019, 2, 634–646. [Google Scholar] [CrossRef]
- De Almeida Rainho, M.; Siqueira, P.B.; de Amorim, Í.S.S.; Mencalha, A.L.; Thole, A.A. Mitochondria in colorectal cancer stem cells—A target in drug resistance. Cancer Drug Resist. 2023, 6, 273–283. [Google Scholar] [CrossRef]
- Prathapan, P. A determination of pan-pathogen antimicrobials? Med. Drug Discov. 2022, 14, 100120. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B. The antifungal potential of niclosamide and structurally related salicylanilides. Int. J. Mol. Sci. 2024, 25, 5977. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Cognetti, M.; Burch-Smith, R.; O’Sullivan Mejia, E.; Mirkin, G. Mycetoma: Development of diagnosis and treatment. J. Fungi 2022, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- Scolding, P.; Fahal, A.; Yotsu, R.R. Drug therapy for mycetoma. Cochrane Database Syst. Rev. 2018, 2018, CD013082. [Google Scholar] [CrossRef]
- World’s First Clinical Trial for Devastating Fungal Disease Mycetoma Shows Efficacy of New, Promising Treatment. Available online: https://dndi.org/press-releases/2023/worlds-first-clinical-trial-for-mycetoma-shows-efficacy-new-promising-treatment/ (accessed on 13 April 2024).
- Mahmoud, A.B.; Abd Algaffar, S.; van de Sande, W.; Khalid, S.; Kaiser, M.; Mäser, P. Niclosamide is active in vitro against mycetoma pathogens. Molecules 2021, 26, 4005. [Google Scholar] [CrossRef]
- Ramos, M.L.M.; Almeida-Silva, F.; de Souza Rabello, V.B.; Nahal, J.; Figueiredo-Carvalho, M.H.G.; Bernardes-Engemann, A.R.; Poester, V.R.; Xavier, M.O.; Meyer, W.; Zancopé-Oliveira, R.M.; et al. In vitro activity of the anthelmintic drug niclosamide against Sporothrix spp. strains with distinct genetic and antifungal susceptibility backgrounds. Braz. J. Microbiol. 2024, 55, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.W.; Elfar, G.A.; Yeo, P.; Phua, N.; Hor, J.H.; Chen, S.; Ho, Y.S.; Cheok, C.F. Nitro-deficient niclosamide confers reduced genotoxicity and retains mitochondrial uncoupling activity for cancer therapy. Int. J. Mol. Sci. 2021, 22, 10420. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšova, J.; Buchta, V.; Horvati, K.; Bösze, S.; Stolaříková, J. New amino acid esters of salicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010, 45, 5106–6113. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J. Antifungal activity of salicylanilides and their esters with 4-(trifluoromethyl)benzoic acid. Molecules 2012, 17, 9426–9442. [Google Scholar] [CrossRef]
- Nair, A.S.; Singh, A.K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-approved trifluoromethyl group-containing drugs: A review of 20 years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Stevens, A.J.; Abraham, R.; Young, K.A.; Russell, C.C.; McCluskey, S.B.; Baker, J.R.; Rusdi, B.; Page, S.W.; O’Handley, R.; O’Dea, M.; et al. Antigiardial activity of novel guanidine compounds. ChemMedChem 2022, 17, e202200341. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, S.; Zanda, M. Synthetic chemistry and biological activity of pentafluorosulphanyl (SF5) organic molecules. J. Fluor. Chem. 2012, 143, 57–93. [Google Scholar] [CrossRef]
- Mo, T.; Mi, X.; Milner, E.E.; Dow, G.S.; Wipf, P. Synthesis of an 8-pentafluorosulfanyl analog of the antimalarial agent mefloquine. Tetrahedron Lett. 2010, 51, 5137–5140. [Google Scholar] [CrossRef]
- Linder, B.; Köhler, L.H.F.; Reisbeck, L.; Menger, D.; Subramaniam, D.; Herold-Mende, C.; Anant, S.; Schobert, R.; Biersack, B.; Kögel, D. A new pentafluorothio-substituted curcuminoid with superior antitumor activity. Biomolecules 2021, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Klefenz, A.; Traenckner, H.; Schneider, P.; Andronache, I.; Schobert, R.; Biersack, B.; Schuppan, D. A new synthetic curcuminoid displays antitumor activities in metastasized melanoma. Cells 2023, 12, 2619. [Google Scholar] [CrossRef] [PubMed]
- Waisser, K.; Petrlikova, E.; Kunes, J.; Vrabcova, P.; Kolar, K.; Stolarikova, J. The antimycobacterial salicylanilides, potential inhibitors of ATP synthesis. Folia Pharm. Univ. Carol. 2011, 39, 17–23. [Google Scholar]
- Zhang, Y.; Wang, Y.; Guo, Y.; Liao, J.; Tu, Z.; Lu, Y.; Ding, K.; Tortorella, M.D.; He, J. Identification and synthesis of low-molecular weight cholecystokinin B receptor (CCKBR) agonists as mediators of long-term synaptic potentiation. Med. Chem. Res. 2019, 28, 387–393. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Y.; Long, D.; Yu, T.; Shen, F.; Lin, X. The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the cytotoxic effects of taxol through Op18/stathmin in non-small cell lung cancer cells in vitro. Int. J. Mol. Med. 2017, 40, 235–242. [Google Scholar] [CrossRef]
- Veeragoni, D.K.; Deshpande, S.; Rachamalla, H.K.; Ande, A.; Misra, S.; Mutheneni, S.R. In vitro and in vivo anticancer and genotoxicity profiles of green synthesized and chemically synthesized silver nanoparticles. ACS Appl. Bio Mater. 2022, 5, 2324–2339. [Google Scholar] [CrossRef]
- Ghosh, H.; Bhattacharyya, S.; Schobert, R.; Dandawate, P.; Biersack, B. Fluorinated and N-acryloyl-modified 3,5-di [(E)-benzylidene] piperidin-4-one curcuminoids for the treatment of pancreatic carcinoma. Pharmaceutics 2023, 15, 1921. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ghosh, H.; Covarrubias-Zambrano, O.; Jain, K.; Swamy, K.V.; Kasi, A.; Hamza, A.; Anant, S.; VanSaun, M.; Weir, S.J.; et al. Anticancer activity of novel difluorinated curcumin analog and its inclusion complex with 2-hydroxypropyl-β-cyclodextrin against pancreatic cancer. Int. J. Mol. Sci. 2023, 24, 6336. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, T.; Du, J.; Zhang, B.; Xiang, D.; Li, W. Xanthohumol, a prenylated flavonoid from hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, H.; Xu, R.; Zhao, Y.; Chinnaswamy, K.; McEachern, D.; Chen, J.; Yang, C.-Y.; Liu, Z.; Wang, M.; et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell 2019, 36, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.A.; Ferkey, D.M.; Mao, F.; Kimelman, D.; Xu, W. Tcf4 can specifically recognize beta-catenin using alternative conformations. Nat. Struct. Biol. 2001, 8, 1048–1052. [Google Scholar] [CrossRef]
- Alexander, N.; Woetzel, N.; Meiler, J. bcl: Cluster: A method for clustering biological molecules coupled with visualization in the Pymol Molecular Graphics System. IEEE Int. Conf. Comput. Adv. Bio Med. Sci. 2011, 2011, 13–18. [Google Scholar] [PubMed]
- Lim, W.; Nyuykonge, B.; Eadie, K.; Konings, M.; Smeets, J.; Fahal, A.; Bonifaz, A.; Todd, M.; Perry, B.; Samby, K.; et al. Screening the pandemic response box identified benzimidazole carbamates, Olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Negl. Trop. Dis. 2022, 16, e0010159. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Eadie, K.; Fahal, A.; Verbon, A.; van de Sande, W.W.J. The performance and costs of XTT, resazurin, MTS and luciferin as viability dyes in in vitro susceptibility testing of Madurella mycetomatis. Trans. R. Soc. Trop. Med. Hyg. 2024, trae030. [Google Scholar] [CrossRef]
- Kloezen, W.; van Helvert-van Poppel, M.; Fahal, A.H.; van de Sande, W.W.J. A Madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Negl. Trop. Dis. 2015, 9, e0003926. [Google Scholar] [CrossRef]
- Camurri, G.; Zaramella, A. High-throughput liquid chromatography/mass spectrometry method for the determination of the chromatographic hydrophobicity index. Anal. Chem. 2001, 73, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.; Nunhuck, S.; Bevan, C.; Abraham, M.H.; Reynolds, D.P. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilized artificial membrane lipophilicity. J. Pharm. Sci. 2003, 92, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Islam, F.; Smith, R.A.; Lam, A.K. Therapeutic strategies against cancer stem cells in esophageal carcinomas. Front. Oncol. 2021, 10, 598957. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Giammarino, A.; Belluci, N.; Angiolella, L. Galleria mellonella as a model for the study of fungal pathogens: Advantages and disadvantages. Pathogens 2024, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, H.; Tan, X.; Jiang, Z.; Wang, P.; Qin, J. Ten-gram scale mechanochemical synthesis of ternary lanthanum coordination polymers for antibacterial and antitumor activities. Front. Chem. 2022, 10, 898324. [Google Scholar] [CrossRef]
- Shimizu, D.; Vallböhmer, D.; Kuramochi, H.; Uchida, K.; Schneider, S.; Chandrasoma, P.T.; Shimada, H.; DeMeester, T.R.; Danenberg, K.D.; Peters, J.H.; et al. Increasing cyclooxygenase-2 (cox-2) gene expression in the progression of Barrett’s esophagus to adenocarcinoma correlates with that of Bcl-2. Int. J. Cancer 2006, 119, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Chatterjee, D.; Deng, D.; Veeranki, O.; Mejia, A.; Ajani, J.A.; Hofstetter, W.; Lin, S.; Guha, S.; Kopetz, S.; et al. Antitumor effects of cyclin dependent kinase 9 inhibition in esophageal adenocarcinoma. Oncotarget 2017, 8, 28696–28710. [Google Scholar] [CrossRef] [PubMed]
- Ong, F.; Kim, K.; Konopleva, M.Y. Venetoclax resistance: Mechanistic insights and future strategies. Cancer Drug Resist. 2022, 5, 380–400. [Google Scholar] [CrossRef]
- Chen, B.; Wei, W.; Ma, L.; Yang, B.; Gill, R.M.; Chua, M.-S.; Butte, A.J.; So, S. Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting CDC37 signaling. Gastroenterology 2017, 152, 2022–2036. [Google Scholar] [CrossRef]
- Su, W.; Guo, C.; Wang, L.; Wang, Z.; Yang, X.; Niu, F.; Tzou, D.; Yang, X.; Huang, X.; Wu, J.; et al. LncRNA MIR22HG abrogation inhibits proliferation and induces apoptosis in esophageal adenocarcinoma cells via activation of the STAT3/c-Myc/FAK signaling. Aging 2019, 11, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Katsha, A.; Arras, J.; Soutto, M.; Belkhiri, A.; El-Rifai, W. AURKA regulates JAK2-STAT3 activity in human gastric and esophageal cancers. Mol. Oncol. 2014, 8, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Vangamudi, B.; Zhu, S.; Soutto, M.; Belkhiri, A.; El-Rifai, W. Regulation of β-catenin by t-DARPP in upper gastrointestinal cancer cells. Mol. Cancer 2011, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Qualman, A.; Garrett, H.; The, E.; Chauhan, A.; Idrovo, J.P.; Cheng, L.; Wani, S.; Meguid, R.A.; Meng, X. Targeting glypican-1 reverses resistance to 5-fluorouracil in esophageal adenocarcinoma cells. Anticancer Res. 2023, 43, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Bild, A.; Teo, J.-L.; Kahn, M. Enhanced Kat3A/catenin transcription: A common mechanism of therapeutic resistance. Cancer Drug Resist. 2019, 2, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, N.; Hu, X. Wnt/b-catenin signaling inhibitors. Curr. Top. Med. Chem. 2023, 23, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef]
- Somchit, N.; Ngee, C.S.; Yaakob, A.; Ahmad, Z.; Zakaria, Z.A. Effects of cytochrome P450 inhibitors on itraconazole and fluconazole induced cytotoxicity in hepatocytes. J. Toxicol. 2009, 2009, 912320. [Google Scholar] [CrossRef] [PubMed]
- Goehringer, N.; Peng, Y.; Nitzsche, B.; Biermann, H.; Pradhan, R.; Schobert, R.; Herling, M.; Höpfner, M.; Biersack, B. Improved anticancer activities of a new pentafluorothio-substituted vorinostat-type histone deacetylase inhibitor. Pharmaceuticals 2021, 14, 1319. [Google Scholar] [CrossRef]
- Haegler, P.; Joerin, L.; Krähenbühl, S.; Bouitbir, J. Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol. Sci. 2017, 157, 183–195. [Google Scholar] [CrossRef]
- Horvat, M.; Kodrič, G.; Jereb, M.; Iskra, J. One pot synthesis of trifluoromethyl aryl sulfoxides by trifluoromethylthiolation of arenes and subsequent oxidation with hydrogen peroxide. RSC Adv. 2020, 10, 34534–34540. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).