Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism
Abstract
1. Introduction
2. Methods
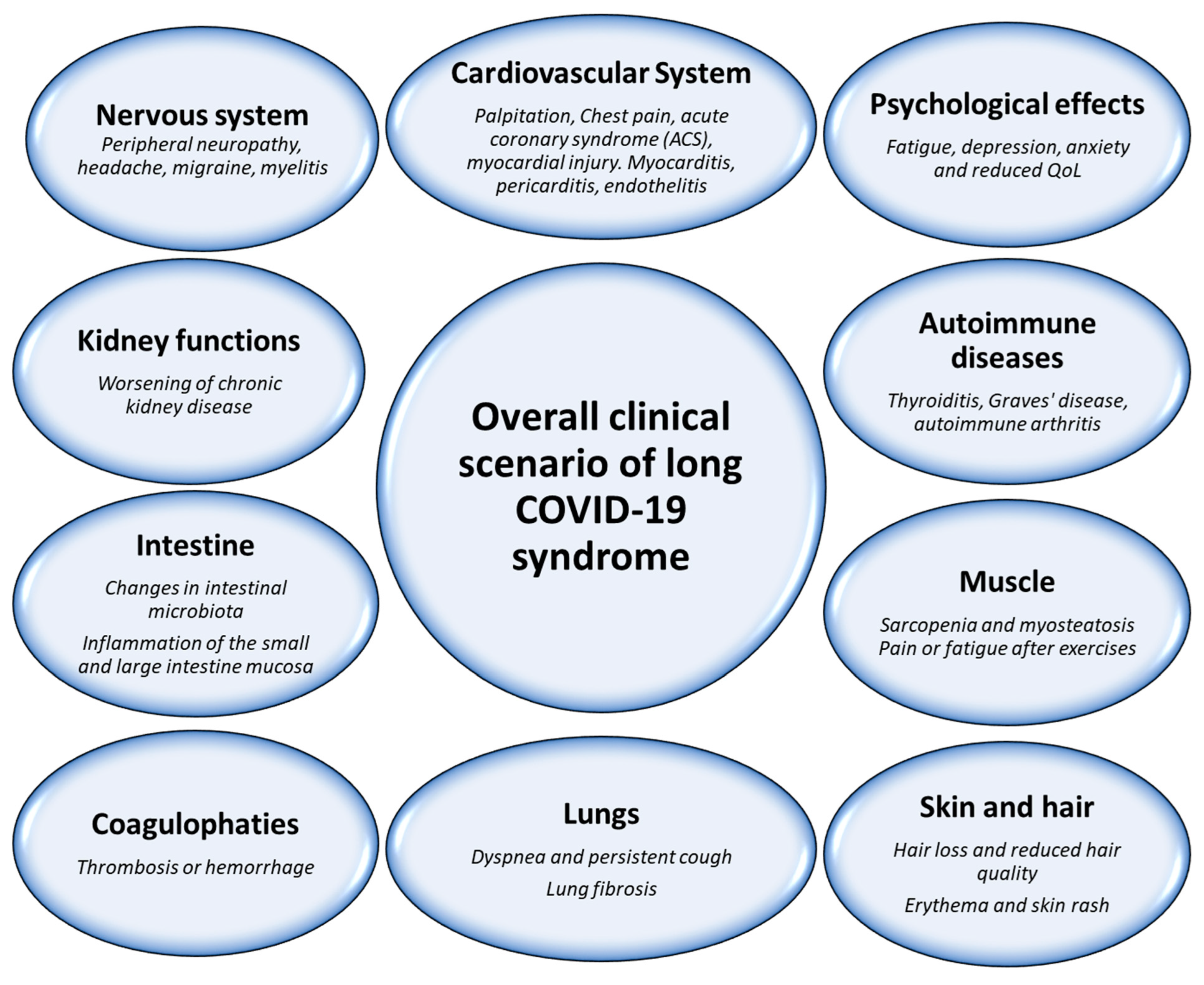
3. Post-Acute COVID-19 Syndrome: A Clinical Scenario
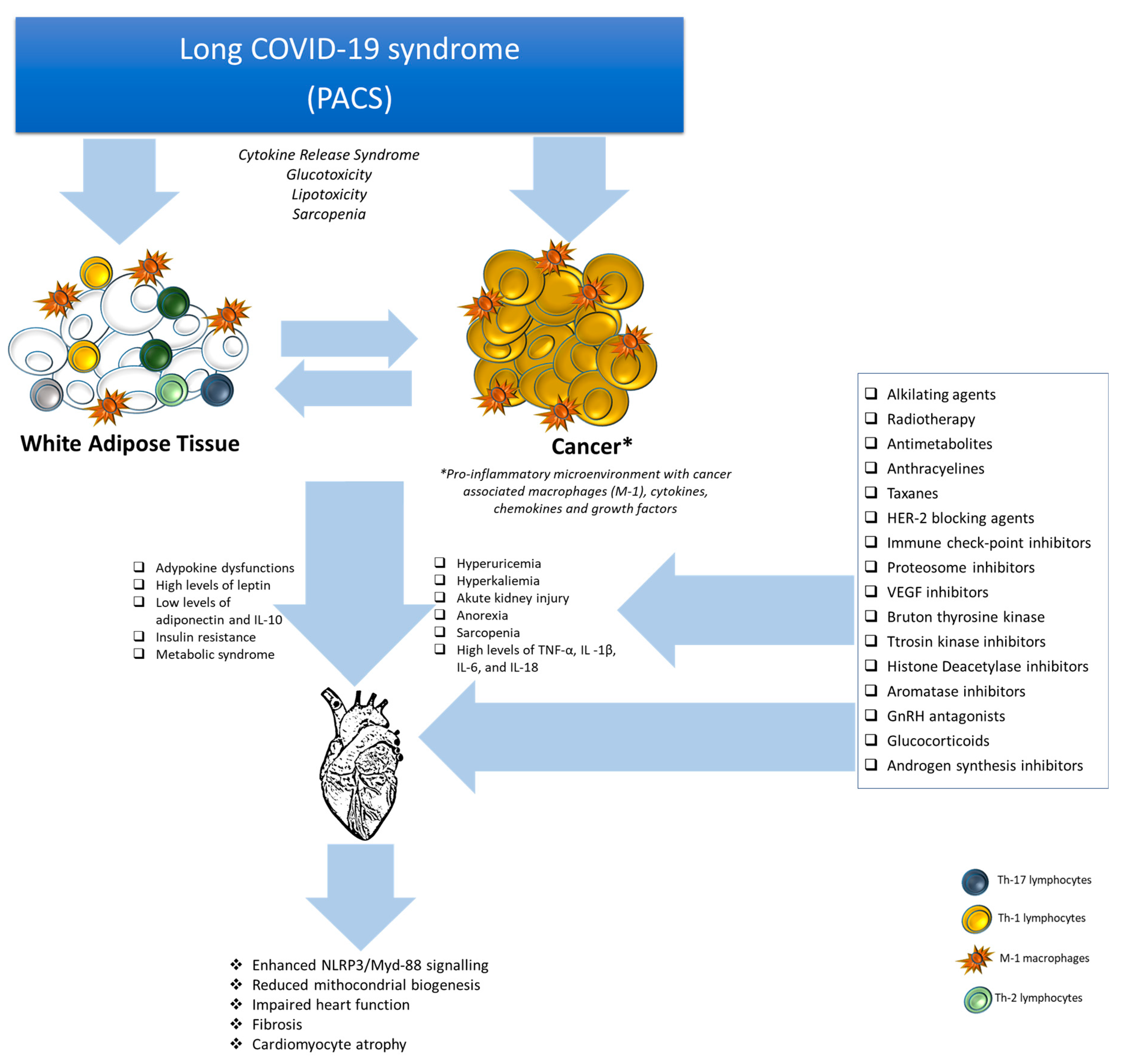
4. Post-Acute COVID-19 Syndrome and Cardiovascular Complications in Cancer Patients
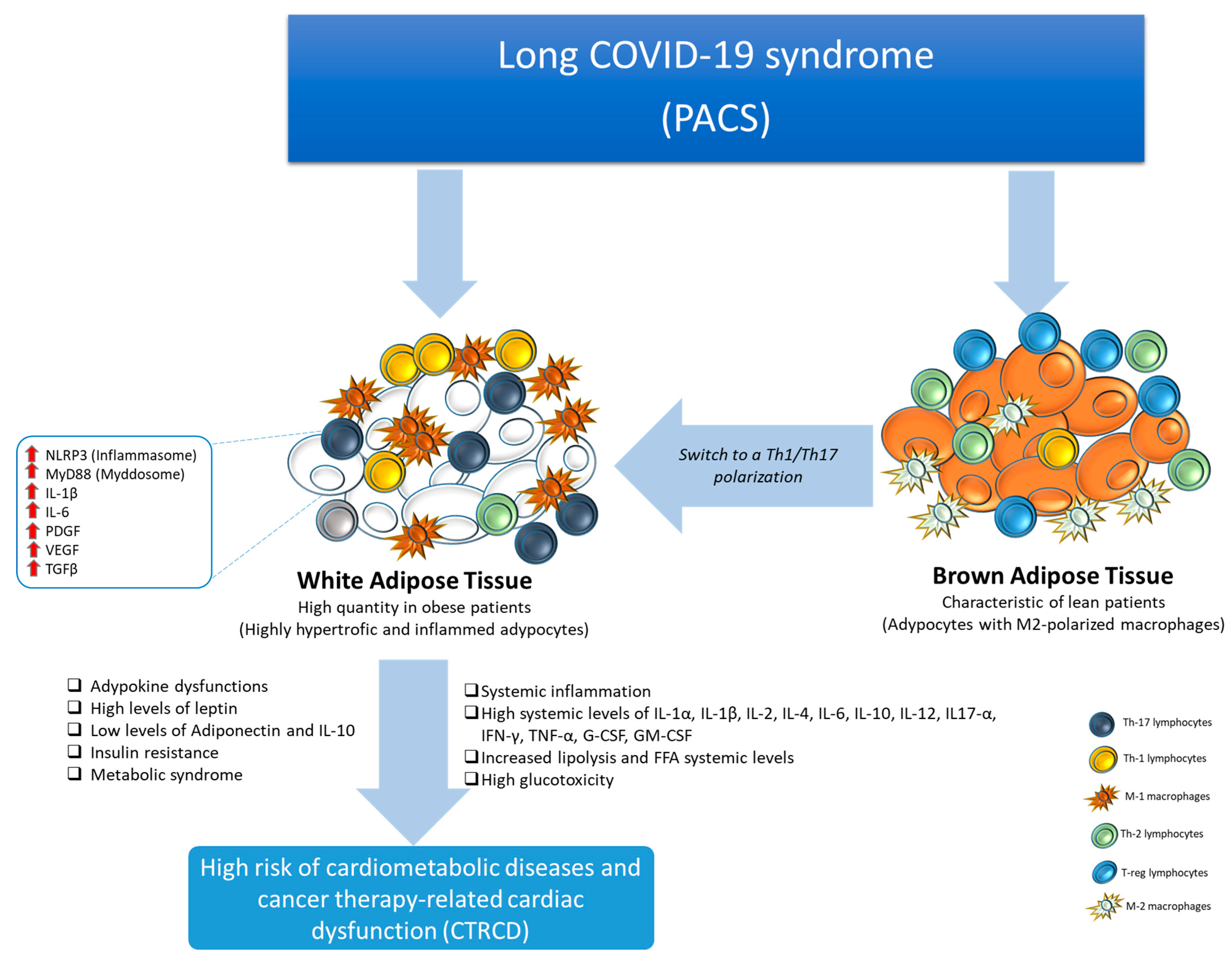
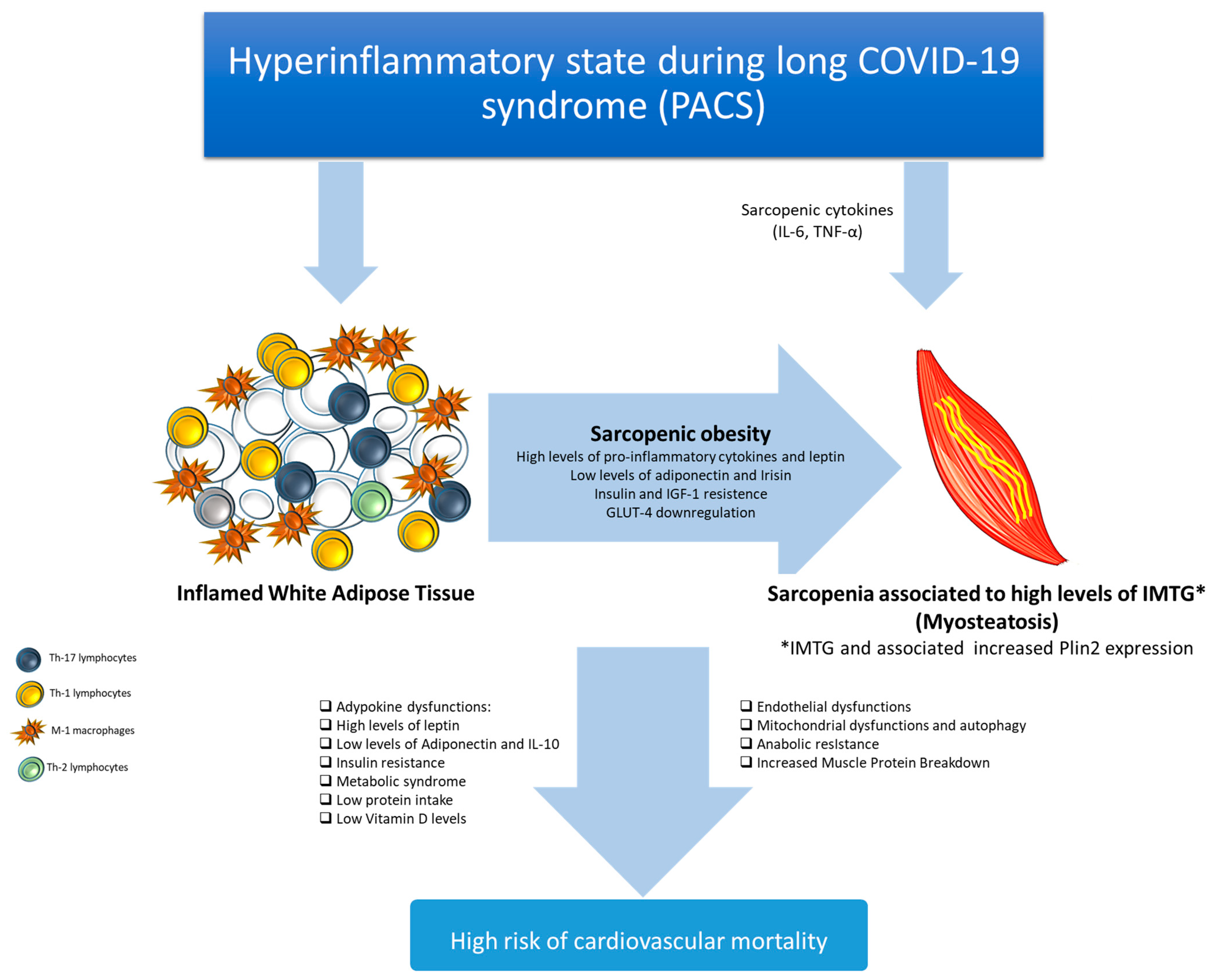
5. Post-Acute COVID-19 and Visceral Obesity
6. Post-Acute COVID-19 and Inflammation
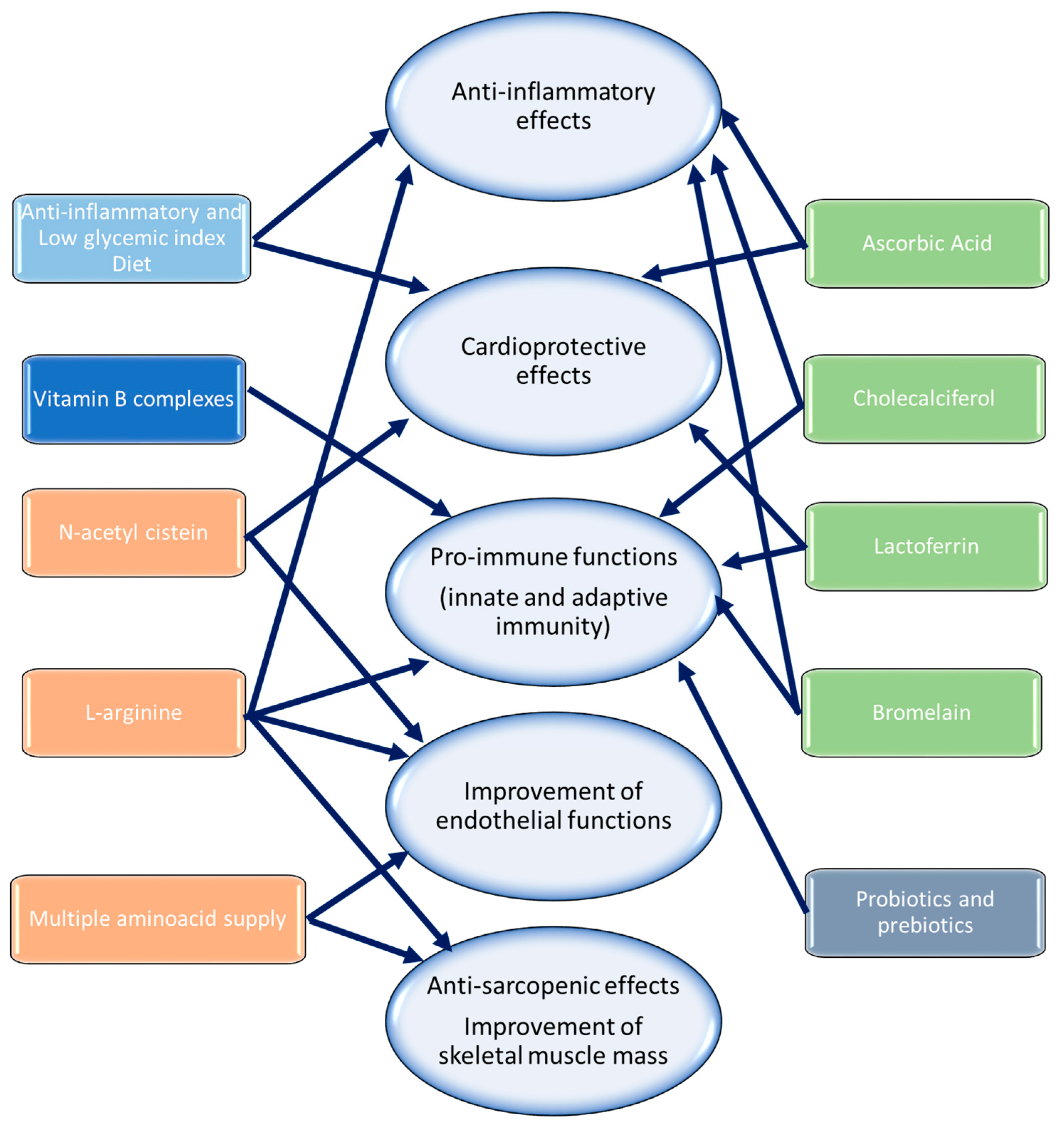
7. Suggestions to Reduce Cardiovascular Complications in Cancer Patients with Post-Acute COVID-19 Syndrome
| Therapeutic Option | Molecular Pathway | Clinical Outcomes | Ref. |
|---|---|---|---|
| Anti-inflammatory diet | ↓ COX-2, NLRP3, MyD88, pro-inflammatory cytokines | Anti-inflammatory systemic effects Cardioprotective effects | [171,172] |
| L-Arginine | ↑ T-lymphocyte survival, ↑ nitric oxide, cGMP, PKG ↓ IL-17, IL-1β | Pro-immune functions (innate and adaptive immunity) Anti-inflammatory systemic effects Improvement of endothelial functions | [173,174,175] |
| N-acetil cystein (NAC) | ↑ Cys, GSH, IL-10 ↓ HOCl, OH-, H2O2 ↓ NLRP3, MyD88 | Anti-inflammatory systemic effects Cardioprotective effects | [175,176] |
| Ascorbic acid, NAC, zinc, and iron | ↑ T-lymphocyte survival ↑ nitric oxide, cGMP, PKG ↓ IL-17, IL-1β, NLRP3, MyD88 | Pro-immune functions (innate and adaptive immunity) Anti-inflammatory systemic effects Improvement of endothelial functions | [177,178,179] |
| Cholecalciferol | ↓ NLRP3, MyD88, IL-6, and IL-17 ↑ CD8+ T-lymphocyte survival; Natural Killer cells ↑ IL-10 | Anti-inflammatory effects Pro-immune functions (innate and adaptive immunity) | [180,181,182,183,184] |
| Liposomal ascorbic acid | ↓ NLRP3, MyD88, IL-6, IL-17, IL-1β ↑ CD8+ T-lymphocyte survival; Natural Killer cells ↑ IL-10 | Anti-inflammatory effects Pro-immune functions (innate and adaptive immunity) | [185] |
| Multiple amino acid supplements (L-Leucine, L-Valine, L-Isoleucine, L-Lysine hydrochloride, L-Phenylalanine, L-Threonine, L-Methionine, L-Tryptophan) | ↓ ROS, IL-6, TNF-α, IL-1 ↑ Mitochondrial biogenesis, motor units, number of fibers ↑ Satellite cells function ↑ NO, PCG1-α | Anti-sarcopenic effects Improvement of skeletal muscle mass Improvement of endothelial functions | [187,188] |
| Lactoferrin | ↓ IL-1, IL-6, and IL-17 ↑ CD8+ T-lymphocyte survival; Natural Killer cells ↑ IL-10 ↓ ROS, MDA, 4-HNA | Antiviral effects Anti-inflammatory effects Pro-immune functions (innate and adaptive immunity) | [189,190,191,192,193] |
| Bromelain | ↓ IL-1, IL-6, IL-17, PGE2, COX-2 ↑ CD8+ T-lymphocyte survival; Natural Killer cells ↑ IL-10 ↓ ACE-2, TMPRSS2 | Antiviral effects Anti-inflammatory effects Pro-immune functions (innate and adaptive immunity) | [194,195,196,197,198,199,200,201,202,203,204,205,206] |
| Probiotics (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii, and lactobacillus reuteri) | ↑ IgA responses ↓ IL-17, IL-6, IL-1, TNF-α ↑ IL-15, IL-12, IL-21 ↑ CD8+ T-lymphocyte survival; Natural Killer cells | Pro-immune functions (innate and adaptive immunity) Enhancement of antibody production | [208,209,210,211] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scholkmann, F.; May, C.A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol. Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albtoosh, A.S.; Toubasi, A.A.; Al Oweidat, K.; Hasuneh, M.M.; Alshurafa, A.H.; Alfaqheri, D.L.; Farah, R.I. New symptoms and prevalence of postacute COVID-19 syndrome among nonhospitalized COVID-19 survivors. Sci. Rep. 2022, 12, 16921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elseidy, S.A.; Awad, A.K.; Vorla, M.; Fatima, A.; Elbadawy, M.A.; Mandal, D.; Mohamad, T. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS). Int. J. Cardiol. Heart Vasc. 2022, 40, 101012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dixit, N.M.; Churchill, A.; Nsair, A.; Hsu, J.J. Post-Acute COVID-19 Syndrome and the cardiovascular system: What is known? Am. Heart J. Plus 2021, 5, 100025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, S.H.; Lim, Y.C.; Zaki, R.A.; Johari, B.M.; Chang, C.Y.; Omar, S.F.S.; Azzeri, A.; Dahlui, M.; Kamarulzaman, A. Prevalence and predictors of post-acute COVID syndrome among infected healthcare workers at University Malaya Medical Centre. PLoS ONE 2024, 19, e0298376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiappelli, F.; Fotovat, L. Post acute COVID-19 syndrome (PACS)—Long COVID. Bioinformation 2022, 18, 908–911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavalcanti, I.D.L.; Soares, J.C.S. Impact of COVID-19 on cancer patients: A review. Asia Pac. J. Clin. Oncol. 2021, 17, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.; Foote, M.B.; Lumish, M.; Stonestrom, A.J.; Wills, B.; Narendra, V.; Avutu, V.; Murciano-Goroff, Y.R.; Chan, J.E.; Derkach, A.; et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J. Clin. Oncol. 2020, 38, 3538–3546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvatore, M.; Hu, M.M.; Beesley, L.J.; Mondul, A.M.; Pearce, C.L.; Friese, C.R.; Fritsche, L.G.; Mukherjee, B. COVID-19 Outcomes by Cancer Status, Site, Treatment, and Vaccination. Cancer Epidemiol. Biomark. Prev. 2023, 32, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Cottenet, J.; Tapia, S.; Arveux, P.; Bernard, A.; Dabakuyo-Yonli, T.S.; Quantin, C. Effect of Obesity among Hospitalized Cancer Patients with or without COVID-19 on a National Level. Cancers 2022, 14, 5660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, H.; Park, Y.; Myung, S.K. Obesity and mortality in patients with COVID-19: A meta-analysis of prospective studies. Asia Pac. J. Clin. Nutr. 2024, 33, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 2019, 8, 662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bełtowski, J. Adiponectin and resistin—New hormones of white adipose tissue. Med. Sci. Monit. 2003, 9, RA55–RA61. [Google Scholar] [PubMed]
- Beppu, L.Y.; Mooli, R.G.R.; Qu, X.; Marrero, G.J.; Finley, C.A.; Fooks, A.N.; Mullen, Z.P.; Frias, A.B., Jr.; Sipula, I.; Xie, B.; et al. Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight. 2021, 6, e140644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castellano-Castillo, D.; Ramos-Molina, B.; Cardona, F.; Queipo-Ortuño, M.I. Epigenetic regulation of white adipose tissue in the onset of obesity and metabolic diseases. Obes. Rev. 2020, 21, e13054. [Google Scholar] [CrossRef] [PubMed]
- Demir, L.; Oflazoğlu, U. The relationship between sarcopenia and serum irisin and TNF-α levels in newly diagnosed cancer patients. Support. Care Cancer 2023, 31, 586. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsimberidou, A.M.; Keating, M.J. Hyperuricemic syndromes in cancer patients. Contrib. Nephrol. 2005, 147, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Lacavalerie, M.R.; Pierre-Francois, S.; Agossou, M.; Inamo, J.; Cabie, A.; Barnay, J.L.; Neviere, R. Obese patients with long COVID-19 display abnormal hyperventilatory response and impaired gas exchange at peak exercise. Future Cardiol. 2022, 18, 577–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; D’Aiuto, G.; Iaffaioli, R.V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cerrone, F.; Bonanno, S.; Nunnari, G.; et al. Reasons why COVID-19 survivors should follow dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations: From hyper-inflammation to cardiac dysfunctions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.; Wong, S.H. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and Post-infective Fatigue Syndrome: A Review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Titze-de-Almeida, R.; Araújo Lacerda, P.H.; de Oliveira, E.P.; de Oliveira, M.E.F.; Vianna, Y.S.S.; Costa, A.M.; Pereira Dos Santos, E.; Guérard, L.M.C.; Ferreira, M.A.M.; Rodrigues Dos Santos, I.C.; et al. Sleep and memory complaints in long COVID: An insight into clustered psychological phenotypes. PeerJ 2024, 12, e16669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martone, A.M.; Tosato, M.; Ciciarello, F.; Galluzzo, V.; Zazzara, M.B.; Pais, C.; Savera, G.; Calvani, R.; Marzetti, E.; Robles, M.C.; et al. Sarcopenia as potential biological substrate of long COVID-19 syndrome: Prevalence, clinical features, and risk factors. J. Cachexia Sarcopenia Muscle 2022, 13, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zong, M.; Zhao, A.; Han, W.; Chen, Y.; Weng, T.; Li, S.; Tang, L.; Wu, J. Sarcopenia, sarcopenic obesity and the clinical outcome of the older inpatients with COVID-19 infection: A prospective observational study. BMC Geriatr. 2024, 24, 578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burges Watson, D.L.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long COVID-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Liu, R.; Ma, H.; Zhang, W. The Pathogenesis of COVID-19-Related Taste Disorder and Treatments. J. Dent. Res. 2023, 102, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Nalroad Sundararaj, S.; Bhatia, J.; Singh Arya, D. Understanding long COVID myocarditis: A comprehensive review. Cytokine 2024, 178, 156584. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Therap. Adv. Gastroenterol. 2022, 15, 17562848221118403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 Depressive Symptoms: Epidemiology, Pathophysiology, and Pharmacological Treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Álvarez-Santacruz, C.; Tyrkalska, S.D.; Candel, S. The Microbiota in Long COVID. Int. J. Mol. Sci. 2024, 25, 1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riad, A.; Kassem, I.; Badrah, M.; Klugar, M. The manifestation of oral mucositis in COVID-19 patients: A case-series. Dermatol. Ther. 2020, 33, e14479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sapkota, H.R.; Nune, A. Long COVID from rheumatology perspective—A narrative review. Clin. Rheumatol. 2022, 41, 337–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Jesus, M.; Chanda, A.; Grabauskas, T.; Kumar, M.; Kim, A.S. Cardiovascular disease and lung cancer. Front. Oncol. 2024, 14, 1258991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926, Erratum in Lancet 2020, 396, 534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Liu, S.; Wang, C.; Wu, Y.; Liu, J. Risk factors for mortality among lung cancer patients with COVID-19 infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0291178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maruyama, S.; Wada, D.; Kanayama, S.; Shimazu, H.; Miyano, Y.; Inoue, A.; Kashihara, M.; Okuda, K.; Saito, F.; Nakamori, Y.; et al. The evaluation of risk factors for prolonged viral shedding during anti-SARS-CoV-2 monoclonal antibodies and long-term administration of antivirals in COVID-19 patients with B-cell lymphoma treated by anti-CD20 antibody. BMC Infect. Dis. 2024, 24, 715. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.L.; Vaddaraju, V.; Venkateswaran, S.; Mansur, A.; Bajaj, S.S.; Kiang, M.V.; Jena, A.B.; Yang, C.J. Deaths Due to COVID-19 in Patients With Cancer During Different Waves of the Pandemic in the, U.S. JAMA Oncol. 2023, 9, 1417–1422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoury, E.; Nevitt, S.; Madsen, W.R.; Turtle, L.; Davies, G.; Palmieri, C. Differences in Outcomes and Factors Associated With Mortality Among Patients With SARS-CoV-2 Infection and Cancer Compared With Those Without Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2210880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salunke, A.A.; Nandy, K.; Pathak, S.K.; Shah, J.; Kamani, M.; Kottakota, V.; Thivari, P.; Pandey, A.; Patel, K.; Rathod, P.; et al. Impact of COVID-19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tleyjeh, I.M.; Kashour, T.; Riaz, M.; Amer, S.A.; AlSwaidan, N.; Almutairi, L.; Halwani, R.; Assiri, A. Persistent COVID-19 symptoms at least one month after diagnosis: A national survey. J. Infect. Public Health 2022, 15, 578–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caranci, N.; Di Girolamo, C.; Bartolini, L.; Fortuna, D.; Berti, E.; Sforza, S.; Giorgi Rossi, P.; Moro, M.L. General and COVID-19-Related Mortality by Pre-Existing Chronic Conditions and Care Setting during 2020 in Emilia-Romagna Region, Italy. Int. J. Environ. Res. Public Health 2021, 18, 13224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duong-Quy, S.; Huynh-Truong-Anh, D.; Nguyen-Thi-Kim, T.; Nguyen-Quang, T.; Tran-Ngoc-Anh, T.; Nguyen-Van-Hoai, N.; Do-Thi-Thu, M.; Nguyen-Chi, T.; Nguyen-Van, T.; Tang-Thi-Thao, T.; et al. Predictive Factors of Mortality in Patients with Severe COVID-19 Treated in the Intensive Care Unit: A Single-Center Study in Vietnam. Pulm Ther. 2023, 9, 377–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meza-Torres, B.; Delanerolle, G.; Okusi, C.; Mayor, N.; Anand, S.; Macartney, J.; Gatenby, P.; Glampson, B.; Chapman, M.; Curcin, V.; et al. Differences in Clinical Presentation With Long COVID After Community and Hospital Infection and Associations With All-Cause Mortality: English Sentinel Network Database Study. JMIR Public Health Surveill. 2022, 8, e37668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipski, D.; Radziemski, A.; Wasiliew, S.; Wyrwa, M.; Szczepaniak-Chicheł, L.; Stryczyński, Ł.; Olasińska-Wiśniewska, A.; Urbanowicz, T.; Perek, B.; Tykarski, A.; et al. Assessment of COVID-19 risk factors of early and long-term mortality with prediction models of clinical and laboratory variables. BMC Infect. Dis. 2024, 24, 685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Righi, E.; Mirandola, M.; Mazzaferri, F.; Razzaboni, E.; Zaffagnini, A.; Erbogasto, A.; Vecchia, I.D.; Auerbach, N.; Ivaldi, F.; Mongardi, M.; et al. Long-Term Patient-Centred Follow-up in a Prospective Cohort of Patients with COVID-19. Infect. Dis. Ther. 2021, 10, 1579–1590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Chen, N.; Zhao, D.; Zhang, J.; Hu, Z.; Tao, Z. Clinical Characteristics of COVID-19 Patients Infected by the Omicron Variant of SARS-CoV-2. Front. Med. 2022, 9, 912367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.H.; Gemünd, I.; Beyer, M.; Bonaguro, L. Lung T cell response in COVID-19. Front. Immunol. 2023, 14, 1108716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Queiroz, M.A.F.; das Neves, P.F.M.; Lima, S.S.; Lopes, J.d.C.; Torres, M.K.d.S.; Vallinoto, I.M.V.C.; de Brito, M.T.F.M.; da Silva, A.L.S.; Leite, M.d.M.; da Costa, F.P.; et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front. Cell. Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, S.M.R.; Brito, A.C.S.; Manfro, W.F.P.; Ribeiro-Alves, M.; Ribeiro, R.S.A.; da Cal, M.S.; Lisboa, V.D.C.; Abreu, D.P.B.; Castilho, L.D.R.; Porto, L.C.M.S.; et al. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients. PLoS ONE 2023, 18, e0283983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Hernández, Y.; Monárrez-Espino, J.; López, D.A.G.; Zheng, J.; Borrego, J.C.; Torres-Calzada, C.; Elizalde-Díaz, J.P.; Mandal, R.; Berjanskii, M.; Martínez-Martínez, E.; et al. The plasma metabolome of long COVID patients two years after infection. Sci. Rep. 2023, 13, 12420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ageev, A.A.; Kozhevnikova, M.V.; Emelyanov, A.V.; Krivova, A.V.; Shumskaya, Y.F.; Musaeva, L.M.; Popova, L.V.; Naymann, Y.I.; Abdullaeva, G.B.; Privalova, E.V.; et al. The Effect of COVID-19 on Long-Term Cardiac Function in Patients With Chronic Heart Failure. Kardiologiia 2022, 62, 23–29, (In Russian, English). [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paruchuri, S.S.H.; Farwa, U.E.; Jabeen, S.; Pamecha, S.; Shan, Z.; Parekh, R.; Lakkimsetti, M.; Alamin, E.; Sharma, V.; Haider, S.; et al. Myocarditis and Myocardial Injury in Long COVID Syndrome: A Comprehensive Review of the Literature. Cureus 2023, 15, e42444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMaster, M.W.; Dey, S.; Fishkin, T.; Wang, A.; Frishman, W.H.; Aronow, W.S. The Impact of Long COVID-19 on the Cardiovascular System. Cardiol. Rev 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Albi, G.; Figini, S.; Basile, S.; Sacchi, P.; Bruno, R.; Pedrazzoli, P. Long-COVID in Patients with Cancer Previously Treated with Early Anti-SARS-CoV-2 Therapies in an Out-of-Hospital Setting: A Single-Center Experience. Cancers 2023, 15, 1269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Zhao, H.; Cui, R.; Ma, L.; Ge, X.; Fu, Q.; Yu, D.; Niu, X. Comparison of clinical outcomes and risk factors for COVID-19 infection in cancer patients without anticancer treatment and noncancer patients. Front. Public Health 2022, 10, 925519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, T.; Nagasu, S.; Furuta, T.; Gobaru, M.; Suzuki, H.; Shimotsuura, Y.; Akiba, J.; Nomura, M.; Fujita, F.; Kawaguchi, T.; et al. Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: Pitfall in managing immune-related adverse events. Front. Oncol. 2023, 13, 1264281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gazzaz, Z.J. Diabetes and COVID-19. Open Life Sci. 2021, 16, 297–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaki, N.; Alashwal, H.; Ibrahim, S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review. Diabetes Metab. Syndr. 2020, 14, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisceglia, I.; Canale, M.L.; Gallucci, G.; Turazza, F.M.; Lestuzzi, C.; Parrini, I.; Russo, G.; Maurea, N.; Quagliariello, V.; Oliva, S.; et al. Cardio-Oncology in the COVID Era (Co & Co): The Never Ending Story. Front. Cardiovasc. Med. 2022, 9, 821193, Erratum in Front. Cardiovasc. Med. 2022, 9, 903766; Erratum in Front. Cardiovasc. Med. 2023, 10, 1169176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krupka, S.; Hoffmann, A.; Jasaszwili, M.; Dietrich, A.; Guiu-Jurado, E.; Klöting, N.; Blüher, M. Consequences of COVID-19 on Adipose Tissue Signatures. Int. J. Mol. Sci. 2024, 25, 2908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Colón, G.J.; Ratnasiri, K.; Chen, H.; Jiang, S.; Zanley, E.; Rustagi, A.; Verma, R.; Chen, H.; Andrews, J.R.; Mertz, K.D.; et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci. Transl. Med. 2022, 14, eabm9151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basolo, A.; Poma, A.M.; Bonuccelli, D.; Proietti, A.; Macerola, E.; Ugolini, C.; Torregrossa, L.; Giannini, R.; Vignali, P.; Basolo, F.; et al. Adipose tissue in COVID-19: Detection of SARS-CoV-2 in adipocytes and activation of the interferon-alpha response. J. Endocrinol. Investig. 2022, 45, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, X.; Wu, J.; Dong, C.; Gao, J.; Seki, T.; Kim, C.; Urgard, E.; Hosaka, K.; Yang, Y.; Long, S.; et al. COVID-19 instigates adipose browning and atrophy through VEGF in small mammals. Nat. Metab. 2022, 4, 1674–1683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muzyka, I.; Revenko, O.; Kovalchuk, I.; Savytska, M.; Bekesevych, A.; Kasko, R.; Zayachkivska, O. What is the role of brown adipose tissue in metabolic health: Lessons learned and future perspectives in the long COVID? Inflammopharmacology 2023, 31, 585–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olivo, A.; Marlin, R.; Lazure, T.; Maisonnasse, P.; Bossevot, L.; Mouanga, C.; Lemaitre, J.; Pourcher, G.; Benoist, S.; Le Grand, R.; et al. Detection of SARS-CoV-2 in subcutaneous fat but not visceral fat, and the disruption of fat lymphocyte homeostasis in both fat tissues in the macaque. Commun. Biol. 2022, 5, 542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haseeb, M.; Shafiq, A.; Sheikh, M.A.; Khan, M.F. Epipericardial Fat Necrosis and COVID-19. Eur. J. Case Rep. Intern. Med. 2024, 11, 004346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5, Erratum in Cell Metab. 2021, 33, 2484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dugail, I.; Amri, E.Z.; Vitale, N. High prevalence for obesity in severe COVID-19: Possible links and perspectives towards patient stratification. Biochimie 2020, 179, 257–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malavazos, A.E.; Corsi Romanelli, M.M.; Bandera, F.; Iacobellis, G. Targeting the Adipose Tissue in COVID-19. Obesity 2020, 28, 1178–1179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Conforti, G.; Iovine, M.; Carbone, A.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients. Cancers 2020, 12, 3316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakhtiari, M.; Asadipooya, K. Metainflammation in COVID-19. Endocr. Metab. Immune Disord Drug Targets 2022, 22, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, G.P.; Singer, B.H.; Singer, K. The Collision of Meta-Inflammation and SARS-CoV-2 Pandemic Infection. Endocrinology 2020, 161, bqaa154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Zhang, C.; Hua, W.; Chen, J. Saying no to SARS-CoV-2: The potential of nitric oxide in the treatment of COVID-19 pneumonia. Med. Gas Res. 2024, 14, 39–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, Y.H. Adipose tissue in communication: Within and without. Nat. Rev. Endocrinol. 2023, 19, 70–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steenblock, C.; Bechmann, N.; Beuschlein, F.; Wolfrum, C.; Bornstein, S.R. Do adipocytes serve as a reservoir for severe acute respiratory symptom coronavirus-2? J. Endocrinol. 2023, 258, e230027. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Mousovich-Neto, F.; Ludwig, R.G.; Carregari, V.C.; Dos Anjos Souza, A.B.; Dos Passos, A.S.C.; Martini, M.C.; Barbosa, P.P.; de Souza, G.F.; Muraro, S.P.; et al. SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner. Nat. Commun. 2022, 13, 5722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, J.; Wilding, J.P.H.; Hu, J. Adipocytes in obesity: A perfect reservoir for SARS-CoV-2? Med. Hypotheses 2023, 171, 111020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; Paccone, A.; Iovine, M.; Cavalcanti, E.; Berretta, M.; Maurea, C.; Canale, M.L.; Maurea, N. Interleukin-1 blocking agents as promising strategy for prevention of anticancer drug-induced cardiotoxicities: Possible implications in cancer patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6797–6812. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Grappasonni, I.; Nguyen, C.T.T.; Tesauro, M.; Pantanetti, P.; Xhafa, S.; Cangelosi, G. Metformin and COVID-19: A systematic review of systematic reviews with meta-analysis. Acta Biomed. 2023, 94, e2023138. [Google Scholar] [CrossRef] [PubMed]
- Zareef, R.; Diab, M.; Al Saleh, T.; Makarem, A.; Younis, N.K.; Bitar, F.; Arabi, M. Aspirin in COVID-19: Pros and Cons. Front. Pharmacol. 2022, 13, 849628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, T.; Zhong, B.; Tang, C.; Qiao, S.; Feng, Y.; Peng, H.; Gu, X. Correlation between epicardial adipose tissue and myocardial injury in patients with COVID-19. Front Physiol. 2024, 15, 1368542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Favre, G.; Legueult, K.; Pradier, C.; Raffaelli, C.; Ichai, C.; Iannelli, A.; Redheuil, A.; Lucidarme, O.; Esnault, V. Visceral fat is associated to the severity of COVID-19. Metabolism 2021, 115, 154440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Health consequences of visceral obesity. Ann. Med. 2001, 33, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, N.; Høier, A.T.Z.B.; Andersen, B.V. A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease. Foods 2021, 10, 892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F. Physical Activity, Long-COVID, and Inactivity: A Detrimental Endless Loop. J. Phys. Act. Health 2024, 21, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Navas-Otero, A.; Calvache-Mateo, A.; Calles-Plata, I.; Valenza-Peña, G.; Hernández-Hernández, S.; Ortiz-Rubio, A.; Valenza, M.C. A lifestyle adjustments program in long COVID-19 improves symptomatic severity and quality of life. A randomized control trial. Patient Educ. Couns. 2024, 122, 108180. [Google Scholar] [CrossRef] [PubMed]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janochova, K.; Haluzik, M.; Buzga, M. Visceral fat and insulin resistance—What we know? Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2019, 163, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased insulin resistance due to Long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Braz. J. Psychiatry 2023, 45, 205–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogdański, A.; Niziołek, P.; Kopeć, S.; Moszak, M. Epicardial Adipose Tissue: A Precise Biomarker for Cardiovascular Risk, Metabolic Diseases, and Target for Therapeutic Interventions. Cardiol. Rev. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Bello-Chavolla, O.Y.; Mancillas-Adame, L.; Rodriguez-Flores, M.; Pedraza, N.R.; Encinas, B.R.; Carrión, C.I.P.; Ávila, M.I.J.; Valladares-García, J.C.; Vanegas-Cedillo, P.E.; et al. Epicardial adipose tissue thickness is associated with increased COVID-19 severity and mortality. Int. J. Obes. 2022, 46, 866–873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cugno, M.; Gualtierotti, R.; Casazza, G.; Tafuri, F.; Ghigliazza, G.; Torri, A.; Costantino, G.; Montano, N.; Peyvandi, F. Mortality in Patients with COVID-19 on Renin Angiotensin System Inhibitor Long-Term Treatment: An Observational Study Showing that Things Are Not Always as They Seem. Adv. Ther. 2021, 38, 2709–2716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, A.P.; Donadello, K.; Schweiger, V.; Zamboni, G.A.; Dalla Valle, Z.; Zamboni, M.; Polati, E.; Gottin, L. Epicardial adipose tissue volume and CT-attenuation as prognostic factors for pulmonary embolism and mortality in critically ill patients affected by COVID-19. Eur. J. Clin. Nutr. 2023, 77, 105–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, H.J.; Aghayev, A.; Hinnrichs, M.; Borggrefe, J.; Surov, A. Epicardial Adipose Tissue as a Prognostic Marker in COVID-19. Vivo 2024, 38, 281–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levy, D.; Giannini, M.; Oulehri, W.; Riou, M.; Marcot, C.; Pizzimenti, M.; Debrut, L.; Charloux, A.; Geny, B.; Meyer, A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients 2022, 14, 912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gambaro, S.E.; Zubiría, M.G.; Portales, A.E.; Rey, M.A.; Rumbo, M.; Giovambattista, A. M1 macrophage subtypes activation and adipocyte dysfunction worsen during prolonged consumption of a fructose-rich diet. J. Nutr. Biochem. 2018, 61, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murnane, L.C.; Forsyth, A.K.; Koukounaras, J.; Pilgrim, C.H.; Shaw, K.; Brown, W.A.; Mourtzakis, M.; Tierney, A.C.; Burton, P.R. Myosteatosis predicts higher complications and reduced overall survival following radical oesophageal and gastric cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Lin, S.; Li, Y.; Zhu, A.J.; Shi, H.; Liu, M. Identification of Ubr1 as an amino acid sensor of steatosis in liver and muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 1454–1467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.P.; Guo, M.J.; Yang, Q.; Li, Y.Y.; Wang, Y.G.; Zhang, M. Myosteatosis is associated with coronary artery calcification in patients with type 2 diabetes. World J. Diabetes 2024, 15, 429–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.M.; Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1270–1282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vedder, I.R.; Levolger, S.; Dierckx, R.A.J.O.; Zeebregts, C.J.; de Vries, J.P.M.; Viddeleer, A.R.; Bokkers, R.P.H. Effect of muscle depletion on survival in peripheral arterial occlusive disease: Quality over quantity. J. Vasc. Surg. 2020, 72, 2006–2016.e1. [Google Scholar] [CrossRef] [PubMed]
- Geladari, E.; Alexopoulos, T.; Kontogianni, M.D.; Vasilieva, L.; Mani, I.; Tenta, R.; Sevastianos, V.; Vlachogiannakos, I.; Alexopoulou, A. The Presence of Myosteatosis Is Associated with Age, Severity of Liver Disease and Poor Outcome and May Represent a Prodromal Phase of Sarcopenia in Patients with Liver Cirrhosis. J. Clin. Med. 2023, 12, 3332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Pozzuto, L.; Silveira, M.N.; Mendes, M.C.S.; Macedo, L.T.; Costa, F.O.; Martinez, C.A.R.; Coy, C.S.R.; da Cunha Júnior, A.D.; Carvalheira, J.B.C. Myosteatosis Differentially Affects the Prognosis of Non-Metastatic Colon and Rectal Cancer Patients: An Exploratory Study. Front. Oncol. 2021, 11, 762444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Body, S.; Ligthart, M.A.P.; Rahman, S.; Ward, J.; May-Miller, P.; Pucher, P.H.; Curtis, N.J.; West, M.A.; Wessex Research Collaborative. Sarcopenia and Myosteatosis Predict Adverse Outcomes After Emergency Laparotomy: A Multi-center Observational Cohort Study. Ann. Surg. 2022, 275, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Ehrengut, C.; Zimmermann, S.; Schramm, D.; Hinnerichs, M.; Bär, C.; Borggrefe, J. Myosteatosis predicts short-term mortality in patients with COVID-19: A multicenter analysis. Nutrition 2024, 120, 112327. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, R.; Palmisano, A.; Esposito, A.; Gnasso, C.; Nicoletti, V.; Leone, R.; Vignale, D.; Falbo, E.; Ferrante, M.; Cilla, M.; et al. Myosteatosis Significantly Predicts Persistent Dyspnea and Mobility Problems in COVID-19 Survivors. Front. Nutr. 2022, 9, 846901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fierro, P.; Martín, D.; Pariente-Rodrigo, E.; Pini, S.F.; Basterrechea, H.; Tobalina, M.; Petitta, B.; Bianconi, C.; Díaz-Salazar, S.; Bonome, M.; et al. Post-COVID-19 syndrome, inflammation and insulin resistance: A retrospective cohort study. Minerva Endocrinol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.; Daimon, M.; Murakami, H.; Otaka, H.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; Matsuhashi, Y.; Yanagimachi, M.; et al. Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PLoS ONE 2016, 11, e0166077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bastin, M.; Andreelli, F. Diabète et corticoïdes: Nouveautés et aspects pratiques [Corticosteroid-induced diabetes: Novelties in pathophysiology and management]. Rev. Med. Interne 2020, 41, 607–616. (In French) [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Khairi Abed, A.; Rouf Moustafa, S.; Almulla, A.F.; Maes, M. Tryptophan catabolites, inflammation, and insulin resistance as determinants of chronic fatigue syndrome and affective symptoms in long COVID. Front Mol Neurosci. 2023, 16, 1194769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.J.; Cho, Y.K.; Jung, H.N.; Kim, E.H.; Lee, M.J.; Jung, C.H.; Park, J.Y.; Kim, H.K.; Lee, W.J. Association Between Insulin Resistance and Myosteatosis Measured by Abdominal Computed Tomography. J. Clin. Endocrinol. Metab. 2023, 108, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyu, K.; Zhang, D.; Song, J.; Li, X.; Perry, R.J.; Samuel, V.T.; Shulman, G.I. Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation. JCI Insight 2021, 6, e139946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef] [PubMed]
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long COVID: A scoping review. EBioMedicine 2023, 91, 104552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr. Med. Res. Opin. 2022, 38, 901–909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, J.X.; Agbana, Y.L.; Sun, Z.S.; Fei, S.W.; Zhao, H.Q.; Zhou, X.N.; Chen, J.H.; Kassegne, K. Increased interleukin-6 is associated with long COVID-19: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannitrapani, L.; Mirarchi, L.; Amodeo, S.; Licata, A.; Soresi, M.; Cavaleri, F.; Casalicchio, S.; Ciulla, G.; Ciuppa, M.E.; Cervello, M.; et al. Can Baseline IL-6 Levels Predict Long COVID in Subjects Hospitalized for SARS-CoV-2 Disease? Int. J. Mol. Sci. 2023, 24, 1731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergantini, L.; Gangi, S.; d’Alessandro, M.; Cameli, P.; Perea, B.; Meocci, M.; Fabbri, G.; Bianchi, F.; Bargagli, E. Altered serum concentrations of IL-8, IL-32 and IL-10 in patients with lung impairment 6 months after COVID-19. Immunobiology 2024, 229, 152813. [Google Scholar] [CrossRef] [PubMed]
- Nigo, M.; Rasmy, L.; May, S.B.; Rao, A.; Karimaghaei, S.; Kannadath, B.S.; De la Hoz, A.; Arias, C.A.; Li, L.; Zhi, D. Real World Long-term Assessment of The Efficacy of Tocilizumab in Patients with COVID-19: Results From A Large De-identified Multicenter Electronic Health Record Dataset in the United States. Int. J. Infect. Dis. 2021, 113, 148–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogard, G.; Barthelemy, J.; Hantute-Ghesquier, A.; Sencio, V.; Brito-Rodrigues, P.; Séron, K.; Robil, C.; Flourens, A.; Pinet, F.; Eberlé, D.; et al. SARS-CoV-2 infection induces persistent adipose tissue damage in aged golden Syrian hamsters. Cell Death Dis. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, A.R.; Chernis, N.; Masschelin, P.M.; Hartig, S.M. Immune Cells Gate White Adipose Tissue Expansion. Endocrinology 2019, 160, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bauzá-Thorbrügge, M.; Vujičić, M.; Chanclón, B.; Palsdottir, V.; Pillon, N.J.; Benrick, A.; Wernstedt Asterholm, I. Adiponectin stimulates Sca1+CD34—Adipocyte precursor cells associated with hyperplastic expansion and beiging of brown and white adipose tissue. Metabolism 2024, 151, 155716. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Scialò, F.; Mallardo, M.; Signoriello, G.; D’Agnano, V.; Bianco, A.; Daniele, A.; Nigro, E. Adiponectin, Leptin, and Resistin Are Dysregulated in Patients Infected by SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 1131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.C.; Lee, C.J.; Yang, C.F.; Chen, Y.C.; Wang, J.H.; Hsu, B.G. Low serum adiponectin level is associated with metabolic syndrome and is an independent marker of peripheral arterial stiffness in hypertensive patients. Diabetol. Metab. Syndr. 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subramanian, S.; Liu, C.; Aviv, A.; Ho, J.E.; Courchesne, P.; Muntendam, P.; Larson, M.G.; Cheng, S.; Wang, T.J.; Mehta, N.N.; et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arter. Thromb. Vasc. Biol. 2014, 34, 2100–2105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cambier, S.; Beretta, F.; Pörtner, N.; Metzemaekers, M.; de Carvalho, A.C.; Martens, E.; Kaes, J.; Aelbrecht, C.; Jacobs, C.; Van Mol, P.; et al. Proteolytic inactivation of CXCL12 in the lungs and circulation of COVID-19 patients. Cell. Mol. Life Sci. 2023, 80, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Fleta, P.; Vera-Tomé, P.; Jiménez-Fernández, M.; Requena, S.; Roy-Vallejo, E.; Sanz-García, A.; Lozano-Prieto, M.; López-Sanz, C.; Vara, A.; Lancho-Sánchez, Á.; et al. A Differential Signature of Circulating miRNAs and Cytokines Between COVID-19 and Community-Acquired Pneumonia Uncovers Novel Physiopathological Mechanisms of COVID-19. Front. Immunol. 2022, 12, 815651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noto, A.; Joo, V.; Mancarella, A.; Suffiotti, M.; Pellaton, C.; Fenwick, C.; Perreau, M.; Pantaleo, G. CXCL12 and CXCL13 Cytokine Serum Levels Are Associated with the Magnitude and the Quality of SARS-CoV-2 Humoral Responses. Viruses 2022, 14, 2665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Avila, H.; Lima, C.N.R.; Rampinelli, P.G.; Mateus, L.C.O.; Sousa Silva, R.V.; Correa, J.R.; Almeida, P.E. Lipid Metabolism Modulation during SARS-CoV-2 Infection: A Spotlight on Extracellular Vesicles and Therapeutic Prospects. Int. J. Mol. Sci. 2024, 25, 640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuen, T.T.; Chan, J.F.; Yan, B.; Shum, C.C.; Liu, Y.; Shuai, H.; Hou, Y.; Huang, X.; Hu, B.; Chai, Y.; et al. Targeting ACLY efficiently inhibits SARS-CoV-2 replication. Int. J. Biol. Sci. 2022, 18, 4714–4730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kridel, S.J.; Lowther, W.T.; Pemble, C.W., 4th. Fatty acid synthase inhibitors: New directions for oncology. Expert Opin. Investig. Drugs 2007, 16, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D. Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer. Cells 2022, 11, 2432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wise, J. COVID-19: Metformin reduces the risk of developing long term symptoms by 40%, study finds. BMJ 2023, 381, 1306. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K. Metformin, cancer, COVID-19, and longevity. Int. J. Clin. Pharmacol. Ther. 2023, 61, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, L.; Jensen, M.H.; Cook, M.E.; Vestergaard, P.; Knop, F.K.; Drewes, A.M.; Olesen, S.S. Metformin treatment is associated with reduced risk of hypoglycaemia, major adverse cardiovascular events, and all-cause mortality in patients with post-pancreatitis diabetes mellitus: A nationwide cohort study. Eur. J. Endocrinol. 2024, 190, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.F.; Hong, C.T.; Chen, W.T.; Chan, L.; Chien, L.N. Metformin adherence and the risk of cardiovascular disease: A population-based cohort study. Ther. Adv. Chronic Dis. 2023, 14, 20406223231163115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Zadjali, J.; Al-Lawati, A.; Al Riyami, N.; Al Farsi, K.; Al Jarradi, N.; Boudaka, A.; Al Barhoumi, A.; Al Lawati, M.; Al Khaifi, A.; Musleh, A.; et al. Reduced HDL-cholesterol in long COVID-19: A key metabolic risk factor tied to disease severity. Clinics 2024, 79, 100344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MCCarthy, M.W. Metformin as a potential treatment for COVID-19. Expert Opin. Pharmacother. 2023, 24, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, S.; Baxter, B.A.; Dooley, G.; LaVergne, S.M.; Gallichotte, E.; Dutt, T.; Tipton, M.; Berry, K.; Haberman, J.; Natter, N.; et al. Relationships between plasma fatty acids in adults with mild, moderate, or severe COVID-19 and the development of post-acute sequelae. Front. Nutr. 2022, 9, 960409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, C.; Jadeja, V.; Zhou, H. Molecular Mechanisms of Palmitic Acid Augmentation in COVID-19 Pathologies. Int. J. Mol. Sci. 2021, 22, 7127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.P.; Chang, C.M.; Yang, C.C.; Pariante, C.M.; Su, K.P. Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav. Immun. 2022, 103, 19–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quaranta, P.; Scabia, G.; Storti, B.; Dattilo, A.; Quintino, L.; Perrera, P.; Di Primio, C.; Costa, M.; Pistello, M.; Bizzarri, R.; et al. SARS-CoV-2 Infection Alters the Phenotype and Gene Expression of Adipocytes. Int. J. Mol. Sci. 2024, 25, 2086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petroni, A.; Paroni, R.; Aloisi, A.M.; Blasevich, M.; Haman, N.; Fessas, D. Thermogenic flux induced by lignoceric acid in peroxisomes isolated from HepG2 cells and from X-adrenoleukodystrophy and control fibroblasts. J. Cell. Physiol. 2019, 234, 18344–18348. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Can essential fatty acids (EFAs) prevent and ameliorate post-COVID-19 long haul manifestations? Lipids Health Dis. 2024, 23, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, T.H.; Ho, C.H.; Chen, D.T.; Wu, J.Y.; Huang, P.Y.; Lai, C.C.; Hsieh, K.Y.; Su, K.P. Omega-3 polyunsaturated fatty acids and the psychiatric post-acute sequelae of COVID-19: A one-year retrospective cohort analysis of 33,908 patients. Brain Behav Immun. 2023, 114, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.; Olliaro, P.; Sigfrid, L.; Carson, G.; Paparella, G.; Hastie, C.; Kaushic, C.; Boily-Larouche, G.; Suett, J.C.; O’Hara, M.; et al. Long COVID: Tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect. Dis. 2021, 21, 601–602, Erratum in Lancet Infect. Dis. 2021, 21, e81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oldani, S.; Petrelli, F.; Dognini, G.; Borgonovo, K.; Parati, M.C.; Ghilardi, M.; Dottorini, L.; Cabiddu, M.; Luciani, A. COVID-19 and Lung Cancer Survival: An Updated Systematic Review and Meta-Analysis. Cancers 2022, 14, 5706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avancini, A.; Trestini, I.; Tregnago, D.; Wiskemann, J.; Lanza, M.; Milella, M.; Pilotto, S. Physical Activity for Oncological Patients in COVID-19 Era: No Time to Relax. JNCI Cancer Spectr. 2020, 4, pkaa071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lope, V.; Guerrero-Zotano, Á.; Fernández de Larrea-Baz, N.; Antolín, S.; Benavent Viñuales, M.; Bermejo, B.; Ruiz-Moreno, E.; Baena-Cañada, J.M.; París, L.; Antón, A.; et al. Cross-sectional and longitudinal associations of adherence to WCRF/AICR cancer prevention recommendations with health-related quality of life in breast cancer survivors. Health-EpiGEICAM study. J. Nutr. Health Aging 2024, 28, 100312. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.A.; Yang, E.H.; Cheng, R.K.; DeCara, J.M.; Dent, S.; Liu, J.E.; Rudski, L.G.; Strom, J.B.; Thavendiranathan, P.; Barac, A.; et al. Cardiovascular Care of the Oncology Patient During COVID-19: An Expert Consensus Document From the ACC Cardio-Oncology and Imaging Councils. J. Natl. Cancer Inst. 2021, 113, 513–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tosato, M.; Ciciarello, F.; Zazzara, M.B.; Pais, C.; Savera, G.; Picca, A.; Galluzzo, V.; Coelho-Júnior, H.J.; Calvani, R.; Marzetti, E.; et al. Nutraceuticals and Dietary Supplements for Older Adults with Long COVID-19. Clin. Geriatr. Med. 2022, 38, 565–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonetti, G.; Medori, M.C.; Fioretti, F.; Farronato, M.; Nodari, S.; Lorusso, L.; Tartaglia, G.M.; Farronato, G.; Bellinato, F.; Gisondi, P.; et al. Dietary supplements for the management of COVID-19 symptoms. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E221–E227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galluzzo, V.; Zazzara, M.B.; Ciciarello, F.; Savera, G.; Pais, C.; Calvani, R.; Picca, A.; Marzetti, E.; Landi, F.; Tosato, M.; et al. Fatigue in COVID-19 survivors: The potential impact of a nutritional supplement on muscle strength and function. Clin. Nutr. ESPEN 2022, 51, 215–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossato, M.S.; Brilli, E.; Ferri, N.; Giordano, G.; Tarantino, G. Observational study on the benefit of a nutritional supplement, supporting immune function and energy metabolism, on chronic fatigue associated with the SARS-CoV-2 post-infection progress. Clin. Nutr. ESPEN 2021, 46, 510–518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; Donkor, K.; Kansakar, U.; Trimarco, V.; Mone, P.; et al. l-Arginine and COVID-19: An Update. Nutrients 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trimarco, V.; Izzo, R.; Lombardi, A.; Coppola, A.; Fiorentino, G.; Santulli, G. Beneficial effects of L-Arginine in patients hospitalized for COVID-19: New insights from a randomized clinical trial. Pharmacol. Res. 2023, 191, 106702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Plata-Menchaca, E.P.; Martin, L.; Bajaña, I.; Martin-Rodríguez, C.; Palmada, C.; Ferrer-Costa, R.; Camos, S.; Villena-Ortiz, Y.; et al. Vitamin C deficiency in critically ill COVID-19 patients admitted to intensive care unit. Front. Med. 2023, 10, 1301001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuźmicka, W.; Manda-Handzlik, A.; Cieloch, A.; Mroczek, A.; Demkow, U.; Wachowska, M.; Ciepiela, O. Zinc Supplementation Modulates NETs Release and Neutrophils’ Degranulation. Nutrients 2020, 13, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasan, R.; Rink, L.; Haase, H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low Vitamin D Levels Are Associated With Long COVID Syndrome in COVID-19 Survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, K.Y.; Lin, C.K.; Chen, N.H. Effects of vitamin D and zinc deficiency in acute and long COVID syndrome. J. Trace Elem. Med. Biol. 2023, 80, 127278. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; Trimarco, V.; Mone, P.; Aloè, T.; Capra Marzani, M.; Diana, A.; Fazio, G.; Mallardo, M.; Maniscalco, M.; Marazzi, G.; et al. Combining L-Arginine with vitamin C improves long-COVID symptoms: The LINCOLN Survey. Pharmacol. Res. 2022, 183, 106360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinopoli, A.; Sciurti, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The Efficacy of Multivitamin, Vitamin A, Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention and Management of COVID-19 and Long-COVID: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 1345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakuma, K.; Hamada, K.; Yamaguchi, A.; Aoi, W. Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia. Cells 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 prevention, treatment, and recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matino, E.; Tavella, E.; Rizzi, M.; Avanzi, G.C.; Azzolina, D.; Battaglia, A.; Becco, P.; Bellan, M.; Bertinieri, G.; Bertoletti, M.; et al. Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial. Nutrients 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berthon, B.S.; Williams, L.M.; Williams, E.J.; Wood, L.G. Effect of Lactoferrin Supplementation on Inflammation, Immune Function, and Prevention of Respiratory Tract Infections in Humans: A Systematic Review and Meta-analysis. Adv. Nutr. 2022, 13, 1799–1819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Owoyele, B.V.; Bakare, A.O.; Ologe, M.O. Bromelain: A Review on its Potential as a Therapy for the Management of COVID-19. Niger. J. Physiol. Sci. 2020, 35, 10–19. [Google Scholar] [PubMed]
- Sagar, S.; Rathinavel, A.K.; Lutz, W.E.; Struble, L.R.; Khurana, S.; Schnaubelt, A.T.; Mishra, N.K.; Guda, C.; Palermo, N.Y.; Broadhurst, M.J.; et al. Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and spike protein. Clin. Transl. Med. 2021, 11, e281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavlidou, E.; Poulios, E.; Papadopoulou, S.K.; Fasoulas, A.; Dakanalis, A.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity. Med. Sci. 2024, 12, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317, Erratum in Life 2024, 11, 317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell. Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Coelho Dos Reis, J.G.A.; Ferreira, G.M.; Lourenço, A.A.; Ribeiro, Á.L.; da Mata, C.P.D.S.M.; de Melo Oliveira, P.; Marques, D.P.A.; Ferreira, L.L.; Clarindo, F.A.; da Silva, M.F.; et al. Ex-vivo mucolytic and anti-inflammatory activity of BromAc in tracheal aspirates from COVID-19. Biomed. Pharmacother. 2022, 148, 112753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kritis, P.; Karampela, I.; Kokoris, S.; Dalamaga, M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol. Open 2020, 8, 100066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Sun, S.; Du, C.; Hu, K.; Zhang, C.; Liu, M.; Wu, Q.; Dong, N. Transmembrane serine protease TMPRSS2 implicated in SARS-CoV-2 infection is autoactivated intracellularly and requires N-glycosylation for regulation. J. Biol. Chem. 2022, 298, 102643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, R.C.; Chen, Y.S.; Huang, J.R.; Jeng, K.C. Cross-linked bromelain inhibits lipopolysaccharide-induced cytokine production involving cellular signaling suppression in rats. J. Agric. Food Chem. 2006, 54, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Soheilifar, S.; Bidgoli, M.; Hooshyarfard, A.; Shahbazi, A.; Vahdatinia, F.; Khoshkhooie, F. Effect of Oral Bromelain on Wound Healing, Pain, and Bleeding at Donor Site Following Free Gingival Grafting: A Clinical Trial. J. Dent. 2018, 15, 309–316. [Google Scholar] [PubMed] [PubMed Central]
- Leelakanok, N.; Petchsomrit, A.; Janurai, T.; Saechan, C.; Sunsandee, N. Efficacy and safety of bromelain: A systematic review and meta-analysis. Nutr. Health 2023, 29, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Jurek, J.M.; Castro-Marrero, J. A Narrative Review on Gut Microbiome Disturbances and Microbial Preparations in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Implications for Long COVID. Nutrients 2024, 16, 1545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.H.; Limaye, A.; Liu, J.R.; Wu, T.N. Potential probiotics for regulation of the gut-lung axis to prevent or alleviate influenza in vulnerable populations. J. Tradit. Complement. Med. 2022, 13, 161–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taufer, C.R.; da Silva, J.; Rampelotto, P.H. The Influence of Probiotic Lactobacilli on COVID-19 and the Microbiota. Nutrients 2024, 16, 1350. [Google Scholar] [CrossRef]
- Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Linton, C.; Wright, H.H.; Wadsworth, D.P.; Schaumberg, M.A. Dietary Inflammatory Index and Associations with Sarcopenia Symptomology in Community-Dwelling Older Adults. Nutrients 2022, 14, 5319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cailleaux, P.E.; Déchelotte, P.; Coëffier, M. Novel dietary strategies to manage sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.; Tintle, N.L.; Myers, M.; Lockshon, L.; Bacareza, H.; Harris, W.S. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fat. Acids 2021, 166, 102250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Grant, W.B.; Frias-Toral, E.; Vetrani, C.; Verde, L.; de Alteriis, G.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients 2022, 14, 1305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Peng, M.M.; Ye, X.; Chen, L.L. Low glycaemic index diets as an intervention for obesity: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 290–315. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Majzoub, J.A.; Al-Zahrani, A.; Dallal, G.E.; Blanco, I.; Roberts, S.B. High glycemic index foods, overeating, and obesity. Pediatrics 1999, 103, E26. [Google Scholar] [CrossRef] [PubMed]
- Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Sigala, D.M.; Bremer, A.A.; Cox, C.L.; Keim, N.L.; Schwarz, J.M.; Pacini, G.; et al. Effects of Consuming Beverages Sweetened with Fructose, Glucose, High-Fructose Corn Syrup, Sucrose, or Aspartame on OGTT-Derived Indices of Insulin Sensitivity in Young Adults. Nutrients 2024, 16, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turesky, R.J. Mechanistic Evidence for Red Meat and Processed Meat Intake and Cancer Risk: A Follow-up on the International Agency for Research on Cancer Evaluation of 2015. Chimia 2018, 72, 718–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Maurea, C.; Gabrielli, D.; Tarantini, L.; Paccone, A.; Inno, A.; Oliva, S.; Cadeddu Dessalvi, C.; et al. Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism. Biomedicines 2024, 12, 1650. https://doi.org/10.3390/biomedicines12081650
Quagliariello V, Canale ML, Bisceglia I, Maurea C, Gabrielli D, Tarantini L, Paccone A, Inno A, Oliva S, Cadeddu Dessalvi C, et al. Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism. Biomedicines. 2024; 12(8):1650. https://doi.org/10.3390/biomedicines12081650
Chicago/Turabian StyleQuagliariello, Vincenzo, Maria Laura Canale, Irma Bisceglia, Carlo Maurea, Domenico Gabrielli, Luigi Tarantini, Andrea Paccone, Alessandro Inno, Stefano Oliva, Christian Cadeddu Dessalvi, and et al. 2024. "Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism" Biomedicines 12, no. 8: 1650. https://doi.org/10.3390/biomedicines12081650
APA StyleQuagliariello, V., Canale, M. L., Bisceglia, I., Maurea, C., Gabrielli, D., Tarantini, L., Paccone, A., Inno, A., Oliva, S., Cadeddu Dessalvi, C., Zito, C., Caraglia, M., Berretta, M., D’Aiuto, G., & Maurea, N. (2024). Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism. Biomedicines, 12(8), 1650. https://doi.org/10.3390/biomedicines12081650













