Memory-Guided Saccades in Subacute and Chronic Stroke: Secondary Data Analysis of the N-PEP-12 Clinical Study
Abstract
:1. Introduction
| Evaluated Domains | Clinical Assessments |
|---|---|
| Neurological Integrity | -Functional status of frontal and parietal lobes post stroke |
| Executive Function | -Evaluates planning and execution of eye movements |
| Working Memory | -Assesses patients’ ability to hold and manipulate spatial information |
2. Materials and Methods
2.1. Study Procedures
2.2. Data Processing
2.3. Neuropsychological Assessment
3. Results
Correlation Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ibhiedu, J.O.; Egbunu, E.O.; Ndam, A.R.; Ogala, F.; Ologunde, T.; Peterson, J.C.; Boluwatife, A.I.; et al. Stroke and cognitive impairment: Understanding the connection and managing symptoms. Ann. Med. Surg. 2023, 85, 6057. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Meschia, J.F.; Gottesman, R.; Wruck, L.; Helmer, K.; Greenberg, S.M.; DISCOVERY Investigators. Cognitive Impairment and Dementia After Stroke: Design and Rationale for the DISCOVERY Study. Stroke 2021, 52, e499–e516. [Google Scholar] [CrossRef] [PubMed]
- Tahmi, M.; Kane, V.A.; Pavol, M.A.; Naqvi, I.A. Neuroimaging biomarkers of cognitive recovery after ischemic stroke. Front. Neurol. 2022, 13, 923942. [Google Scholar] [CrossRef] [PubMed]
- Hofgren, C.; Samuelsson, H.; Klasson, S.; Jern, C.; Sunnerhagen, K.S.; Jood, K. Cognitive screen and employment long-term after infratentorial stroke. Acta Neurol. Scand. 2022, 145, 610–618. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ji, Y.; Wei, X.; Wang, F.; Xu, F.; Lu, C.; Ma, Q.; Wang, K. Eye Movement Technique to Improve Executive Function in Patients With Stroke: A Randomized Controlled Trial. Front. Neurol. 2021, 12, 599850. [Google Scholar] [CrossRef]
- Ionescu, A.; Ștefănescu, E.; Strilciuc, Ș.; Rafila, A.; Mureșanu, D. Correlating Eye-Tracking Fixation Metrics and Neuropsychological Assessment after Ischemic Stroke. Medicina 2023, 59, 1361. [Google Scholar] [CrossRef] [PubMed]
- Opwonya, J.; Doan, D.N.T.; Kim, S.G.; Kim, J.I.; Ku, B.; Kim, S.; Park, S.; Kim, J.U. Saccadic Eye Movement in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2022, 32, 193–227. [Google Scholar] [CrossRef]
- Tynterova, A.; Perepelitsa, S.; Golubev, A. Personalized Neurophysiological and Neuropsychological Assessment of Patients with Left and Right Hemispheric Damage in Acute Ischemic Stroke. Brain Sci. 2022, 12, 554. [Google Scholar] [CrossRef]
- Devereux, N.; Berns, A.M. Evaluation & Treatment of Psychological Effects of Stroke. Del. J. Public Health 2023, 9, 62–69. [Google Scholar] [CrossRef]
- Dautzenberg, G.; Lijmer, J.; Beekman, A. Diagnostic accuracy of the Montreal Cognitive Assessment (MoCA) for cognitive screening in old age psychiatry: Determining cutoff scores in clinical practice. Avoiding spectrum bias caused by healthy controls. Int. J. Geriatr. Psychiatry 2020, 35, 261–269. [Google Scholar] [CrossRef]
- Sagnier, S.; Renou, P.; Olindo, S.; Debruxelles, S.; Poli, M.; Rouanet, F.; Munsch, F.; Tourdias, T.; Sibon, I. Gait Change Is Associated with Cognitive Outcome after an Acute Ischemic Stroke. Front. Aging Neurosci. 2017, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Sun, Y. A newly designed intensive caregiver education program reduces cognitive impairment, anxiety, and depression in patients with acute ischemic stroke. Braz. J. Med. Biol. Res. 2019, 52, e8533. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, M.; Ma, W.; Zhang, Z.; Zhang, M.; Li, X. The Performance of Saccade Tasks Correlates with Cognitive Test Scores in Elderly Population: Evidence for the Usefulness of Oculomotor Tests in Cognitive Assessment. Gerontology 2023, 69, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Stepanyan, A.; Zakharyan, R.; Simonyan, A.; Tsakanova, G.; Arakelyan, A. Involvement of polymorphisms of the nerve growth factor and its receptor encoding genes in the etiopathogenesis of ischemic stroke. BMC Med. Genet. 2018, 19, 33. [Google Scholar] [CrossRef]
- Wen, M.; Dong, Z.; Zhang, L.; Li, B.; Zhang, Y.; Li, K. Depression and Cognitive Impairment: Current Understanding of Its Neurobiology and Diagnosis. Neuropsychiatr. Dis. Treat. 2022, 18, 2783–2794. [Google Scholar] [CrossRef]
- Leng, Q.; Deng, B.; Ju, Y. Application and progress of advanced eye movement examinations in cognitive impairment. Front. Aging Neurosci. 2024, 16, 1377406. [Google Scholar] [CrossRef]
- Herwig, A.; Beisert, M.; Schneider, W.X. On the spatial interaction of visual working memory and attention: Evidence for a global effect from memory-guided saccades. J. Vis. 2010, 10, 8. [Google Scholar] [CrossRef]
- Rizzo, J.-R.; Hudson, T.E.; Abdou, A.; Lui, Y.W.; Rucker, J.C.; Raghavan, P.; Landy, M.S. Disrupted Saccade Control in Chronic Cerebral Injury: Upper Motor Neuron-Like Disinhibition in the Ocular Motor System. Front. Neurol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Elfeky, A.; D’Août, K.; Lawson, R.; Hepworth, L.R.; Thomas, N.D.A.; Clynch, A.; Rowe, F.J. Biomechanical adaptation to post-stroke visual field loss: A systematic review. Syst. Rev. 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Birle, C.; Slavoaca, D.; Balea, M.; Livint Popa, L.; Muresanu, I.; Stefanescu, E.; Vacaras, V.; Dina, C.; Strilciuc, S.; Popescu, B.O.; et al. Cognitive function: Holarchy or holacracy? Neurol. Sci. 2021, 42, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Balea, M.; Birle, C.; Costin, C.; Marton, J.; Muresanu, I.A.; Jemna, N.; Popa, L.L.; Slavoaca, D.; Rosu, O.V.; Stan, A.; et al. Effects of N-Pep-12 dietary supplementation on neurorecovery after ischemic stroke. Neurol. Sci. 2021, 42, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.L.; Iancu, M.; Livint, G.; Balea, M.; Dina, C.; Vacaras, V.; Vladescu, C.; Balanescu, L.; Buzoianu, A.D.; Strilciuc, S.; et al. N-Pep-12 supplementation after ischemic stroke positively impacts frequency domain QEEG. Neurol. Sci. 2022, 43, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, T.L.; Ezard, G.; Hermens, F. Eye Movements in Neuropsychological Tasks. In Processes of Visuospatial Attention and Working Memory; Hodgson, T., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 393–418. ISBN 978-3-030-31026-4. [Google Scholar]
- Zaino, D.; Serchi, V.; Giannini, F.; Pucci, B.; Veneri, G.; Pretegiani, E.; Rosini, F.; Monti, L.; Rufa, A. Different saccadic profile in bulbar versus spinal-onset amyotrophic lateral sclerosis. Brain 2023, 146, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Willeke, K.F.; Tian, X.; Buonocore, A.; Bellet, J.; Ramirez-Cardenas, A.; Hafed, Z.M. Memory-guided microsaccades. Nat. Commun. 2019, 10, 3710. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A. The Tobii I-VT Fixation Filter. Tobii Technol. 2012, 20, 4–19. [Google Scholar]
- Olsen, A.; Matos, R. Identifying parameter values for an I-VT fixation filter suitable for handling data sampled with various sampling frequencies. In Proceedings of the Symposium on Eye Tracking Research and Applications, Santa Barbara, CA, USA, 28–30 March 2012; Association for Computing Machinery: New York, NY, USA, 2012; pp. 317–320. [Google Scholar]
- Ettinger, U.; Kumari, V.; Crawford, T.J.; Davis, R.E.; Sharma, T.; Corr, P.J. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 2003, 40, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, U.; Kumari, V.; Crawford, T.J.; Flak, V.; Sharma, T.; Davis, R.E.; Corr, P.J. Saccadic eye movements, schizotypy, and the role of neuroticism. Biol. Psychol. 2005, 68, 61–78. [Google Scholar] [CrossRef]
- Meyhöfer, I.; Bertsch, K.; Esser, M.; Ettinger, U. Variance in saccadic eye movements reflects stable traits. Psychophysiology 2016, 53, 566–578. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Mariz, J.; Bull, L.; Mehta, Z.; Rothwell, P.M. Impact of Different Operational Definitions on Mild Cognitive Impairment Rate and MMSE and MoCA Performance in Transient Ischaemic Attack and Stroke. Cerebrovasc. Dis. 2013, 36, 355–362. [Google Scholar] [CrossRef]
- Aben, I.; Verhey, F.; Lousberg, R.; Lodder, J.; Honig, A. Validity of the Beck Depression Inventory, Hospital Anxiety and Depression Scale, SCL-90, and Hamilton Depression Rating Scale as Screening Instruments for Depression in Stroke Patients. Psychosomatics 2002, 43, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, A.T.; Townes, B.D.; Mahurin, R.K. Equivalence of the Color Trails Test and Trail Making Test in Nonnative English-Speakers. Arch. Clin. Neuropsychol. 2000, 15, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.L.; Kishiyama, M.M.; Yund, E.W.; Herron, T.J.; Edwards, B.; Poliva, O.; Hink, R.F.; Reed, B. Improving digit span assessment of short-term verbal memory. J. Clin. Exp. Neuropsychol. 2011, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rivera Mindt, M.; Marquine, M.J.; Aghvinian, M.; Scott, T.M.; Cherner, M.; Morlett Paredes, A.; Taylor, M.J.; Umlauf, A.; Suarez, P.; Diaz-Santos, M.; et al. Demographically-adjusted norms for the processing speed subtests of the WAIS-III in a Spanish-speaking adult population: Results from the Neuropsychological Norms for the U.S.-Mexico Border Region in Spanish (NP-NUMBRS) project. Clin. Neuropsychol. 2021, 35, 293–307. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 20 June 2024).
- Posit Team. RStudio: Integrated Development Environment for R [Internet]; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 20 June 2024).
- Schauberger, P.; Walker, A. openxlsx: Read, Write and Edit xlsx Files [Internet]. 2023. Available online: https://CRAN.R-project.org/package=openxlsx (accessed on 20 June 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis [Internet]; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 20 June 2024).
- Pedersen, T.L. patchwork: The Composer of Plots [Internet]. 2024. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 20 June 2024).
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, X.; An, R.; Xiao, W.; Wan, Q. The application of saccades to assess cognitive impairment among older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2023, 35, 2307–2321. [Google Scholar] [CrossRef]
- Płomecka, M.B.; Barańczuk-Turska, Z.; Pfeiffer, C.; Langer, N. Aging Effects and Test–Retest Reliability of Inhibitory Control for Saccadic Eye Movements. eNeuro 2020, 7, ENEURO.0459-19.2020. [Google Scholar] [CrossRef]
- Abel, L.A.; Douglas, J. Effects of age on latency and error generation in internally mediated saccades. Neurobiol. Aging 2007, 28, 627–637. [Google Scholar] [CrossRef]
- Hikosaka, O. Changes and disorders in voluntary saccades during development and aging. No Hattatsu Brain Dev. 1997, 29, 213–219. [Google Scholar]
- Di Stasi, L.L.; Renner, R.; Staehr, P.; Helmert, J.R.; Velichkovsky, B.M.; Cañas, J.J.; Catena, A.; Pannasch, S. Saccadic peak velocity sensitivity to variations in mental workload. Aviat. Space Environ. Med. 2010, 81, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Basanovic, J.; Todd, J.; van Bockstaele, B.; Notebaert, L.; Meeten, F.; Clarke, P.J.F. Assessing anxiety-linked impairment in attentional control without eye-tracking: The masked-target antisaccade task. Behav. Res. Methods 2023, 55, 135–142. [Google Scholar] [CrossRef]
- Myles, O.; Grafton, B.; MacLeod, C. Anxiety & inhibition: Dissociating the involvement of state and trait anxiety in inhibitory control deficits observed on the anti-saccade task. Cogn. Emot. 2020, 34, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Basanovic, J.; Notebaert, L.; Clarke, P.J.F.; MacLeod, C.; Jawinski, P.; Chen, N.T.M. Inhibitory attentional control in anxiety: Manipulating cognitive load in an antisaccade task. PLoS ONE 2018, 13, e0205720. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Noiret, N.; Vandel, P.; Monnin, J.; Chopard, G.; Laurent, E. Saccadic Eye Movements in Depressed Elderly Patients. PLoS ONE 2014, 9, e105355. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Wu, Y.; Fitzgerald, P.B.; Zalesky, A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Hum. Brain Mapp. 2021, 42, 4155–4172. [Google Scholar] [CrossRef] [PubMed]
- Blohm, G.; Missal, M.; Lefèvre, P. Processing of Retinal and Extraretinal Signals for Memory-Guided Saccades During Smooth Pursuit. J. Neurophysiol. 2005, 93, 1510–1522. [Google Scholar] [CrossRef]
- Nyffeler, T.; Pierrot-Deseilligny, C.; Pflugshaupt, T.; von Wartburg, R.; Hess, C.W.; Müri, R.M. Information processing in long delay memory-guided saccades: Further insights from TMS. Exp. Brain Res. 2004, 154, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Somers, J.T.; Das, V.E.; Dell’Osso, L.F.; Leigh, R.J. Saccades to Sounds: Effects of Tracking Illusory Visual Stimuli. J. Neurophysiol. 2000, 84, 96–101. [Google Scholar] [CrossRef]
- Smith, E.S.; Crawford, T.J. Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location. Brain Sci. 2021, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Moscovitch, M.; Alain, C. A systematic review and meta-analysis of memory-guided attention: Frontal and parietal activation suggests involvement of fronto-parietal networks. WIREs Cogn. Sci. 2021, 12, e1546. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.C.; Jensen, O.; Doeller, C.F.; Staudigl, T. Saccades are coordinated with directed circuit dynamics and stable but distinct hippocampal patterns that promote memory formation 2022. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. JINS 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Senior, G.; Piovesana, A.; Beaumont, P. Discrepancy analysis and Australian norms for the Trail Making Test. Clin. Neuropsychol. 2018, 32, 510–523. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Mous, S.; Karatekin, C. Memory-guided saccades in youth-onset psychosis and attention deficit hyperactivity disorder (ADHD). Early Interv. Psychiatry 2014, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Etherton, J.L.; Bianchini, K.J.; Heinly, M.T.; Greve, K.W. Pain, Malingering, and Performance on the WAIS-III Processing Speed Index. J. Clin. Exp. Neuropsychol. 2006, 28, 1218–1237. [Google Scholar] [CrossRef]
- Bombassaro, T.; Carrilho, C.G.; Peixoto, C.; Alves, G.S.; Kahn, J.P.; Nardi, A.E.; Veras, A.B. Cognition in Schizophrenia: A Systematic Review of Wechsler Adult Intelligence Scale Studies. Prim. Care Companion CNS Disord. 2023, 25, 49391. [Google Scholar] [CrossRef]
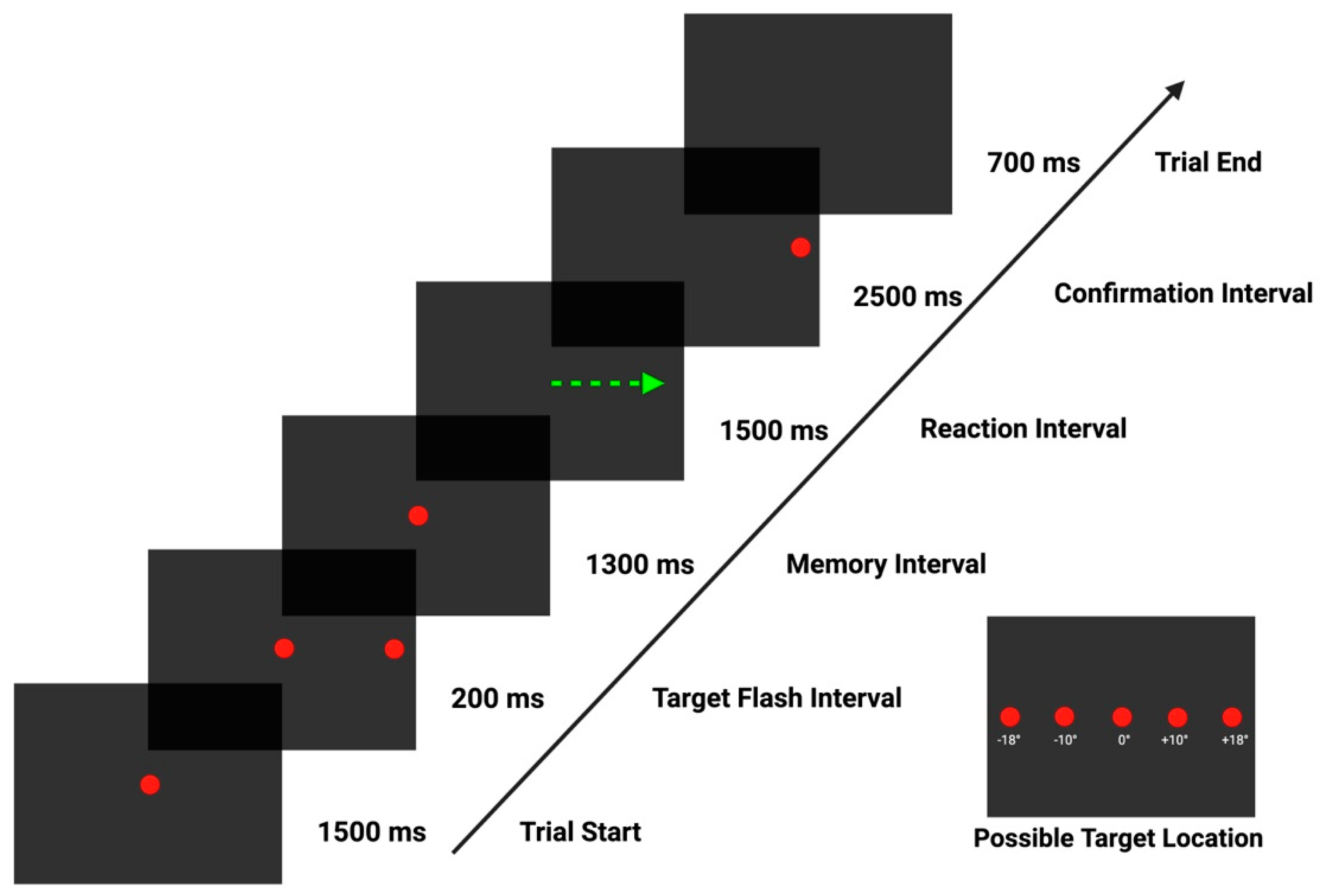
| Metric | Description |
|---|---|
| %MGS | % of correctly executed memory-guided saccades towards the remembered target location from the total number of trials |
| %CorrVGS | % of corrective visually guided saccades made toward the confirmation target location from the total number of valid trials |
| %EarlyErrorRate | % early errors—saccades made during the first 300 ms of the memorisation phase from the total number of valid trials |
| %LateErrorRate | % of late errors—saccades made during the second part of the memorisation phase (>301 ms) from the total number of valid trials |
| %TotalErrorRate | Total % saccades made during the memorisation phase from the total number of valid trials |
| Age at Stroke Onset | N. of Patients/% | Mean | Median | SD |
|---|---|---|---|---|
| All subjects | 62 (100%) | 59.94 | 61.5 | 10.87664 |
| Female | 14 (22.59%) | 61.92 | 63 | 9.12700 |
| Male | 48 (77.41%) | 59.23 | 60.5 | 11.34983 |
| Variables of Interest | Eye-Tracking Parameter | Correlation Coefficient (ρ) | p Value |
|---|---|---|---|
| Age | %LateErrorRate | 0.327 | 0.009 |
| %MGS | −0.274 | 0.003 | |
| Time to Peak Velocity of NEAR VGS | 0.273 | 0.035 | |
| MoCA (Montreal Cognitive Assessment) | Mean Duration of NEAR VGS | 0.259 | 0.045 |
| HADS-A (Anxiety score) | %EarlyErrorRate | 0.325 | 0.01 |
| Mean Velocity of NEAR MGS | 0.226 | 0.037 | |
| Peak Velocity of NEAR MGS | 0.279 | 0.028 | |
| Peak Velocity of MGS FAR | 0.259 | 0.042 | |
| Duration of MGS FAR (SD) | −0.262 | 0.04 | |
| HADS-D (Depression score) | %EarlyErrorRate | 0.331 | 0.014 |
| %TotalErrorRate | 0.294 | 0.021 | |
| CLRES01-1 (Color Trails 1—time in seconds) | Mean Velocity of MGS NEAR (SD) | 0.31 | 0.014 |
| Peak Velocity of MGS NEAR (SD) | 0.21 | 0.09 | |
| CLRES02-1 (Color Trails 1—number of errors) | Latency of MGS FAR (SD) | −0.373 | 0.003 |
| Mean Velocity of MGS FAR (SD) | −0.324 | 0.01 | |
| Peak Velocity of MGS FAR (SD) | −0.282 | 0.026 | |
| Mean Velocity of VGS NEAR | −0.268 | 0.038 | |
| Peak Velocity of VGS NEAR (SD) | −0.293 | 0.023 | |
| Amplitude of VGS NEAR (SD) | −0.408 | 0.001 | |
| Amplitude of VGS FAR (SD) | −0.302 | 0.019 | |
| CLRES03-1 (Color Trails 1—near misses) | Latency of MGS NEAR | 0.236 | 0.065 |
| Peak Velocity of MGS FAR | −0.288 | 0.023 | |
| %CorrVGS | 0.315 | 0.013 | |
| Amplitude of VGS FAR | 0.313 | 0.014 | |
| Mean Velocity of VGS FAR | 0.335 | 0.008 | |
| Time to Peak Velocity of VGS FAR | 0.391 | 0.002 | |
| Duration of VGS FAR | 0.328 | 0.001 | |
| CLRES04-1 (Color Trails 1—number of prompts) | %EarlyErrorRate | 0.275 | 0.03 |
| %MGS | −0.256 | 0.045 | |
| CLRES05-1 (Color Trails 2—time in seconds) | Mean Velocity of MGS NEAR(SD) | 0.324 | 0.01 |
| Mean Velocity of MGS FAR | −0.343 | 0.006 | |
| CLRES06-1 (Color Trails 2—number of color errors) | Peak Velocity of MGS FAR | −0.41 | <0.001 |
| Peak Velocity of MGS FAR (SD) | −0.373 | 0.003 | |
| CLRES09-1 (Color Trails 2—number of prompts) | %EarlyErrorRate | 0.301 | 0.018 |
| DGFRES-1 (Digit Span Forward) | Amplitude of MGS NEAR (SD) | 0.258 | 0.043 |
| Gain of MGS NEAR (SD) | 0.673 | 0.055 | |
| DGBRES-1 (Digit Span Backward) | Time to Peak Velocity of MGS NEAR | −0.387 | 0.002 |
| %CorrVGS | 0.353 | 0.005 | |
| PSSCNUM-1 (Processing Speed—number correct) | Time to Peak Velocity of MGS NEAR | −0.26 | 0.042 |
| Mean Velocity of MGS NEAR (SD) | −0.284 | 0.025 | |
| Latency of MGS FAR (SD) | −0.264 | 0.038 | |
| PSSINUM-1 (Processing Speed—number incorrect) | Duration of MGS NEAR (SD) | −0.229 | 0.074 |
| Time to Peak Velocity of VGS NEAR | 0.34 | 0.008 |
| Predictor | B | SE | t | p |
|---|---|---|---|---|
| Intercept | −35.603 | 40.089 | −0.888 | 0.378 |
| Age | 0.634 | 0.259 | 2.447 | 0.018 |
| Gender | 10.561 | 6.053 | 1.745 | 0.087 |
| MOTS-1 | 0.103 | 1.015 | 0.101 | 0.920 |
| HDTSA-1 | 0.349 | 1.096 | 0.319 | 0.751 |
| HDTSD-1 | −0.555 | 0.964 | −0.576 | 0.567 |
| CLRES01-1 | 0.066 | 0.155 | 0.422 | 0.675 |
| CLRES02-1 | −8.840 | 5.703 | −1.550 | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, E.; Balea, M.; Chelaru, V.-F.; Jemna, N.; Verișezan Roșu, O.; Truță, A.; Stan, A.D.; Chira, D.; Strilciuc, Ș.; Mureșanu, D. Memory-Guided Saccades in Subacute and Chronic Stroke: Secondary Data Analysis of the N-PEP-12 Clinical Study. Biomedicines 2024, 12, 1678. https://doi.org/10.3390/biomedicines12081678
Ștefănescu E, Balea M, Chelaru V-F, Jemna N, Verișezan Roșu O, Truță A, Stan AD, Chira D, Strilciuc Ș, Mureșanu D. Memory-Guided Saccades in Subacute and Chronic Stroke: Secondary Data Analysis of the N-PEP-12 Clinical Study. Biomedicines. 2024; 12(8):1678. https://doi.org/10.3390/biomedicines12081678
Chicago/Turabian StyleȘtefănescu, Emanuel, Maria Balea, Vlad-Florin Chelaru, Nicoleta Jemna, Olivia Verișezan Roșu, Anamaria Truță, Adina Dora Stan, Diana Chira, Ștefan Strilciuc, and Dafin Mureșanu. 2024. "Memory-Guided Saccades in Subacute and Chronic Stroke: Secondary Data Analysis of the N-PEP-12 Clinical Study" Biomedicines 12, no. 8: 1678. https://doi.org/10.3390/biomedicines12081678







