An Observational Longitudinal Study on Seasonal Variations in Tourette Syndrome: Evidence for a Role of Ambient Temperature in Tic Exacerbation
Abstract
:1. Introduction
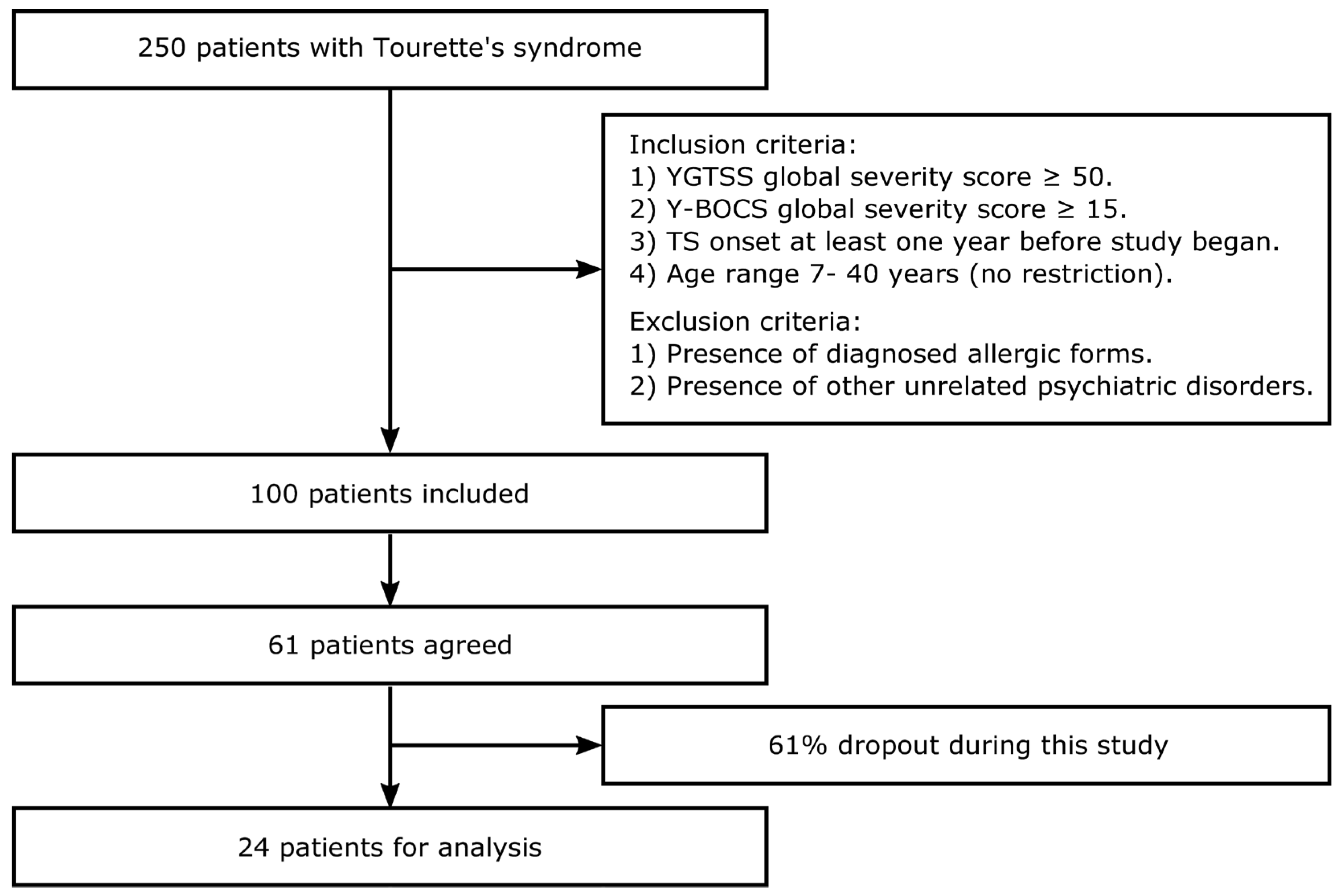
2. Materials and Methods
2.1. Subjects
2.2. Ethical Statement
2.3. Statistical Analysis
3. Results
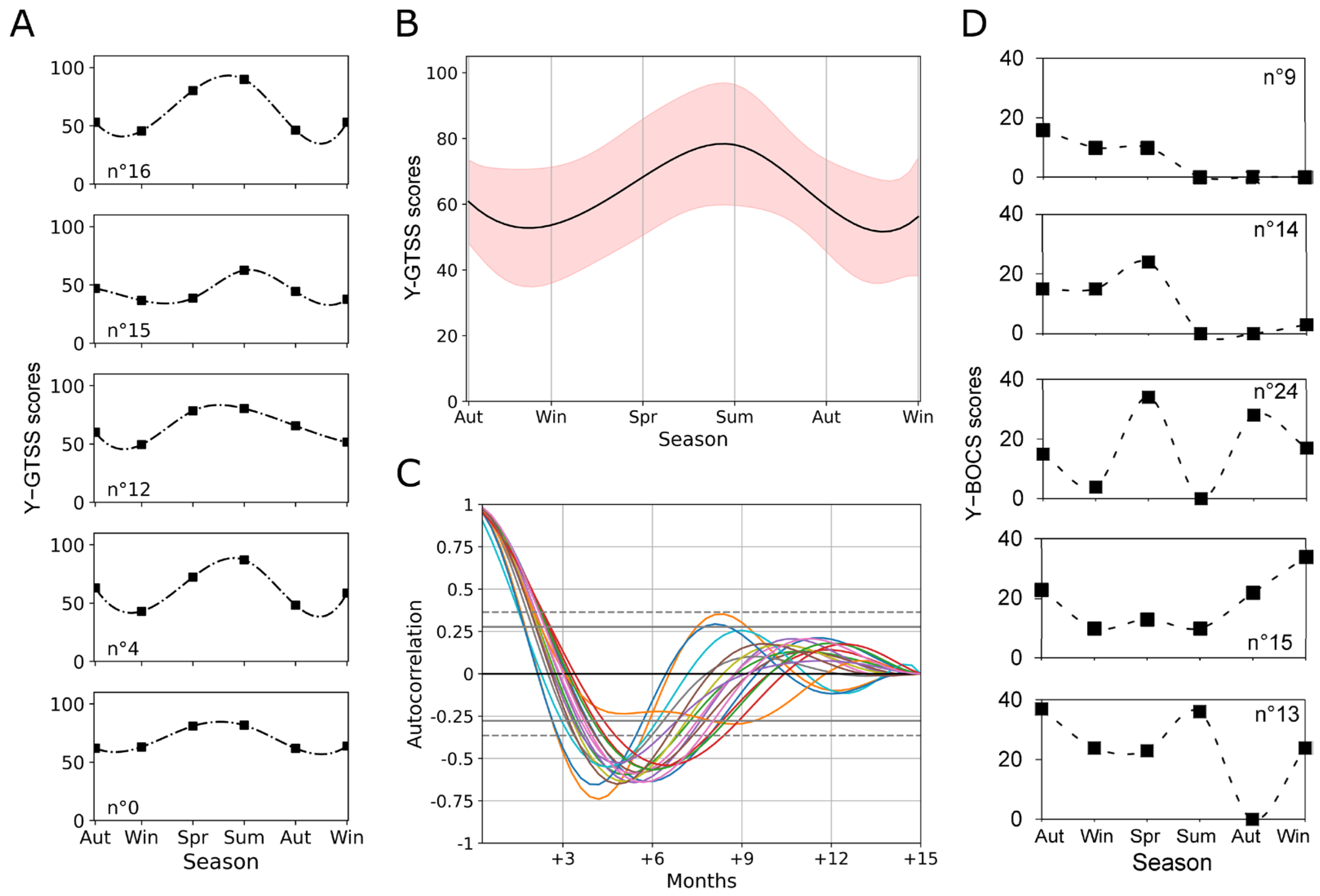
3.1. Progression of Tic and OCD Symptomatology along Seasons in Patients with Obsessive–Compulsive Tic Disorder
3.2. Effects of Environmental Variables on Seasonal Changes in Tic and OCD Symptomatology
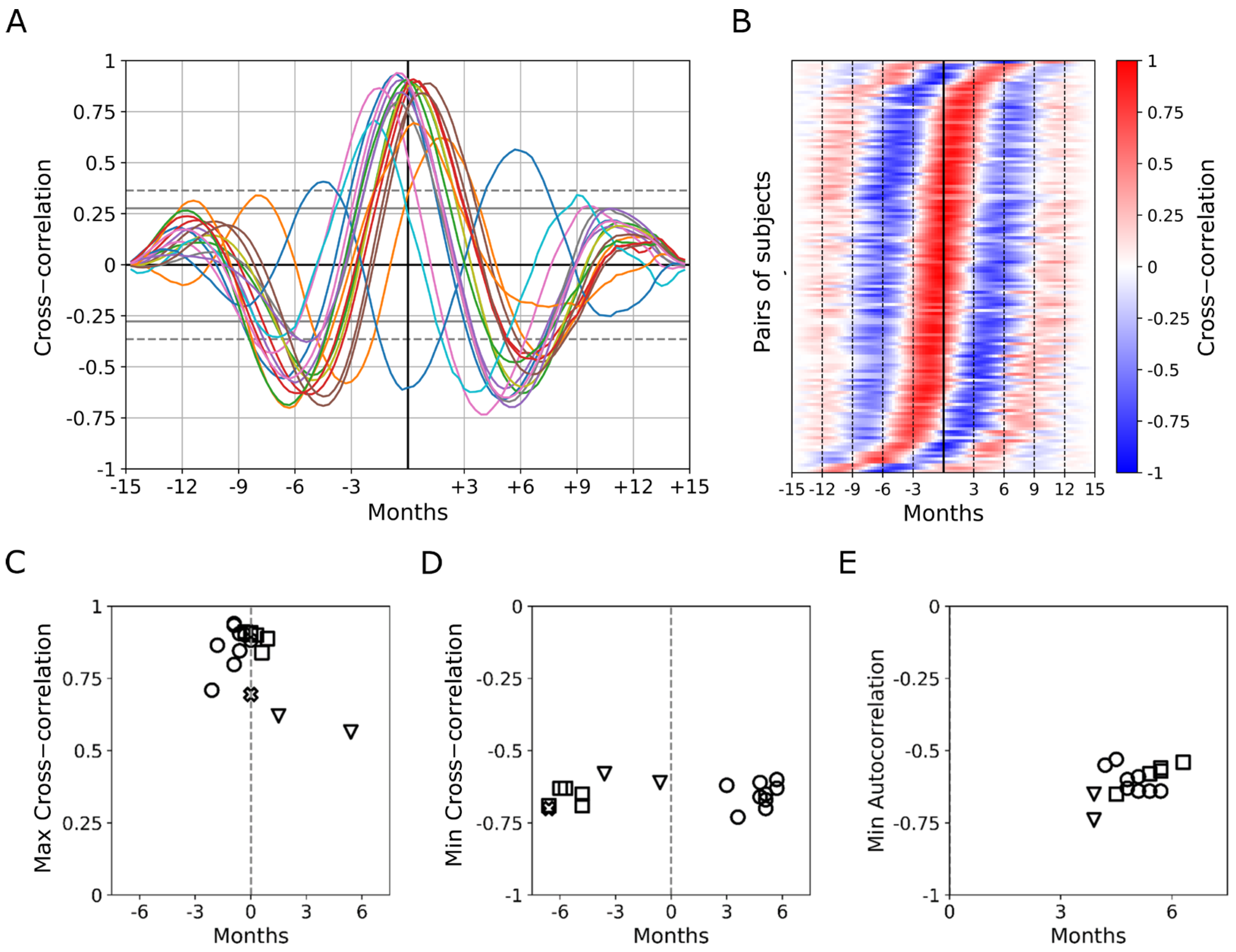
3.3. Seasonal Periodicity in Y-GTSS Scores as Assessed by Autocorrelation and Cross-Correlation Analysis
3.4. Different Patterns in Seasonal Periodicity Emerge in Tic and OCD Symptomatology
4. Discussion
Limitations and Future Developments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the Dynamics of Infectious Diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Kaasinen, V.; Jokinen, P.; Joutsa, J.; Eskola, O.; Rinne, J.O. Seasonality of Striatal Dopamine Synthesis Capacity in Parkinson’s Disease. Neurosci. Lett. 2012, 530, 80–84. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; Volume 280. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Marazziti, D.; Albert, U.; Pallanti, S.; Gambini, O.; Tundo, A.; Zanaboni, C.; Servello, D.; Rizzo, R.; Scalone, L.; et al. Parsing the Phenotype of Obsessive-Compulsive Tic Disorder (OCTD): A Multidisciplinary Consensus. Int. J. Psychiatry Clin. Pract. 2017, 21, 156–159. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, R.; Van Dorsselaer, S.; Ten Have, M.; Schoemaker, C.; Vollebergh, W.A.M. Seasonal Variations in Mental Disorders in the General Population of a Country with a Maritime Climate: Findings from the Netherlands Mental Health Survey and Incidence Study. Am. J. Epidemiol. 2005, 162, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Bloch, M.H.; Scahill, L.; King, R.A. Tourette Syndrome: The Self under Siege. J. Child Neurol. 2006, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Malgaroli, A.; Cerutti, S.; Signorini, M.G. Detection of Fractal Behavior in Temporal Series of Synaptic Quantal Release Events: A Feasibility Study. Comput. Intell. Neurosci. 2012, 2012, 704673. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Signorini, M.G.; Cerutti, S.; Malgaroli, A. A Pre-Docking Source for the Power-Law Behavior of Spontaneous Quantal Release: Application to the Analysis of LTP. Front. Cell. Neurosci. 2015, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Briguglio, M.; Galentino, R.; Dell’Osso, B.; Malgaroli, A.; Banfi, G.; Porta, M. Exploring Circannual Rhythms and Chronotype Effect in Patients with Obsessive-Compulsive Tic Disorder (OCTD): A Pilot Study. J. Affect. Disord. 2020, 262, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kircanski, K.; Woods, D.W.; Chang, S.W.; Ricketts, E.J.; Piacentini, J.C. Cluster Analysis of the Yale Global Tic Severity Scale (YGTSS): Symptom Dimensions and Clinical Correlates in an Outpatient Youth Sample. J. Abnorm. Child Psychol. 2010, 38, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Maust, D.; Cristancho, M.; Gray, L.; Rushing, S.; Tjoa, C.; Thase, M.E. Psychiatric Rating Scales, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 106. [Google Scholar]
- Green, J.A. Too Many Zeros and/or Highly Skewed? A Tutorial on Modelling Health Behaviour as Count Data with Poisson and Negative Binomial Regression. Health Psychol. Behav. Med. 2021, 9, 436–455. [Google Scholar] [CrossRef] [PubMed]
- Bhikram, T.; Arnold, P.; Crawley, A.; Abi-Jaoude, E.; Sandor, P. The Functional Connectivity Profile of Tics and Obsessive-Compulsive Symptoms in Tourette Syndrome. J. Psychiatr. Res. 2020, 123, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Ferro, M.; Spadini, S.; Racchetti, G.; Malgaroli, A. The Dysfunctional Mechanisms Throwing Tics: Structural and Functional Changes in Tourette Syndrome. Behav. Sci. 2023, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Grados, M.; Huselid, R.; Duque-Serrano, L. Transcranial Magnetic Stimulation in Tourette Syndrome: A Historical Perspective, Its Current Use and the Influence of Comorbidities in Treatment Response. Brain Sci. 2018, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lan, Y.; Zhu, H.; Yu, L.; Wu, S.; Wan, W.; Shu, Y.; Xiang, H.; Hou, T.; Zhang, H.; et al. Effects of Chemogenetic Inhibition of D1 or D2 Receptor-Containing Neurons of the Substantia Nigra and Striatum in Mice with Tourette Syndrome. Front. Mol. Neurosci. 2021, 14, 779436. [Google Scholar] [CrossRef] [PubMed]
- Lombroso, P.J.; Mack, G.; Scahill, L.; King, R.A.; Leckman, J.F. Exacerbation of Gilles de La Tourette’s Syndrome Associated with Thermal Stress. Neurology 1991, 41, 1984. [Google Scholar] [CrossRef] [PubMed]
- Scahill, L.; Lombroso, P.J.; Mack, G.; Van Wattum, P.J.; Zhang, H.; Vitale, A.; Leckman, J.F. Thermal Sensitivity in Tourette Syndrome: Preliminary Report. Percept. Mot. Ski. 2001, 92, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.R. Tourette Syndrome Associated with Body Temperature Dysregulation: Possible Involvement of an Idiopathic Hypothalamic Disorder. J. Child Neurol. 2002, 17, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Bundo, M.; de Schrijver, E.; Federspiel, A.; Toreti, A.; Xoplaki, E.; Luterbacher, J.; Franco, O.H.; Müller, T.; Vicedo-Cabrera, A.M. Ambient Temperature and Mental Health Hospitalizations in Bern, Switzerland: A 45-Year Time-Series Study. PLoS ONE 2021, 16, e0258302. [Google Scholar] [CrossRef] [PubMed]
- Tansey, E.A.; Johnson, C.D. Recent Advances in Thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, P.; Landgrebe, M.; Wittmann, M.; Schecklmann, M.; Poeppl, T.B.; Hajak, G.; Langguth, B. Hypothermia Associated with Antipsychotic Drug Use: A Clinical Case Series and Review of Current Literature. J. Clin. Pharmacol. 2012, 52, 1090–1097. [Google Scholar] [CrossRef]
- Micieli, G.; Tosi, P.; Marcheselli, S.; Cavallini, A. Autonomic Dysfunction in Parkinson’s Disease. Neurol. Sci. 2003, 24, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.F.; Mora, F.; Myers, R.D. Dopamine and Thermoregulation: An Evaluation with Special Reference to Dopaminergic Pathways. Neurosci. Biobehav. Rev. 1985, 9, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T. Effects of Dopaminergic Antagonist and Agonist on Thermoregulation in Rabbits. J. Physiol. 1979, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Hu, S.; Lu, M.; Liu, R.; Li, B.; Shi, B.; Liu, G. Association between Thermal Response and Endogenous Dopamine: Step-Change Environments in Winter. J. Therm. Biol. 2023, 112, 103447. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.P.; Kohn, P.D.; Baller, E.B.; Bronstein, J.A.; Masdeu, J.C.; Berman, K.F. Seasonal Effects on Human Striatal Presynaptic Dopamine Synthesis. J. Neurosci. 2010, 30, 14691–14694. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Isotti, F.; Ferro, M.; Racchetti, G.; Anchora, L.; Rucco, D.; Malgaroli, A. Facilitation of Dopamine-Dependent Long-Term Potentiation in the Medial Prefrontal Cortex of Male Rats Follows the Behavioral Effects of Stress. J. Neurosci. Res. 2021, 99, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Isotti, F.; Ferro, M.; Spadini, S.; Racchetti, G.; Musazzi, L.; Malgaroli, A. Occlusion of Dopamine-Dependent Synaptic Plasticity in the Prefrontal Cortex Mediates the Expression of Depressive-like Behavior and Is Modulated by Ketamine. Sci. Rep. 2022, 12, 11055. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Bansal, R.; Hao, X.; Sanchez-Pena, J.P.; Sobel, L.J.; Liu, J.; Xu, D.; Zhu, H.; Mallar Chakravarty, M.; Durkin, K.; et al. Enlargement of Thalamic Nuclei in Tourette Syndrome. Arch. Gen. Psychiatry 2010, 67, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Montesano, G.; Belfiore, M.; Ripamonti, M.; Arena, A.; Lamanna, J.; Ferro, M.; Zimarino, V.; Ambrosi, A.; Malgaroli, A. Effects of the Concomitant Activation of ON and OFF Retinal Ganglion Cells on the Visual Thalamus: Evidence for an Enhanced Recruitment of GABAergic Cells. Front. Neural Circuits 2015, 9, 00077. [Google Scholar] [CrossRef]
- Norsted, E.; Gömüç, B.; Meister, B. Protein Components of the Blood–Brain Barrier (BBB) in the Mediobasal Hypothalamus. J. Chem. Neuroanat. 2008, 36, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Zis, P.; Buttiglione, M. The Role of Immune Mechanisms in Tourette Syndrome. Brain Res. 2015, 1617, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Eapen, V.; Singer, H.S.; Martino, D.; Scharf, J.M.; Paschou, P.; Roessner, V.; Woods, D.W.; Hariz, M.; Mathews, C.A.; et al. Gilles de La Tourette Syndrome. Nat. Rev. Dis. Prim. 2017, 3, 16097. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, R.J.; Fahey, S.; Frick, L.; Leckman, J.; Vaccarino, F.; Duman, R.S.; Williams, K.; Swedo, S.; Pittenger, C. Antibodies from Children with PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am. J. Psychiatry 2021, 178, 48–64. [Google Scholar] [CrossRef] [PubMed]


| Sociodemographic Data | |||
|---|---|---|---|
| N | 24 | ||
| Sex (male–female) | 20:4 | ||
| Age (mean ± SD) | 20.58 ± 9.18 | ||
| Y-GTSS | Y-BOCS | Social Impairment Y-GTSS | |
| Autumn 2017 | 59.96 ± 12.85 | 10.75 ± 11.04 | 33.33 ± 7.61 |
| Winter 2018 | 49.83 ± 15.44 | 8.21 ± 8.60 | 28.54 ± 8.90 |
| Spring 2018 | 63.58 ± 16.92 | 8.53 ± 12.06 | 33.25 ± 12.95 |
| Summer 2018 | 67.89 ± 17.08 | 6.71 ± 11.41 | 36.40 ± 11.78 |
| Autumn 2018 | 43.64 ± 17.19 | 6.15 ± 11.41 | 2.11 ± 13.18 |
| Winter 2019 | 37.67 ± 16.52 | 8.95 ± 11.62 | 18.14 ± 13.40 |
| Significant p-Values | Autumn 17 | Winter 18 | Spring 18 | Summer 18 | Autumn 18 | Winter 19 |
|---|---|---|---|---|---|---|
| Autumn 17 | 0.011 | <0.001 | ||||
| Winter 18 | 0.005 | |||||
| Spring 18 | 0.002 | <0.001 | ||||
| Summer 18 | 0.005 | <0.001 | <0.001 | |||
| Autumn 18 | 0.011 | 0.002 | <0.001 | |||
| Winter 19 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamanna, J.; Mazzoleni, R.; Farina, R.; Ferro, M.; Galentino, R.; Porta, M.; Malgaroli, A. An Observational Longitudinal Study on Seasonal Variations in Tourette Syndrome: Evidence for a Role of Ambient Temperature in Tic Exacerbation. Biomedicines 2024, 12, 1668. https://doi.org/10.3390/biomedicines12081668
Lamanna J, Mazzoleni R, Farina R, Ferro M, Galentino R, Porta M, Malgaroli A. An Observational Longitudinal Study on Seasonal Variations in Tourette Syndrome: Evidence for a Role of Ambient Temperature in Tic Exacerbation. Biomedicines. 2024; 12(8):1668. https://doi.org/10.3390/biomedicines12081668
Chicago/Turabian StyleLamanna, Jacopo, Riccardo Mazzoleni, Ramona Farina, Mattia Ferro, Roberta Galentino, Mauro Porta, and Antonio Malgaroli. 2024. "An Observational Longitudinal Study on Seasonal Variations in Tourette Syndrome: Evidence for a Role of Ambient Temperature in Tic Exacerbation" Biomedicines 12, no. 8: 1668. https://doi.org/10.3390/biomedicines12081668






