Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer
Abstract
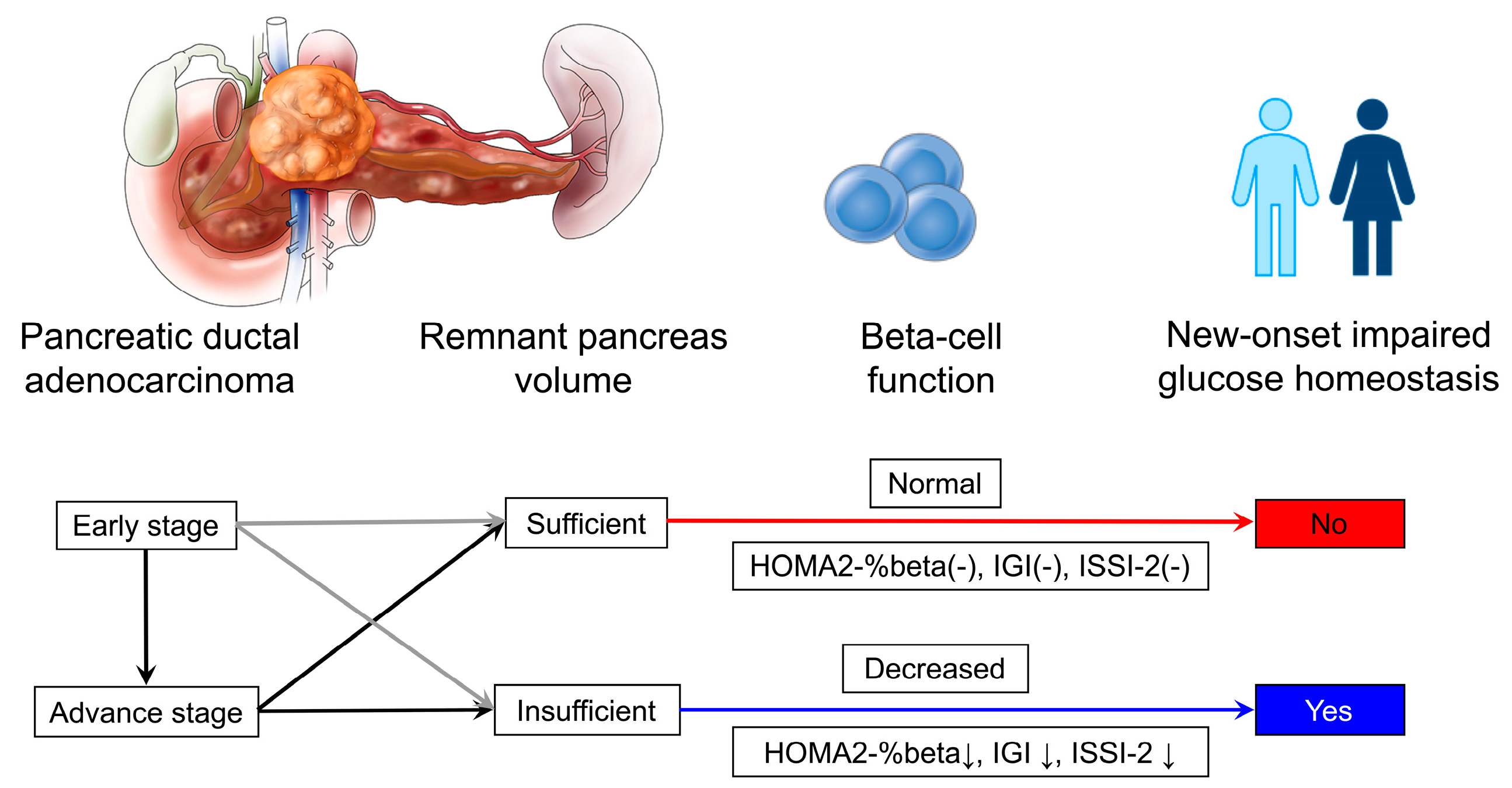
1. Introduction
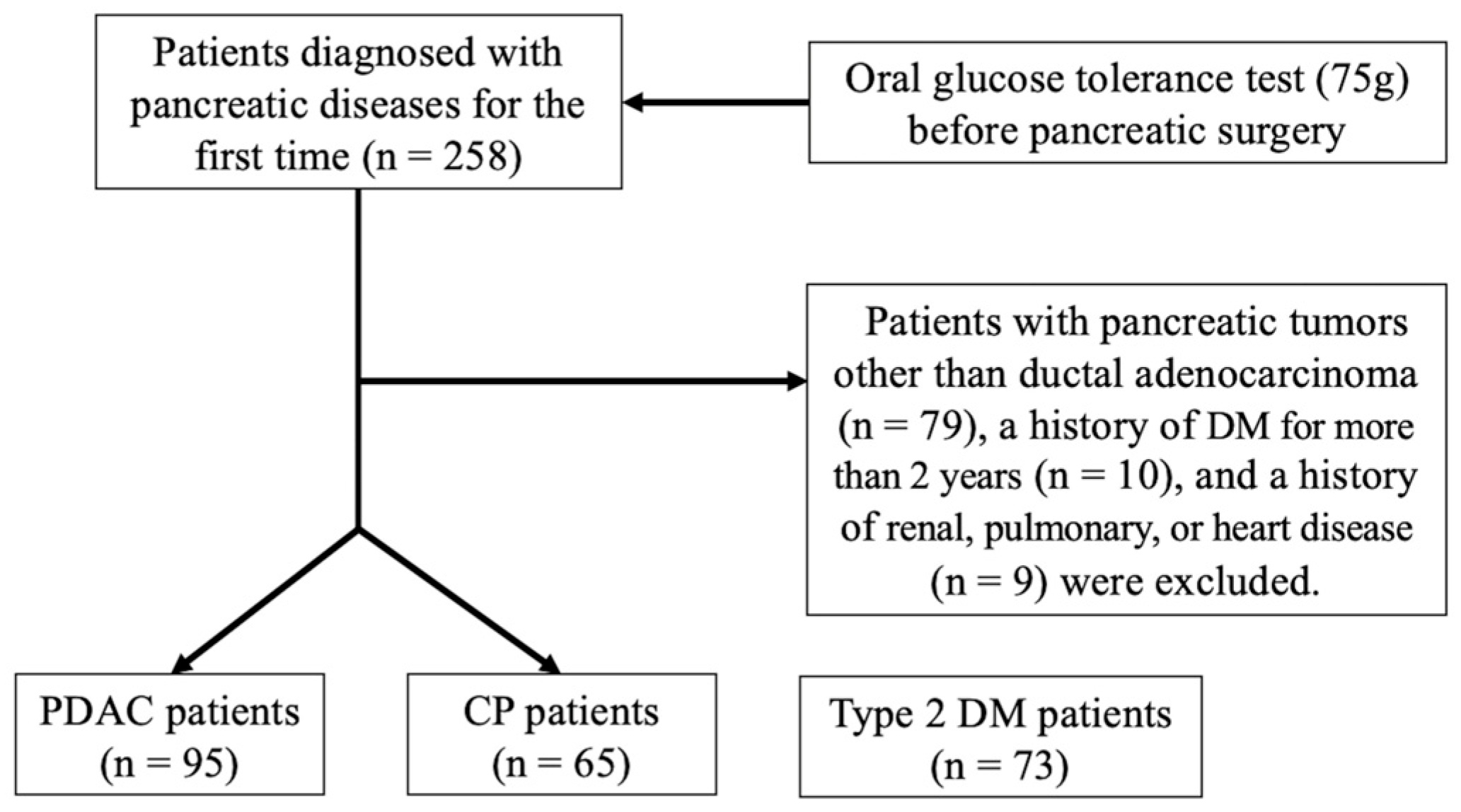
2. Materials and Methods
2.1. Study Design
2.2. Biochemical Parameters
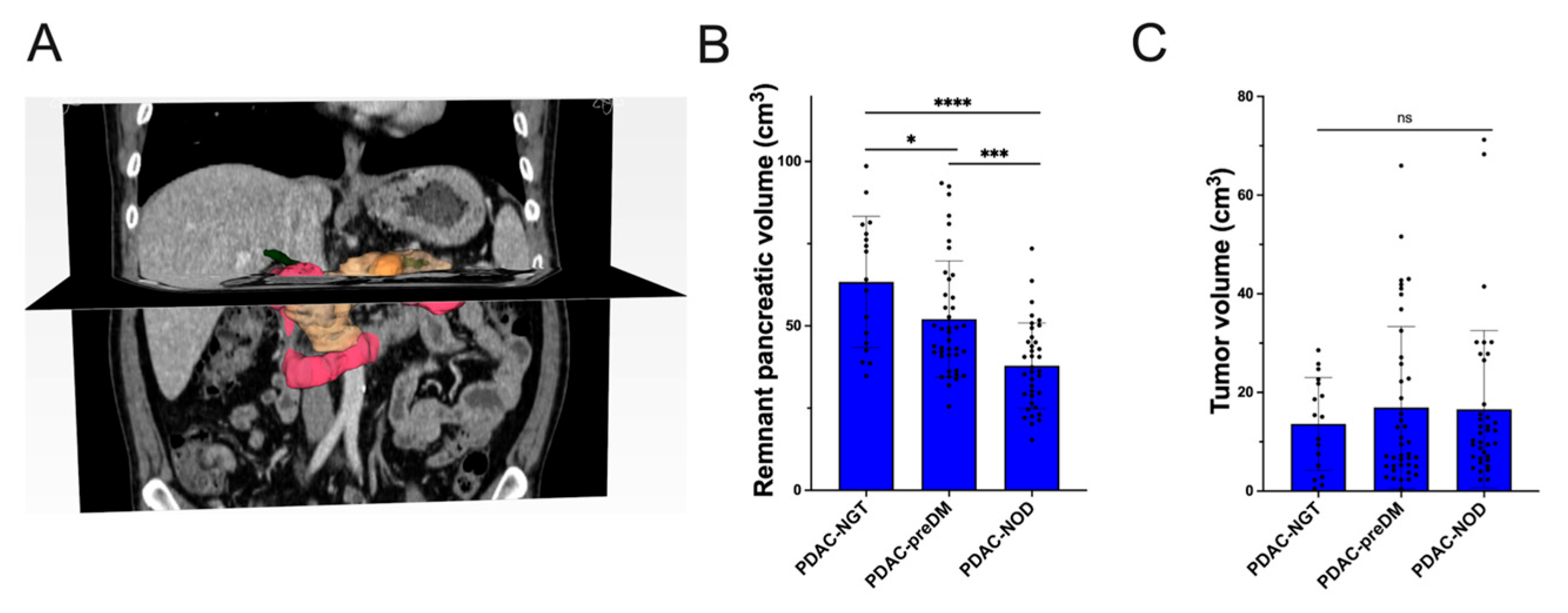
2.3. Calculation of Remnant Pancreas Volume
2.4. Statistical Analysis
3. Results
3.1. Glycemic Traits of PDAC Patients
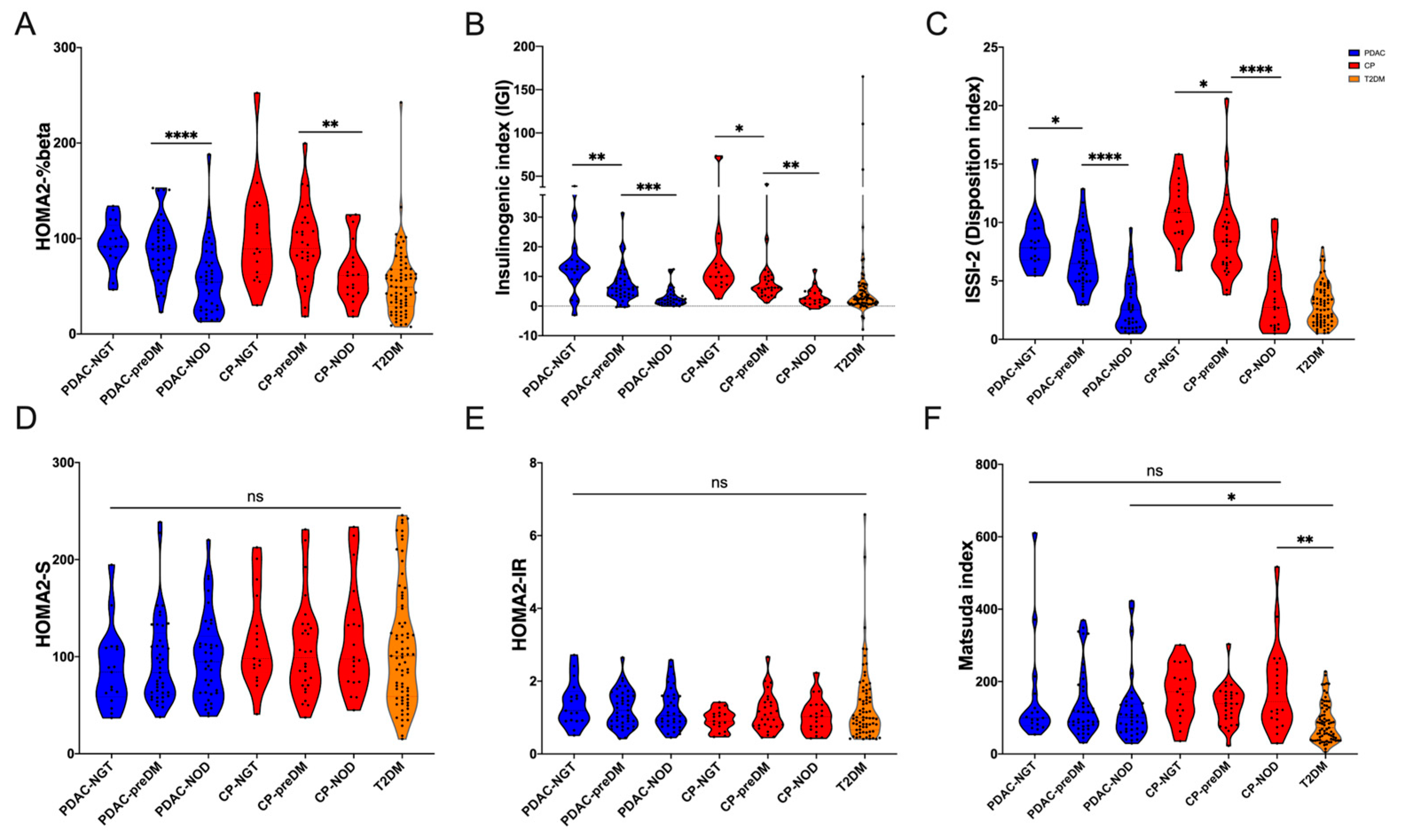
3.2. Glucose Metabolism Traits of PDAC Patients with Different Glucose Tolerance Statuses
3.3. Risk Factors of preDM and NOD Secondary to PDAC
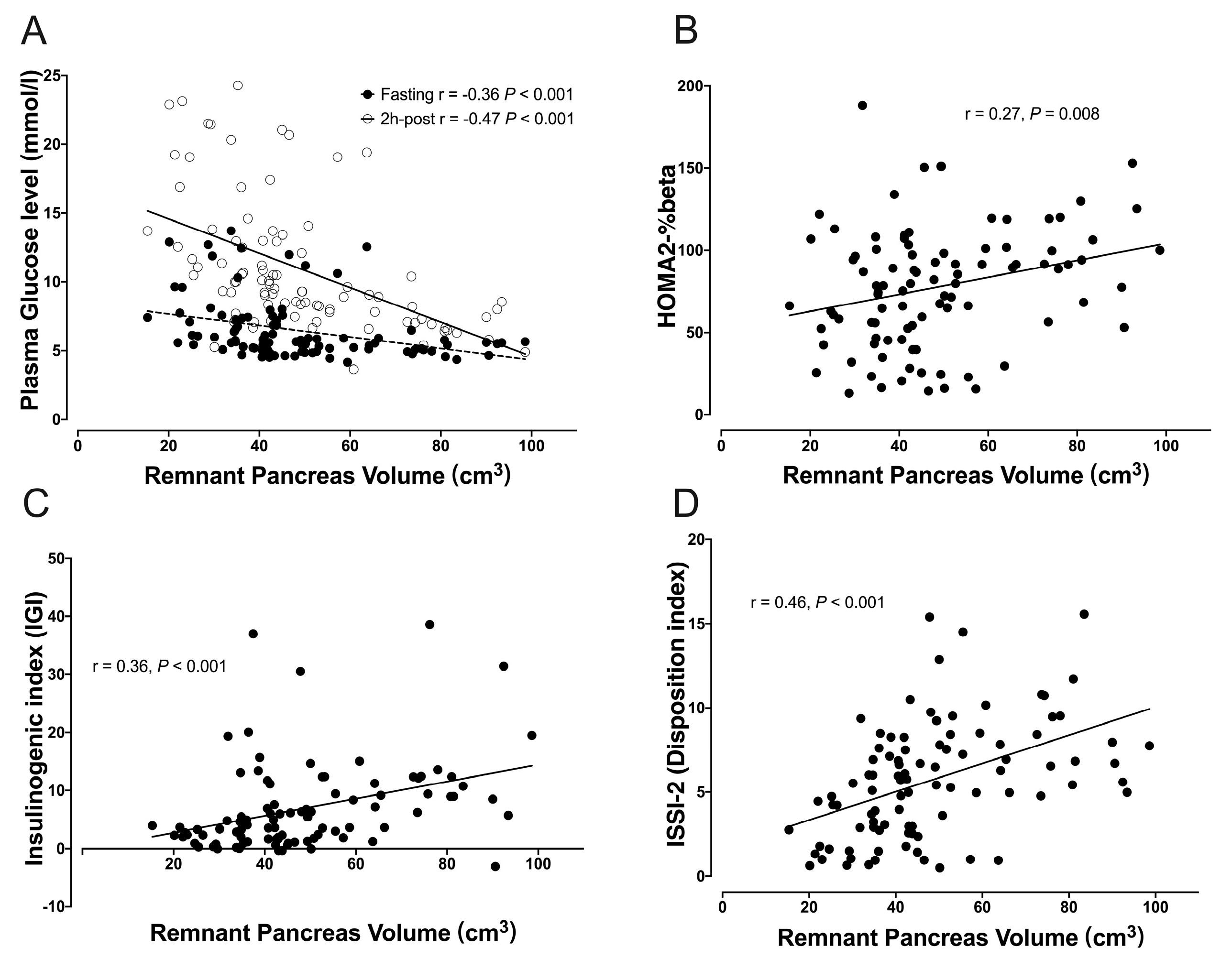
3.4. Correlation between RPV and Glucose Metabolism Indexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Ransom, J.; de Andrade, M.; Petersen, G.M. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology 2005, 129, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Kang, D.; Kang, M.; Kim, S.; Lee, E.K.; Kim, Y.A.; Chang, Y.J.; Choi, K.S.; Jung, S.Y.; Woo, S.M.; et al. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018, 4, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Grand, M.K.; von Heymann, A.; Persson, F.; Siersma, V.; Kriegbaum, M.; Lykkegaard Andersen, C.; Johansen, C. Incidence of New-Onset Type 2 Diabetes after Cancer: A Danish Cohort Study. Diabetes Care 2022, 45, e105–e106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Smyrk, T.C.; Levy, M.J.; Topazian, M.A.; Chari, S.T. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer before Diagnosis. Gastroenterology 2018, 155, 490–500.e492. [Google Scholar] [CrossRef]
- Cersosimo, E.; Pisters, P.W.; Pesola, G.; McDermott, K.; Bajorunas, D.; Brennan, M.F. Insulin secretion and action in patients with pancreatic cancer. Cancer 1991, 67, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Permert, J.; Ihse, I.; Jorfeldt, L.; von Schenck, H.; Arnqvist, H.J.; Larsson, J. Pancreatic cancer is associated with impaired glucose metabolism. Eur. J. Surg. 1993, 159, 101–107. [Google Scholar]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Aggarwal, G.; Rabe, K.G.; Petersen, G.M.; Chari, S.T. New-onset diabetes in pancreatic cancer: A study in the primary care setting. Pancreatology 2012, 12, 156–161. [Google Scholar] [CrossRef]
- Pelaez-Luna, M.; Takahashi, N.; Fletcher, J.G.; Chari, S.T. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: A retrospective review of CT scans and fasting glucose values prior to diagnosis. Am. J. Gastroenterol. 2007, 102, 2157–2163. [Google Scholar] [CrossRef]
- Basso, D.; Plebani, M.; Fogar, P.; Del Favero, G.; Briani, G.; Meggiato, T.; Panozzo, M.P.; Ferrara, C.; D’Angeli, F.; Burlina, A. Beta-cell function in pancreatic adenocarcinoma. Pancreas 1994, 9, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.N.; Frier, B.M.; Armitage, M.; Ashby, J.P. Abnormal insulin secretion in carcinoma of the pancreas: Response to glucagon stimulation. Diabet. Med. 1985, 2, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.P.; Nagpal, S.J.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Virostko, J.; Williams, J.; Hilmes, M.; Bowman, C.; Wright, J.J.; Du, L.; Kang, H.; Russell, W.E.; Powers, A.C.; Moore, D.J. Pancreas Volume Declines During the First Year After Diagnosis of Type 1 Diabetes and Exhibits Altered Diffusion at Disease Onset. Diabetes Care 2019, 42, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Thompson, M.L.; Filipp, S.L.; Grajo, J.R.; Nambam, B.; Beegle, R.; Middlebrooks, E.H.; Gurka, M.J.; Atkinson, M.A.; Schatz, D.A.; Haller, M.J. Relative Pancreas Volume Is Reduced in First-Degree Relatives of Patients With Type 1 Diabetes. Diabetes Care 2019, 42, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Sorokin, E.P.; Thomas, E.L.; Sattar, N.; Cule, M.; Bell, J.D.; Yaghootkar, H. Estimating the Effect of Liver and Pancreas Volume and Fat Content on Risk of Diabetes: A Mendelian Randomization Study. Diabetes Care 2022, 45, 460–468. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch. Pathol. Lab. Med. 2021, 145, 543–553. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, H.S.; Allen, E.W.; Herron, A.L., Jr.; Brennan, M.T. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Defronzo, R.A.; Tripathy, D.; Schwenke, D.C.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.C.; Henry, R.R.; Kitabchi, A.E.; Mudaliar, S.; et al. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care 2013, 36, 3607–3612. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Yoshida, A.; Suganami, H.; Ishiguro, H.; Yamamoto, M.; Fujihara, K.; Kodama, S.; Tanaka, S.; Kaku, K.; Sone, H. Role of fatty liver in the association between obesity and reduced hepatic insulin clearance. Diabetes Metab. 2018, 44, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Butler, A.E.; Meier, J.J.; Monchamp, T.; Allen-Auerbach, M.; Rizza, R.A.; Butler, P.C. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin. Anat. 2007, 20, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Cervantes, A.; Kim, J.U.; Nguyen, N.N.; DeSouza, S.V.; Dokpuang, D.; Lu, J.; Petrov, M.S. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G806–G815. [Google Scholar] [CrossRef]
- Wu, B.U.; Lustigova, E.; Chen, Q.; Dong, E.Y.; Maitra, A.; Chari, S.T.; Feng, Z.; Rinaudo, J.A.; Matrisian, L.M.; Parker, R.A. Imaging of the Pancreas in New-Onset Diabetes: A Prospective Pilot Study. Clin. Transl. Gastroenterol. 2022, 13, e00478. [Google Scholar] [CrossRef]
- Liao, W.C.; Chen, P.R.; Huang, C.C.; Chang, Y.T.; Huang, B.S.; Chang, C.C.; Wu, M.S.; Chow, L.P. Relationship between pancreatic cancer-associated diabetes and cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 899–908. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Noda, T.; Harada, N.; Miyamoto, T.; Tomioka, T.; Yamamoto, K.; Yamaguchi, T.; Izawa, K.; Tsunoda, T.; Yoshino, R.; et al. Collective review of small carcinomas of the pancreas. Ann. Surg. 1986, 203, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Misawa, R.; Zielinski, M.C.; Cowen, P.; Jo, J.; Periwal, V.; Ricordi, C.; Khan, A.; Szust, J.; Shen, J.; et al. Regional differences in islet distribution in the human pancreas—Preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE 2013, 8, e67454. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Hessami, N.; Smith, K.D.; Ripple, G.H.; Barth, R.J.; Klibansky, D.A.; Colacchio, T.A.; Zaki, B.; Tsapakos, M.J.; Suriawinata, A.A.; et al. The effect of neoadjuvant chemoradiation on pancreatic cancer-associated diabetes mellitus. Pancreas 2014, 43, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Ramachandran, V.; Javeed, N.; Arumugam, T.; Dutta, S.; Klee, G.G.; Klee, E.W.; Smyrk, T.C.; Bamlet, W.; Han, J.J.; et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology 2012, 143, 1510–1517.e1. [Google Scholar] [CrossRef]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Binang, H.B.; Perera, C.J.; Apte, M.V. Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes. Int. J. Mol. Sci. 2023, 24, 10203. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef]
- Sharma, S.; Tapper, W.J.; Collins, A.; Hamady, Z.Z.R. Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus. Gastroenterology 2022, 162, 1665–1674.e2. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 95) | NGT (n = 17) | preDM (n = 41) | NOD (n = 37) | p Value 1 | p Value 2 | p Value 3 |
|---|---|---|---|---|---|---|---|
| Sex (male) | 60 | 7 | 30 | 23 | 0.035 | 0.338 | 0.238 |
| Age (year) | 59.8 ± 11.6 | 59.3 ± 11.5 | 56.9 ± 13.3 | 63.2 ± 8.6 | 0.519 | 0.014 | 0.169 |
| BMI (kg/m2) | 22.17 ± 2.97 | 22.84 ± 2.99 | 21.82 ± 2.64 | 22.28 ± 3.35 | 0.220 | 0.498 | 0.587 |
| Smoking history | 36 | 4 | 17 | 15 | 0.241 | 0.255 | 0.061 |
| Drinking history | 34 | 6 | 15 | 13 | >0.999 | >0.999 | >0.999 |
| Tumor location | 0.765 | 0.815 | >0.999 | ||||
| Head and neck | 60 | 10 | 27 | 23 | |||
| Body and tail | 35 | 7 | 14 | 14 | |||
| Diameter of the main pancreatic duct | |||||||
| In the pancreatic neck (mm) | 4.7 ± 3.8 | 5.1 ± 4.3 | 4.1 ± 3.5 | 5.1 ± 3.9 | 0.388 | 0.267 | 0.998 |
| In the pancreatic tail (mm) | 3.0 ± 2.2 | 2.4 ± 1.3 | 2.8 ± 2.3 | 3.6 ± 2.4 | 0.458 | 0.146 | 0.016 |
| Tumor poorly differentiated | 33 | 7 | 11 | 15 | 0.354 | 0.235 | >0.999 |
| Tumor stage | 0.022 | 0.824 | 0.017 | ||||
| I~II | 51 | 14 | 20 | 17 | |||
| III~IV | 44 | 3 | 21 | 20 | |||
| CA19-9 (U/mL) | 385.8 ± 395.7 | 380.5 ± 397.3 | 361.5 ± 392.8 | 415.2 ± 407.0 | 0.868 | 0.555 | 0.771 |
| Total bilirubin (μmol/L) | 60.5 ± 95.2 | 52.5 ± 95.3 | 66.0 ± 96.3 | 57.9 ± 96.2 | 0.626 | 0.712 | 0.846 |
| Total bile acids (μmol/L) | 37.1 ± 76.5 | 9.3 ± 19.8 | 5.7 ± 5.2 | 5.7 ± 5.4 | 0.279 | 0.674 | 0.824 |
| Creatine (μmol/L) | 65.2 ± 13.3 | 62.7 ± 11.5 | 68.2 ± 14.6 | 62.9 ± 12.0 | 0.164 | 0.083 | 0.944 |
| eGFR (mL/min/1.73m2) | 96.8 ± 12.7 | 97.5 ± 10.8 | 97.4 ± 15.4 | 95.9 ± 10.3 | 0.970 | 0.622 | 0.602 |
| Variables | PDAC | |
|---|---|---|
| p Value | OR (95%CI) | |
| Sex (male) | 0.627 | 1.24 (0.52, 2.95) |
| Age (≥65 years) | 0.550 | 1.32 (0.53, 3.33) |
| BMI (<18.5, >23.9 kg/m2) | 0.042 | 2.54 (1.03, 6.23) |
| Remnant pancreas volume (cm3) | <0.001 | 0.95 (0.92, 0.97) |
| Tumor volume (cm3) | 0.231 | 1.03 (0.98, 1.09) |
| Tumor location (head and neck) | 0.895 | 1.07 (0.40, 2.83) |
| MPD dilation | 0.928 | 1.05 (0.39, 2.84) |
| Tumor well-differentiated | 0.804 | 0.89 (0.37, 2.17) |
| Tumor stage (IIB~IV) | 0.039 | 2.56 (1.05, 6.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Tan, C.; Liu, Y.; Zheng, Z.; Liu, X.; Chen, Y. Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer. Biomedicines 2024, 12, 1653. https://doi.org/10.3390/biomedicines12081653
Yang J, Tan C, Liu Y, Zheng Z, Liu X, Chen Y. Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer. Biomedicines. 2024; 12(8):1653. https://doi.org/10.3390/biomedicines12081653
Chicago/Turabian StyleYang, Jie, Chunlu Tan, Ya Liu, Zhenjiang Zheng, Xubao Liu, and Yonghua Chen. 2024. "Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer" Biomedicines 12, no. 8: 1653. https://doi.org/10.3390/biomedicines12081653
APA StyleYang, J., Tan, C., Liu, Y., Zheng, Z., Liu, X., & Chen, Y. (2024). Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer. Biomedicines, 12(8), 1653. https://doi.org/10.3390/biomedicines12081653






