Altered Brain Reactivity to Food Cues in Undergraduate Students with Disordered Eating Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Disordered Eating Behavior
2.3. Stimulus and Procedure
2.4. Electrophysiological Assessment
2.4.1. EEG Recording and Analysis
2.4.2. Principal Component Analysis
2.5. Statistical Analysis
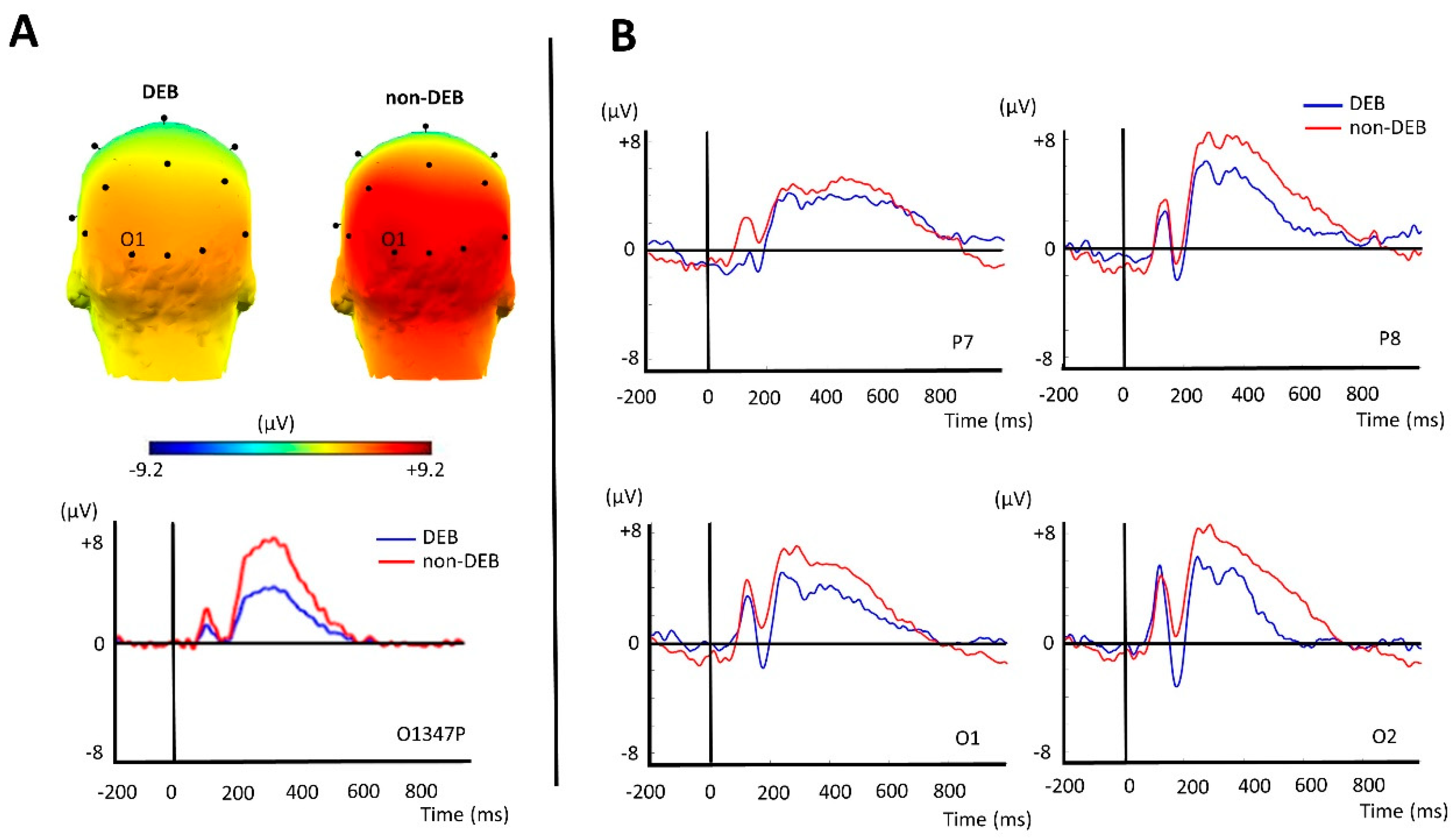
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chami, R.; Cardi, V.; Lautarescu, A.; Mallorquí-Bagué, N.; McLoughlin, G. Neural responses to food stimuli among individuals with eating and weight disorders: A systematic review of event-related potentials. Int. Rev. Psychiatry 2019, 31, 318–331. [Google Scholar] [CrossRef]
- Hiluy, J.C.; David, I.A.; Daquer, A.F.C.; Duchesne, M.; Volchan, E.; Appolinario, J.C. A Systematic Review of Electrophysiological Findings in Binge-Purge Eating Disorders: A Window Into Brain Dynamics. Front. Psychol. 2021, 12, 619780. [Google Scholar] [CrossRef] [PubMed]
- Blechert, J.; Feige, B.; Joos, A.; Zeeck, A.; Tuschen-Caffier, B. Electrocortical processing of food and emotional pictures in anorexia nervosa and bulimia nervosa. Psychosom. Med. 2011, 73, 415–421. [Google Scholar] [CrossRef]
- Quick, V.M.; Byrd-Bredbenner, C. Disturbed eating behaviours and associated psychographic characteristics of college students. J. Hum. Nutr. Diet. 2013, 26 (Suppl. 1), 53–63. [Google Scholar] [CrossRef]
- Carbine, K.A.; Rodeback, R.; Modersitzki, E.; Miner, M.; LeCheminant, J.D.; Larson, M.J. The utility of event-related potentials (ERPs) in understanding food-related cognition: A systematic review and recommendations. Appetite 2018, 128, 58–78. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique, 2nd ed.; The MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Kappenman, E.S.; Luck, S.J. (Eds.) The Oxford Handbook of Event-Related Potential Components; Oxford University Press: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Kessler, R.C.; Shahly, V.; Hudson, J.I.; Supina, D.; Berglund, P.A.; Chiu, W.T.; Gruber, M.; Aguilar-Gaxiola, S.; Alonso, J.; Andrade, L.H.; et al. A comparative analysis of role attainment and impairment in binge-eating disorder and bulimia nervosa: Results from the WHO World Mental Health Surveys. Epidemiol. Psychiatr. Sci. 2014, 23, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.P.; Appolinario, J.C.; Mattos, P.; Treasure, J.; Nazar, B.P. Eating disorder symptoms in Brazilian university students: A systematic review and meta-analysis. Braz. J. Psychiatry 2019, 41, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, A.J.; Yasuda, M.; Burant, C.F.; Gregor, N.; Khatri, S.; Sweet, M.; Falk, E.B. Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite 2012, 59, 738–747. [Google Scholar] [CrossRef]
- Stockburger, J.; Renner, B.; Weike, A.I.; Hamm, A.O.; Schupp, H.T. Vegetarianism and food perception. Selective visual attention to meat pictures. Appetite 2009, 52, 513–516. [Google Scholar] [CrossRef]
- Garner, D.M.; Garfinkel, P.E. The Eating Attitudes Test: An index of the symptoms of anorexia nervosa. Psychol. Med. 1979, 9, 273–279. [Google Scholar] [CrossRef]
- Bighetti, F.; dos Santos, C.B.; dos Santos, J.E.; Ribeiro, R.P.P. Tradução e validação do Eating Attitudes Test em adolescentes do sexo feminino de Ribeirão Preto, São Paulo. J. Bras. Psiquiatr. 2004, 53, 339–346. [Google Scholar]
- Freitas, S.; Gorenstein, C.; Appolinario, J.C. Instrumentos para a avaliação dos transtornos alimentares. Rev. Bras. Psiquiatr. 2002, 24 (Suppl. 3), 34–38. [Google Scholar] [CrossRef]
- Mintz, L.B.; O’Halloran, M.S. The Eating Attitudes Test: Validation With DSM-IV Eating Disorder Criteria. J. Pers. Assess. 2000, 74, 489–503. [Google Scholar] [CrossRef]
- da Silva Leal, G.V.; Philippi, S.T.; Polacow, V.O.; Cordás, T.A.; dos Santos Alvarenga, M. O que é comportamento de risco para transtornos alimentares em adolescentes? J. Bras. Psiquiatr. 2013, 62, 62–75. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The Eating Attitudes Test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Orbitello, B.; Ciano, R.; Corsaro, M.; Rocco, P.L.; Taboga, C.; Tonutti, L.; Balestrieri, M. The EAT-26 as screening instrument for clinical nutrition unit attenders. Int. J. Obes. 2006, 30, 977–981. [Google Scholar] [CrossRef]
- David, I.A.; Krutman, L.; Fernández-Santaella, M.C.; Andrade, J.R.; Andrade, E.B.; Oliveira, L.; Pereira, M.G.; Gomes, F.S.; Gleiser, S.; Oliveira, J.M.; et al. Appetitive drives for ultra-processed food products and the ability of text warnings to counteract consumption predispositions. Public Health Nutr. 2018, 21, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Increasing consumption of ultra-processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutr. 2010, 14, 5–13. [Google Scholar] [CrossRef]
- Hasnain, M.K.; Fox, P.T.; Woldorff, M.G. Intersubject variability of functional areas in the human visual cortex. Hum. Brain Mapp. 1998, 6, 301–315. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Dien, J.; Frishkoff, G.A. Principal Components Analysis of ERP Data. In Event-Related Potentials: A Methods Handbook; Handy, T.C., Ed.; MIT Press: Cambridge, MA, USA, 2005; pp. 189–207. [Google Scholar]
- Kujawa, A.; Weinberg, A.; Hajcak, G.; Klein, D.N. Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal-spatial PCA. Dev. Psychobiol. 2013, 55, 539–550. [Google Scholar] [CrossRef]
- Lobo, I.; David, I.A.; Figueira, I.; Campagnoli, R.R.; Volchan, E.; Pereira, M.G.; de Oliveira, L. Brain reactivity to unpleasant stimuli is associated with severity of posttraumatic stress symptoms. Biol. Psychol. 2014, 103, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Foti, D.; Hajcak, G.; Dien, J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology 2009, 46, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology 2010, 47, 170–183. [Google Scholar] [CrossRef]
- Dien, J.; Beal, D.J.; Berg, P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clin. Neurophysiol. 2005, 116, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Tenke, C.E. Optimizing PCA methodology for ERP component identification and measurement: Theoretical rationale and empirical evaluation. Clin. Neurophysiol. 2003, 114, 2307–2325. [Google Scholar] [CrossRef]
- Hajcak, G.; Weinberg, A.; MacNamara, A.; Foti, D. ERPs and the Study of Emotion; Oxford University Press: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Hajcak, G.; Foti, D. Significance?… Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology 2020, 57, e13570. [Google Scholar] [CrossRef]
- Blechert, J.; Goltsche, J.E.; Herbert, B.M.; Wilhelm, F.H. Eat your troubles away: Electrocortical and experiential correlates of food image processing are related to emotional eating style and emotional state. Biol. Psychol. 2014, 96, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Junghöfer, M.; Weike, A.I.; Hamm, A.O. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport 2003, 14, 1107–1110. [Google Scholar] [CrossRef]
- Schupp, H.T.; Markus, J.; Weike, A.I.; Hamm, A.O. Emotional Facilitation of Sensory Processing in the Visual Cortex. Psychol. Sci. 2003, 14, 7–13. [Google Scholar] [CrossRef]
- Flaisch, T.; Schupp, H.T. Tracing the time course of emotion perception: The impact of stimulus physics and semantics on gesture processing. Soc. Cogn. Affect Neurosci. 2013, 8, 820–827. [Google Scholar] [CrossRef]
- Maffei, A.; Goertzen, J.; Jaspers-Fayer, F.; Kleffner, K.; Sessa, P.; Liotti, M. Spatiotemporal Dynamics of Covert versus Overt Processing of Happy, Fearful and Sad Facial Expressions. Brain Sci. 2021, 11, 942. [Google Scholar] [CrossRef]
- Lemos, T.C.; Almo, A.; Campagnoli, R.R.; Pereira, M.G.; Oliveira, L.; Volchan, E.; Krutman, L.; Delgado, R.; Fernández-Santaella, M.C.; Khandpur, N.; et al. A red code triggers an unintended approach motivation toward sweet ultra-processed foods: Possible implications for front-of-pack labels. Food Qual. Prefer. 2020, 79, 103784. [Google Scholar] [CrossRef]
- Sawada, R.; Sato, W.; Uono, S.; Kochiyama, T.; Toichi, M. Electrophysiological correlates of the efficient detection of emotional facial expressions. Brain Res. 2014, 1560, 60–72. [Google Scholar] [CrossRef]
- Wolz, I.; Sauvaget, A.; Granero, R.; Mestre-Bach, G.; Bano, M.; Martin-Romera, V.; de las Heras, M.V.; Jiménez-Murcia, S.; Jansen, A.; Roefs, A.; et al. Subjective craving and event-related brain response to olfactory and visual chocolate cues in binge-eating and healthy individuals. Sci. Rep. 2017, 7, 41736. [Google Scholar] [CrossRef] [PubMed]
- Svaldi, J.; Tuschen-Caffier, B.; Peyk, P.; Blechert, J. Information processing of food pictures in binge eating disorder. Appetite 2010, 55, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rodríguez, R.; Hernández-Rivero, I.; Fernández-Santaella, M.C.; Vila, J.; Guerra, P.; Miccoli, L. Neural Processing of Food and Erotic Cues in Bulimia Nervosa. Psychosom. Med. 2019, 81, 527–535. [Google Scholar] [CrossRef]
- MacNamara, A.; Foti, D.; Hajcak, G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion 2009, 9, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.M.; Shah, R.; McCullough, M.L.; Gapstur, S.M.; Patel, A.V. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLoS ONE 2020, 15, e0231229. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.K.; Machizawa, M.G. Neural activity predicts individual differences in visual working memory capacity. Nature 2004, 428, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Meyer, A.; Kotov, R. Psychometrics and the neuroscience of individual differences: Internal consistency limits between-subjects effects. J. Abnorm. Psychol. 2017, 126, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Werle, D.; Schroeder, P.A.; Wolz, I.; Svaldi, J. Incentive sensitization in binge behaviors: A mini review on electrophysiological evidence. Addict. Behav. Rep. 2021, 13, 100344. [Google Scholar] [CrossRef]
- Bartholdy, S.; Musiat, P.; Campbell, I.C.; Schmidt, U. The Potential of Neurofeedback in the Treatment of Eating Disorders: A Review of the Literature. Eur. Eat. Disord. Rev. 2013, 21, 456–463. [Google Scholar] [CrossRef]

| EAT-26 Group | ||
|---|---|---|
| DEB | Non-DEB | |
| Sample size (n) | 11 | 15 |
| Age, mean (SD) | 22.73 (±2.80) | 21.14 (±5.75) |
| Sex (% female) | 72.7% | 73.3% |
| Mean BMI (kg/m2) | 21.83 (±2.43) | 21.88 (±4.85) |
| PCA Factors | Peak Amplitude (µV) (Mean (SD)) | Test | t or U | p-Value | Effect Size | |
|---|---|---|---|---|---|---|
| DEB (n = 11) | Non-DEB (n = 15) | |||||
| 347O1P | 3.94 (3.31) | 7.39 (4.58) | Student | 2.12 | 0.04 | 0.84 |
| 347C4N | −0.98 (1.91) | −0.38 (2.50) | Student | 0.66 | 0.52 | - |
| 622O2P | −0.371 (2.68) | 2.23 (3.51) | Mann–Whitney | 112 | 0.13 | - |
| 250O2N | −0.10 (3.98) | −0.13 (2.58) | Student | −0.022 | 0.98 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiluy, J.C.; David, I.A.; Lobo, I.; Braga, F.; Fernandes, T.; Ferreira, N.B.; Mauro, M.F.F.P.; Appolinario, J.C. Altered Brain Reactivity to Food Cues in Undergraduate Students with Disordered Eating Behaviors. Biomedicines 2024, 12, 1656. https://doi.org/10.3390/biomedicines12081656
Hiluy JC, David IA, Lobo I, Braga F, Fernandes T, Ferreira NB, Mauro MFFP, Appolinario JC. Altered Brain Reactivity to Food Cues in Undergraduate Students with Disordered Eating Behaviors. Biomedicines. 2024; 12(8):1656. https://doi.org/10.3390/biomedicines12081656
Chicago/Turabian StyleHiluy, Joao C., Isabel A. David, Isabela Lobo, Filipe Braga, Thayane Fernandes, Naiane Beatriz Ferreira, Maria Francisca F. P. Mauro, and Jose C. Appolinario. 2024. "Altered Brain Reactivity to Food Cues in Undergraduate Students with Disordered Eating Behaviors" Biomedicines 12, no. 8: 1656. https://doi.org/10.3390/biomedicines12081656






