Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence
Abstract
:1. Introduction
2. Clinical Evaluation and Laboratory Evaluation of Patients with Thyroid Nodules
- Patients younger than 20 or older than 70 years of age;
- Male patients;
- Signs of dysphagia or dysphonia;
- History of neck irradiation;
- Previous thyroid carcinoma in the same patient;
- A firm, hard, or immobile nodule upon palpation;
- Cervical lymphadenopathy upon palpation.
- Family history of autoimmune disease (e.g., Hashimoto’s thyroiditis);
- Family history of benign thyroid nodule or goiter;
- Thyroid hormonal dysfunction (hypothyroidism or hyperthyroidism);
- Nodule that provokes pain or tenderness;
- A soft, smooth, and mobile nodule upon palpation [12].
3. Imaging
3.1. Ultrasound
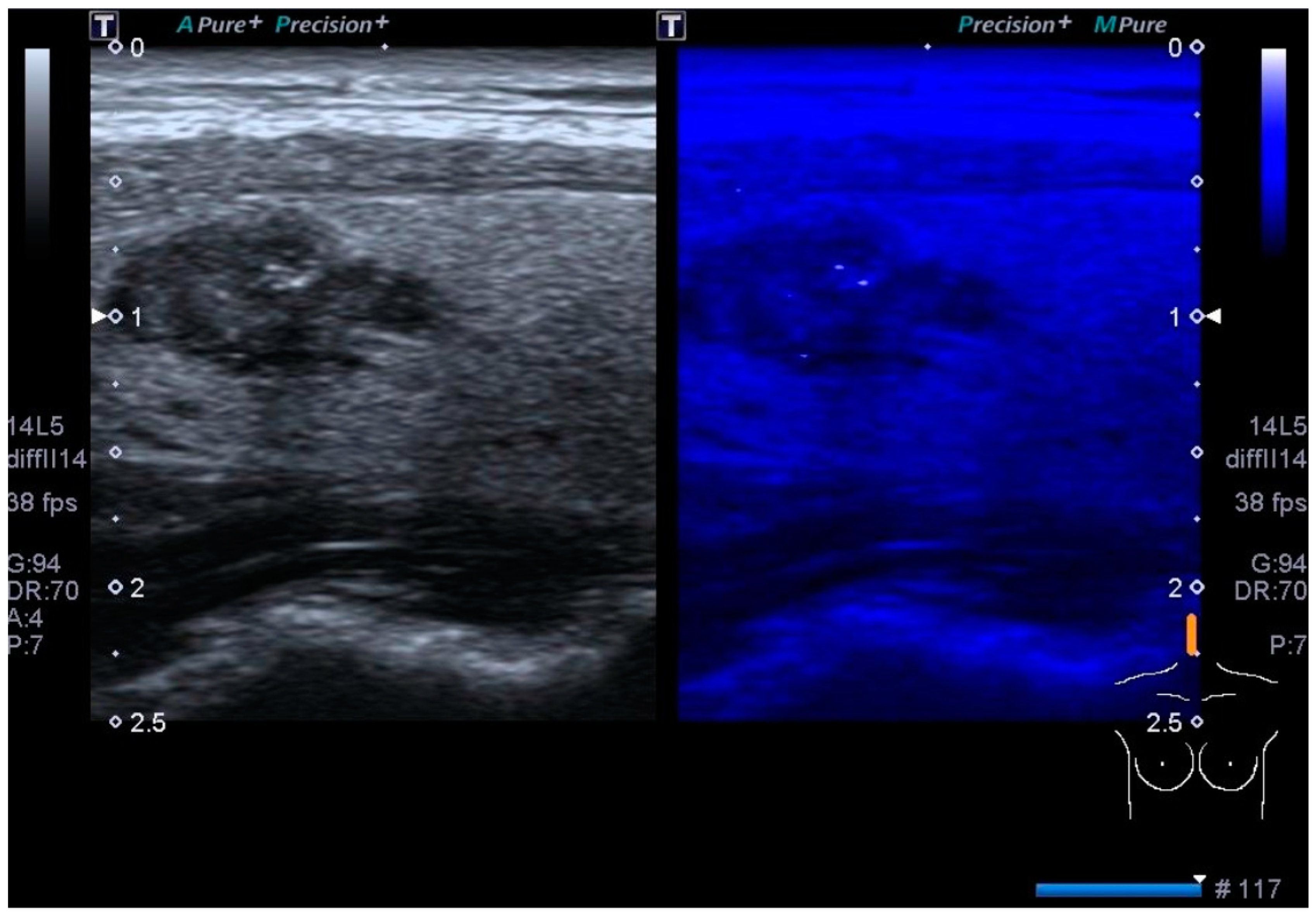
3.2. Color Doppler US
3.3. Contrast-Enhanced US
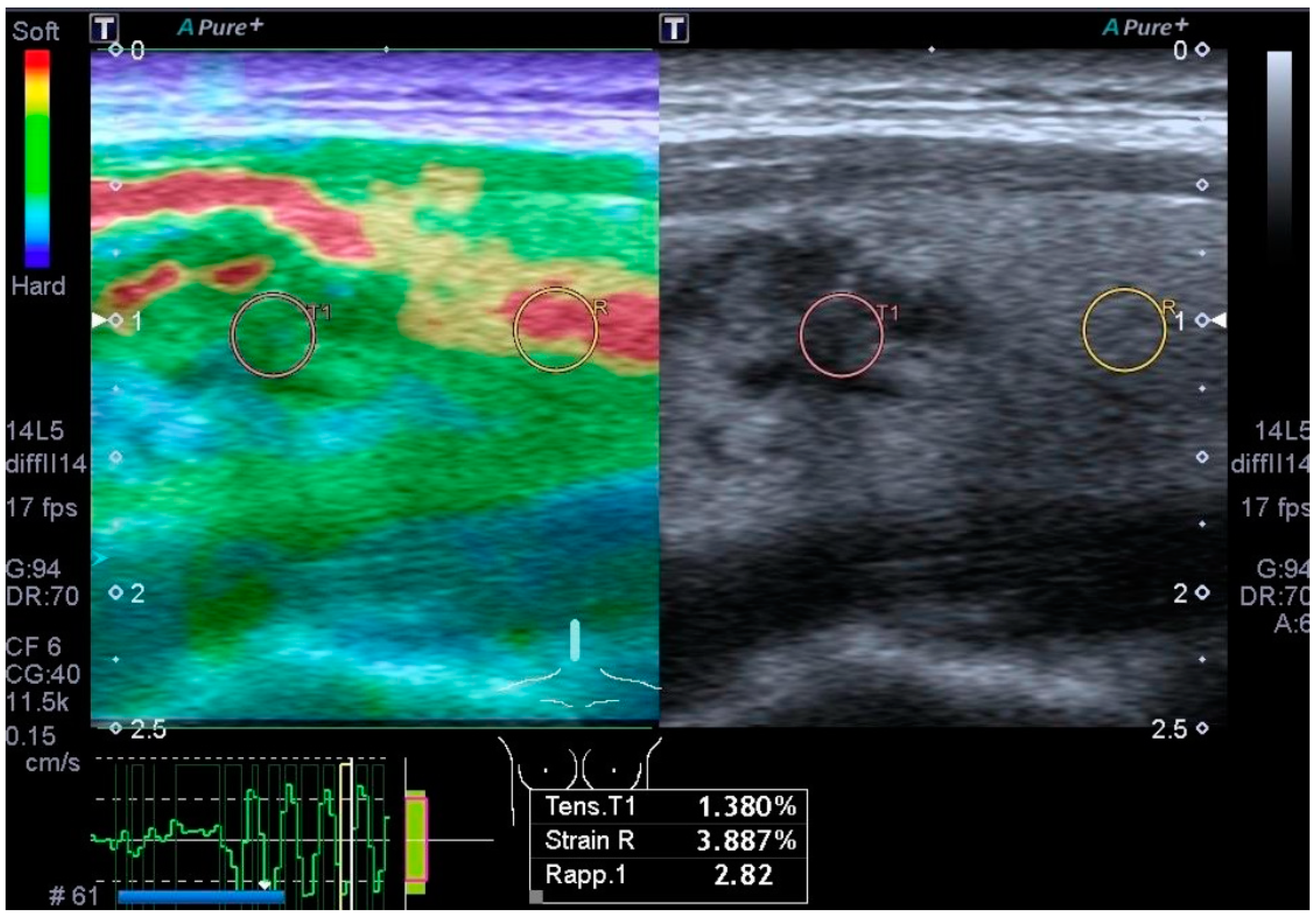
3.4. Elastography
4. US Classification Systems
4.1. The Thyroid Imaging Reporting and Data System of the American College of Radiology (ACR TI-RADS)
- Composition: cystic or completely cystic, 0 points; spongiform, 0 points; mixed cystic and solid, 1 point; solid or almost completely solid, 2 points.
- Echogenicity: anechoic, 0 points; hyper- or isoechoic, 1 point; hypoechoic, 2 points; very hypoechoic, 3 points.
- Shape (assessed on the transverse plane): wider than tall, 0 points; taller than wide, 3 points.
- Margins: smooth, 0 points; ill-defined, 0 points; lobulated or irregular, 2 points; extrathyroidal extension, 3 points.
- Echogenic foci: none, 0 points; large comet tail artifacts, 0 points; macrocalcification, 1 point; peripheral or rim calcifications, 2 points; punctate echogenic foci, 3 points [9].
- TR1: no FNA;
- TR2: no FNA;
- TR3: if ≥1.5 cm, follow-up; if ≥2.5 cm, FNA; follow-up US at 1, 3, and 5 years;
- TR4: if ≥1.0 cm, follow-up; if ≥1.5 cm, FNA; follow-up US at 1, 2, 3, and 5 years;
- TR5: if ≥0.5 cm, follow-up; if ≥1.0 cm, FNA; US follow-up every year for the next 5 years.
4.2. American Thyroid Association (ATA) Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer
- Benign US pattern (0% risk of malignancy): no FNAB required;
- Extremely low-suspicion US pattern (<3% risk of malignancy): FNAB if ≥2 cm (or US follow-up);
- Low-suspicion pattern (5–10% risk of malignancy): FNAB if ≥1.5 cm;
- Intermediate-suspicion pattern (10–20% risk of malignancy): FNAB if ≥1 cm;
- High-suspicion pattern (>70–90% risk of malignancy): FNAB if ≥1 cm.
- Benign pattern (0% risk of malignancy): completely cystic nodules with fine walls;
- Extremely low-suspicion pattern (<3% risk of malignancy): spongiform nodules and nodules with interspersed cystic spaces and no features of the higher-level patterns;
- Low-suspicion pattern (5–10% risk of malignancy): isoechoic or hyperechoic nodules, or partially cystic nodules with a peripheral solid component, and no features of the higher-level patterns;
- Intermediate-suspicion pattern (10–20% risk of malignancy): hypoechoic solid nodules with smooth margins and no features of the higher-level patterns;
- High-suspicion pattern (>70–90% risk): solid hypoechoic nodules or solid hypoechoic components of partially cystic nodules, with at least one of these features: microcalcifications, irregular margins (infiltrative, microlobulated), extrathyroidal extension, a taller-than-wide shape, rim calcifications with an extrusive soft tissue component, or lymphadenopathy.
4.3. Korean Society of Thyroid Radiology (KSThR): Thyroid Imaging, Reporting and Data System (K-TIRADS)
- K-TIRADS 1: No nodule.
- K-TIRADS 2: Benign category:
- Iso-/hyperechoic spongiform;
- Partially cystic nodule with intracystic echogenic foci and comet tail artifact;
- Pure cyst malignancy risk is <3%. No biopsy indicated.
- K-TIRADS 3: Low-suspicion category: Partially cystic or iso-/hyperechoic nodule without any of the three suspicious US features. Malignancy risk is 3–10%. Biopsy indicated at >2 cm.
- K-TIRADS 4: Intermediate suspicion:
- Solid hypoechoic nodules with no other suspicious US pattern;
- Partially cystic or iso-/hyperechoic nodule with any of the three suspicious US characteristics;
- Completely calcified nodule malignancy risk is 10–40%. Biopsy indicated at >1 or 1.5 cm.
- K-TIRADS 5: High suspicion: Solid hypoechoic nodule with any of the three suspicious US features (punctate echogenic foci, nonparallel orientation, and irregular margins). Malignancy risk is >60%. Biopsy indicated at >1 cm.
4.4. European Thyroid Association TIRADS (EU-TIRADS)
5. Secondary Imaging Methods
5.1. Thyroid Scintigraphy: Nuclear Imaging Study of the Thyroid
5.2. Thyroid Computed Tomography (CT)
6. FNAB
Post-FNAB Management of Thyroid Nodules
7. Advancements in Cytologic Analysis
Molecular Testing
8. Artificial Intelligence (AI) Systems
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FNAB | Fine-needle aspiration biopsy |
| TSH | Thyroid-stimulating hormone |
| Incidentalomas | Nodules incidentally found on high-resolution ultrasonography |
| PTC | Papillary thyroid carcinoma |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| anti-TPO | Antibody antithyroid peroxidase |
| anti-Tg | Antibody antithyroglobulin |
| US | Ultrasound |
| CDUS | Color Doppler US |
| CEUS | Contrast-enhanced US |
| ACR | American College of Radiology |
| TI-RADS | Thyroid Imaging Reporting and Data System |
| K-TIRADS | Korean Society of Thyroid Radiology Thyroid Imaging, Reporting and Data System |
| EU-TIRADS | European Thyroid Association TIRADS |
| ATA | American Thyroid Association |
| FNA | Fine-needle aspiration |
| CT | Computed tomography |
| AI | Artificial intelligence |
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Gharib, H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann. Intern. Med. 1997, 126, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Vander, J.B.; Gaston, E.A.; Dawber, T.R. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann. Intern. Med. 1968, 69, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, W.M.; Evered, D.C.; Hall, R.; Appleton, D.; Brewis, M.; Clark, F.; Evans, J.G.; Young, E.; Bird, T.; Smith, P.A. The spectrum of thyroid disease in a community: The Whickham survey. Clin. Endocrinol. 1977, 7, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, J.J.; Drake, T.M.; Uttley, L.; Balasubramanian, S.P. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid 2016, 26, 1541–1552. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Dankle, S.K.; Talavera, F.; Schalch, D.S.; Griffing, G.T.; Gambert, S.R. Thyroid Nodule. Available online: https://emedicine.medscape.com/article/127491-overview (accessed on 25 April 2024).
- Yildirim Simsir, I.; Cetinkalp, S.; Kabalak, T. Review of Factors Contributing to Nodular Goiter and Thyroid Carcinoma. Med. Princ. Pract. 2020, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Reiners, C. The association between multinodular goiter and thyroid cancer. Minerva Endocrinol. 2010, 35, 187–192. [Google Scholar]
- Hong, Y.J.; Son, E.J.; Kim, E.-K.; Kwak, J.Y.; Hong, S.W.; Chang, H.-S. Positive predictive values of sonographic features of solid thyroid nodule. Clin. Imaging 2010, 34, 127–133. [Google Scholar] [CrossRef]
- Moon, W.J.; Jung, S.L.; Lee, J.H.; Na, D.G.; Baek, J.H.; Lee, Y.H.; Kim, J.; Kim, H.S.; Byun, J.S.; Lee, D.H.; et al. Benign and malignant thyroid nodules: US differentiation—Multicenter retrospective study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Atkinson, H.; Jones, J. Assessment of Thyroid Lesions (Ultrasound). Reference Article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/35024 (accessed on 25 April 2024).
- Hoang, J.; Lee, W.; Lee, M.; Johnson, D.; Farrell, S. US Features of Thyroid Malignancy: Pearls and Pitfalls. Radiographics 2007, 27, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Ciledag, N.; Arda, K.; Aribas, B.K.; Aktas, E.; Köse, S.K. The utility of ultrasound elastography and MicroPure imaging in the differentiation of benign and malignant thyroid nodules. AJR Am. J. Roentgenol. 2012, 198, W244–W249. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.H.; Kim, S.Y.; Jeon, W.K.; Kang, J.H.; An, S.K.; Jun, W.S. Radiologic and Pathologic Findings of Nonpalpable Thyroid Carcinomas Detected by Ultrasonography in a Medical Screening Center. J. Ultrasound Med. 2008, 27, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, J.; Smith, A. Parathyroid Adenoma. Head Neck Pathol. 2008, 2, 305–308. [Google Scholar] [CrossRef]
- Reading, C.; Charboneau, J.; Hay, I.; Sebo, T. Sonography of Thyroid Nodules: A “Classic Pattern” Diagnostic Approach. Ultrasound Q. 2005, 21, 157–165. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frates, M.C.; Benson, C.B.; Doubilet, P.M.; Cibas, E.S.; Marqusee, E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J. Ultrasound Med. 2003, 22, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kwak, J.Y.; Kim, M.J.; Son, E.J.; Kim, E.K. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology 2010, 255, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, E.; Briganti, S.I.; Crescenzi, A.; Beretta Anguissola, G.; Perrella, E.; Taffon, C.; Palermo, A.; Manfrini, S.; Pozzilli, P.; Lauria Pantano, A. Usefulness of Color Doppler Ultrasonography in the Risk Stratification of Thyroid Nodules. Eur. Thyroid. J. 2021, 10, 339–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantisani, V.; David, E.; Ferrari, D.; Fanelli, F.; Di Marzo, L.; Catalano, C.; Benedetto, F.; Spinelli, D.; Katsargyris, A.; Blandino, A.; et al. Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. Ann. Vasc. Surg. 2017, 40, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Dolcetti, V.; Fresilli, D.; Del Gaudio, G.; Pacini, P.; Huang, P.; Camponovo, C.; Leoncini, A.; D’Andrea, V.; Pironi, D.; et al. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J. Clin. Med. 2021, 10, 4559. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Elfeky, M.; Iqbal, S. Strain Elastography. Reference Article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/32099 (accessed on 25 April 2024).
- Cantisani, V.; Grazhdani, H.; Drakonaki, E.; D’Andrea, V.; Di Segni, M.; Kaleshi, E.; Calliada, F.; Catalano, C.; Redler, A.; Brunese, L.; et al. Strain US Elastography for the Characterization of Thyroid Nodules: Advantages and Limitation. Int. J. Endocrinol. 2015, 2015, 908575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, M.; Weerakkody, Y.; Goel, A. Shear Wave Elastography. Reference Article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/32100 (accessed on 25 April 2024).
- Cantisani, V.; David, E.; Grazhdani, H.; Rubini, A.; Radzina, M.; Dietrich, C.F.; Durante, C.; Lamartina, L.; Grani, G.; Valeria, A.; et al. Prospective Evaluation of Semiquantitative Strain Ratio and Quantitative 2D Ultrasound Shear Wave Elastography (SWE) in Association with TIRADS Classification for Thyroid Nodule Characterization. Ultraschall Med. 2019, 40, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Dighe, M.K. Elastography of Thyroid Masses. Ultrasound Clin. 2014, 9, 13–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swan, K.Z.; Nielsen, V.E.; Bonnema, S.J. Evaluation of thyroid nodules by shear wave elastography: A review of current knowledge. J. Endocrinol. Investig. 2021, 44, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.H.; Mansor, M.; Sheriba, N.; Assem, M.; Abdelfattah, Y.; Ashoush, O.A.; Rakha, M.; Abdelfattah, D.; El-Sawy, S.S.; Elshenoufy, M.; et al. Role of elastography strain ratio and TIRADS score in predicting malignant thyroid nodule. Arch. Endocrinol. Metab. 2021, 64, 735–742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tappouni, R.R.; Itri, J.N.; McQueen, T.S.; Lalwani, N.; Ou, J.J. ACR TI-RADS: Pitfalls, Solutions, and Future Directions. Radiographics 2019, 39, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Middleton, W.D.; Farjat, A.E.; Langer, J.E.; Reading, C.C.; Teefey, S.A.; Abinanti, N.; Boschini, F.J.; Bronner, A.J.; Dahiya, N.; et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 2018, 287, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Eidt, L.B.; Nunes de Oliveira, C.; Lagos, Y.B.B.; Solera, G.L.M.; Izquierdo, R.; Meyer, E.L.S.; Mattevi, V.S.; Golbert, L. A prospective comparison of ACR-TIRADS and EU-TIRADS in thyroid nodule assessment for FNA-US. Clin. Endocrinol. 2023, 98, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Marukatat, N.; Parklug, P.; Chanasriyotin, C. Comparison of the diagnostic accuracy of K-TIRADS and EU-TIRADS guidelines for detection of thyroid malignancy on ultrasound. Radiography 2023, 29, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Knipe, H.; Weerakkody, Y. ACR Thyroid Imaging Reporting and Data System (ACR TI-RADS). Reference article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/52374 (accessed on 25 April 2024).
- Middleton, W.D.; Teefey, S.A.; Reading, C.C.; Langer, J.E.; Beland, M.D.; Szabunio, M.M.; Desser, T.S. Multiinstitutional Analysis of Thyroid Nodule Risk Stratification Using the American College of Radiology Thyroid Imaging Reporting and Data System. AJR Am. J. Roentgenol. 2017, 208, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 2019, 104, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, Y.; Wu, R.-X.; Zhang, Y.-Z.; Gu, J.-Y.; Ye, X.-H.; Tang, W.; Xu, S.-H.; Liu, C.; Wu, X.-H. Validation and Comparison of Three Newly-Released Thyroid Imaging Reporting and Data Systems for Cancer Risk Determination. Endocrine 2019, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Na, D.; Baek, J.; Sung, J.; Kim, J.; Kang, S. US Fine-Needle Aspiration Biopsy for Thyroid Malignancy: Diagnostic Performance of Seven Society Guidelines Applied to 2000 Thyroid Nodules. Radiology 2018, 287, 893–900. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Cantisani, V.; Maranghi, M.; Lucia, P.; Durante, C. Interobserver Agreement of Various Thyroid Imaging Reporting and Data Systems. Endocr. Connect. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Ioakim, S.; Syed, A.A.; Zavros, G.; Picolos, M.; Persani, L.; Kyriacou, A. Real-world application of ATA Guidelines in over 600 aspirated thyroid nodules: Is it time to change the size cut-offs for FNA? Eur. Thyroid J. 2022, 11, e220163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Na, D.G.; Joo, L.; Lee, J.Y.; Ha, E.J.; Kim, J.H.; Jung, S.L.; Baek, J.H. Standardized Imaging and Reporting for Thyroid Ultrasound: Korean Society of Thyroid Radiology Consensus Statement and Recommendation. Korean J. Radiol. 2023, 24, 22–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russ, G.; Bonnema, S.; Erdogan, M.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Schenke, S.; Seifert, P.; Zimny, M.; Winkens, T.; Binse, I.; Görges, R. Risk Stratification of Thyroid Nodules Using the Thyroid Imaging Reporting and Data System (TIRADS): The Omission of Thyroid Scintigraphy Increases the Rate of Falsely Suspected Lesions. J. Nucl. Med. 2019, 60, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kundel, A.; Zarnegar, R.; Kato, M.; Moo, T.-A.; Zhu, B.; Scognamiglio, T.; Fahey, T.J. Comparison of microarray analysis of fine needle aspirates and tissue specimen in thyroid nodule diagnosis. Diagn. Mol. Pathol. 2010, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The Bethesda System For Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Wesoła, M.; Jeleń, M. Bethesda System in the evaluation of thyroid nodules: Review. Adv. Clin. Exp. Med. 2017, 26, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rullo, E.; Minelli, G.; Bosco, D.; Nardi, F.; Ascoli, V. Evaluation of the Italian cytological subclassification of thyroid indeterminate nodules into TIR-3A and TIR-3B: A retrospective study of 290 cases with histological correlation from a single institution. J. Endocrinol. Investig. 2018, 41, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Raparia, K.; Min, S.K.; Mody, D.R.; Anton, R.; Amrikachi, M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: Patient’s sex and nodule size are possible predictors of malignancy. Arch. Pathol. Lab. Med. 2009, 133, 787–790. [Google Scholar] [CrossRef]
- Alexander, E.K.; Schorr, M.; Klopper, J.; Kim, C.; Sipos, J.; Nabhan, F.; Parker, C.; Steward, D.L.; Mandel, S.J.; Haugen, B.R. Multicenter clinical experience with the Afirma gene expression classifier. J. Clin. Endocrinol. Metab. 2014, 99, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Thompson, L.D.R.; Jing, X.; Rossi, E.D. Is thyroid core needle biopsy a valid compliment to fine-needle aspiration? J. Am. Soc. Cytopathol. 2020, 9, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Layfield, L.J.; Cibas, E.S.; Gharib, H.; Mandel, S.J. Thyroid aspiration cytology: Current status. CA Cancer J. Clin. 2009, 59, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Krane, J.F. Role of Ancillary Techniques in Thyroid Cytology Specimens. Acta Cytol. 2020, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The diagnosis and management of thyroid nodules: A review. JAMA 2018, 319, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Lepe, M.; Tolino, L.A.; Miller, M.E.; Ohori, N.P.; Wald, A.I.; Landau, M.S.; Kaya, C.; Malapelle, U.; Bellevicine, C.; et al. Thyroid cytology smear slides: An untapped resource for ThyroSeq testing. Cancer Cytopathol. 2021, 129, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Nguyen, T.P.X.; Hassell, L.A.; Jung, C.K. Diagnostic performances of the Afirma Gene Sequencing Classifier in comparison with the Gene Expression Classifier: A meta-analysis. Cancer Cytopathol. 2021, 129, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.A.; Walts, A.E.; Sistrunk, J.W.; Giordano, T.J.; Sadow, P.M.; Massoll, N.; Campbell, R.; Jackson, S.A.; Toney, N.; Narick, C.M.; et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn. Cytopathol. 2020, 48, 1254–1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guiot, J.; Vaidyanathan, A.; Deprez, L.; Zerka, F.; Danthine, D.; Frix, A.N.; Lambin, P.; Bottari, F.; Tsoutzidis, N.; Miraglio, B.; et al. A review in radiomics: Making personalized medicine a reality via routine imaging. Med. Res. Rev. 2022, 42, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Dolcetti, V.; Radzina, M.; Bellini, M.I.; Frezza, F.; Munir, K.; Grani, G.; Durante, C.; D’Andrea, V.; David, E.; et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers 2022, 14, 3357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Grazhdani, H.; Tattaresu, G.; Pittari, A.; Foti, P.V.; Palmucci, S.; Spatola, C.; Lo Greco, M.C.; Inì, C.; Tiralongo, F.; et al. Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence. Biomedicines 2024, 12, 1676. https://doi.org/10.3390/biomedicines12081676
David E, Grazhdani H, Tattaresu G, Pittari A, Foti PV, Palmucci S, Spatola C, Lo Greco MC, Inì C, Tiralongo F, et al. Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence. Biomedicines. 2024; 12(8):1676. https://doi.org/10.3390/biomedicines12081676
Chicago/Turabian StyleDavid, Emanuele, Hektor Grazhdani, Giuliana Tattaresu, Alessandra Pittari, Pietro Valerio Foti, Stefano Palmucci, Corrado Spatola, Maria Chiara Lo Greco, Corrado Inì, Francesco Tiralongo, and et al. 2024. "Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence" Biomedicines 12, no. 8: 1676. https://doi.org/10.3390/biomedicines12081676
APA StyleDavid, E., Grazhdani, H., Tattaresu, G., Pittari, A., Foti, P. V., Palmucci, S., Spatola, C., Lo Greco, M. C., Inì, C., Tiralongo, F., Castiglione, D., Mastroeni, G., Gigli, S., & Basile, A. (2024). Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence. Biomedicines, 12(8), 1676. https://doi.org/10.3390/biomedicines12081676






