Review of Myocardial Ischemia, Scar, and Viability Estimation with Molecular Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Current Guidelines for Diagnostics and Treatment
3. Biological Background of MRI Imaging for Cardiac Ischemia, Scarring, and Viability
4. Principles of Molecular Imaging in Cardiac MRI
4.1. Cardiac Anatomy and Function
4.2. Contrast Agent for Myocardial Ischemia, Scar, and Viability Imaging
4.3. Conventional Relaxation Time Methods in Myocardial Ischemia, Scar, and Viability Imaging
4.4. Rotating Frame Relaxation Time Methods for Ischemic and Scar Imaging
4.5. Magnetization Transfer Method for Ischemia, Scar, and Viability Imaging
4.6. Diffusion Techniques for Ischemia, Scar, and Viability Imaging
4.7. Hyperpolarization MRI for Ischemic, Scar, and Viability Imaging
5. Molecular MRI Contrast and Imaging Agents
5.1. Imaging Myocardial Perfusion
5.2. Imaging Cellular Hypoxemia and Metabolism
5.3. Protein-Targeted Gadolinium-Based Contrast Agents
6. Comparison and Integration of mMRI with PET
PET Imaging Tracers
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Molecular Magnetic Resonance Imaging in Cardiovascular Medicine. Circulation 2007, 115, 2076–2086. [Google Scholar] [CrossRef]
- Saraste, A.; Knuuti, J. ESC 2019 Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes: Recommendations for Cardiovascular Imaging. Herz 2020, 45, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.P.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, E9–E119. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Davies, T.S. Cytokines in Atherosclerosis: Key Players in All Stages of Disease and Promising Therapeutic Targets. Cytokine Growth Factor. Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Walpola, P.L.; Gotlieb, A.I.; Cybulsky, M.I.; Langille, B.L. Expression of ICAM-1 and VCAM-1 and Monocyte Adherence in Arteries Exposed to Altered Shear Stress. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2–10. [Google Scholar] [CrossRef]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, E.; Saraste, A.; Knuuti, J.; Liimatainen, T.; Ylä-Herttuala, S. Molecular Imaging to Monitor Left Ventricular Remodeling in Heart Failure. Curr. Cardiovasc. Imaging Rep. 2019, 12, 11. [Google Scholar] [CrossRef]
- Curley, D.; Lavin Plaza, B.; Shah, A.M.; Botnar, R.M. Molecular Imaging of Cardiac Remodelling after Myocardial Infarction. Basic Res. Cardiol. 2018, 113, 10. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Manisty, C.; Moon, J.C. Cardiac MRI Evaluation of Myocardial Disease. Heart 2016, 102, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Lombardi, F. Postinfarct Left Ventricular Remodelling: A Prevailing Cause of Heart Failure. Cardiol. Res. Pract. 2016, 2016, 2579832. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Commerford, P.J.; Gersh, B.J.; Pfeffer, M.A. Controversies in Ventricular Remodelling. Lancet 2006, 367, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Cheong, B.; Muthupillai, R. Tools for Cardiovascular Magnetic Resonance Imaging. Cardiovasc. Diagn. Ther. 2014, 4, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.R.; Berry, C.; Kellman, P.; Anderson, R.; Hsu, L.Y.; Chen, M.Y.; McPhaden, A.R.; Watkins, S.; Schenke, W.; Wright, V.; et al. Bright-Blood T 2-Weighted MRI Has High Diagnostic Accuracy for Myocardial Hemorrhage in Myocardial Infarction a Preclinical Validation Study in Swine. Circ. Cardiovasc. Imaging 2011, 4, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Rehwald, W.G.; Jenista, E.R.; Wendell, D.C.; Filev, P.; van Assche, L.; Jensen, C.J.; Parker, M.A.; Chen, E.-L.; Crowley, A.L.C.; et al. Dark-Blood Delayed Enhancement Cardiac Magnetic Resonance of Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Larroza, A.; López-Lereu, M.P.; Monmeneu, J.V.; Gavara, J.; Chorro, F.J.; Bodí, V.; Moratal, D. Texture Analysis of Cardiac Cine Magnetic Resonance Imaging to Detect Nonviable Segments in Patients with Chronic Myocardial Infarction. Med. Phys. 2018, 45, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.N.; Singh, A.; Nazir, S.A.; Kanagala, P.; Gershlick, A.H.; McCann, G.P. Comparison of Cardiovascular Magnetic Resonance Feature Tracking and Tagging for the Assessment of Left Ventricular Systolic Strain in Acute Myocardial Infarction. Eur. J. Radiol. 2015, 84, 840–848. [Google Scholar] [CrossRef]
- Sillanmäki, S.; Vainio, H.L.; Ylä-Herttuala, E.; Husso, M.; Hedman, M. Measuring Cardiac Dyssynchrony with DENSE (Displacement Encoding with Stimulated Echoes)—A Systematic Review. Rev. Cardiovasc. Med. 2023, 24, 261. [Google Scholar] [CrossRef]
- Polacin, M.; Karolyi, M.; Eberhard, M.; Gotschy, A.; Baessler, B.; Alkadhi, H.; Kozerke, S.; Manka, R. Segmental Strain Analysis for the Detection of Chronic Ischemic Scars in Non-Contrast Cardiac MRI Cine Images. Sci. Rep. 2021, 11, 12376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sood, A.; Mittal, B.R.; Vijayvergiya, R. Assessment of Myocardial Viability Using Echocardiographic Strain Imaging in Patients with ST-Elevation Myocardial Infarction: Comparison with Cardiac PET Imaging. J. Echocardiogr. 2020, 18, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Saha, M.; Guha, S.; Roy, T.; Kumar, R.; Sinha, A.K. Strain Imaging as an Early Predictor in Acute Myocardial Infarction—An Augmented Cross-Sectional Study. Indian. Heart J. 2024, 76, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, E.; Laidinen, S.; Laakso, H.; Liimatainen, T. Quantification of Myocardial Infarct Area Based on T RAFFn Relaxation Time Maps—Comparison with Cardiovascular Magnetic Resonance Late Gadolinium Enhancement, T1ρ and T2 in Vivo. J. Cardiovasc. Magn. Reson. 2018, 20, 34. [Google Scholar] [CrossRef]
- Lewis, A.J.M.; Tyler, D.J.; Rider, O. Clinical Cardiovascular Applications of Hyperpolarized Magnetic Resonance. Cardiovasc. Drugs Ther. 2020, 34, 231–240. [Google Scholar] [CrossRef]
- Do, H.P.; Ramanan, V.; Qi, X.; Barry, J.; Wright, G.A.; Ghugre, N.R.; Nayak, K.S. Non-Contrast Assessment of Microvascular Integrity Using Arterial Spin Labeled Cardiovascular Magnetic Resonance in a Porcine Model of Acute Myocardial Infarction. J. Cardiovasc. Magn. Reson. 2018, 20, 45. [Google Scholar] [CrossRef]
- Pan, J.; Ng, S.M.; Neubauer, S.; Rider, O.J. Phenotyping Heart Failure by Cardiac Magnetic Resonance Imaging of Cardiac Macro- and Microscopic Structure: State of the Art Review. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1302–1317. [Google Scholar] [CrossRef]
- Fahmy, A.S.; Neisius, U.; Tsao, C.W.; Berg, S.; Goddu, E.; Pierce, P.; Basha, T.A.; Ngo, L.; Manning, W.J.; Nezafat, R. Gray Blood Late Gadolinium Enhancement Cardiovascular Magnetic Resonance for Improved Detection of Myocardial Scar. J. Cardiovasc. Magn. Reson. 2018, 20, 22. [Google Scholar] [CrossRef]
- Payne, A.R.; Casey, M.; McClure, J.; McGeoch, R.; Murphy, A.; Woodward, R.; Saul, A.; Bi, X.; Zuehlsdorff, S.; Oldroyd, K.G.; et al. Bright-Blood T2-Weighted MRI Has Higher Diagnostic Accuracy Than Dark-Blood Short Tau Inversion Recovery MRI for Detection of Acute Myocardial Infarction and for Assessment of the Ischemic Area at Risk and Myocardial Salvage. Circ. Cardiovasc. Imaging 2011, 4, 210–219. [Google Scholar] [CrossRef]
- Kis, E.; Ablonczy, L.; Reusz, G.S. Cardiac Magnetic Resonance Imaging of the Myocardium in Chronic Kidney Disease. Kidney Blood Press. Res. 2018, 43, 134–142. [Google Scholar] [CrossRef]
- Klein, C.; Nekolla, S.G.; Balbach, T.; Schnackenburg, B.; Nagel, E.; Fleck, E.; Schwaiger, M. The Influence of Myocardial Blood Flow and Volume of Distribution on Late Gd-DTPA Kinetics in Ischemic Heart Failure. J. Magn. Reson. Imaging 2004, 20, 588–594. [Google Scholar] [CrossRef]
- Brown, R.W.; Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging, Physical Principles and Sequence Design; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in Clinical Practice: A Comprehensive Review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; LaRocca, G.; Mirelis, J.G. Myocardial Mapping with Cardiac Magnetic Resonance: The Diagnostic Value of Novel Sequences. Rev. Española Cardiol. (Engl. Ed.) 2016, 69, 849–861. [Google Scholar] [CrossRef]
- Aletras, A.H.; Tilak, G.S.; Natanzon, A.; Hsu, L.Y.; Gonzalez, F.M.; Hoyt, R.F.; Arai, A.E. Retrospective Determination of the Area at Risk for Reperfused Acute Myocardial Infarction with T2-Weighted Cardiac Magnetic Resonance Imaging: Histopathological and Displacement Encoding with Stimulated Echoes (DENSE) Functional Validations. Circulation 2006, 113, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Lota, A.S.; Gatehouse, P.D.; Mohiaddin, R.H. T2 Mapping and T2* Imaging in Heart Failure. Heart Fail. Rev. 2017, 22, 431–440. [Google Scholar] [CrossRef]
- Maestrini, V.; Treibel, T.A.; White, S.K.; Fontana, M.; Moon, J.C. T1 Mapping for Characterization of Intracellular and Extracellular Myocardial Diseases in Heart Failure. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9287. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Apostolou, D.; Argyriou, P.; Velitsista, S.; Papa, L.; Efentakis, S.; Vernardos, E.; Kanoupaki, M.; Kanoupakis, G.; Manginas, A. T1 and T2 Mapping in Cardiology: “Mapping the Obscure Object of Desire”. Cardiology 2017, 138, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Cokic, I.; Tang, R.L.Q.; Yang, H.J.; Sharif, B.; Marbán, E.; Li, D.; Berman, D.S.; Dharmakumar, R. Determination of Location, Size, and Transmurality of Chronic Myocardial Infarction without Exogenous Contrast Media by Using Cardiac Magnetic Resonance Imaging at 3 T. Circ. Cardiovasc. Imaging 2014, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Stoffers, R.H.; Madden, M.; Shahid, M.; Contijoch, F.; Solomon, J.; Pilla, J.J.; Gorman, J.H.; Gorman, R.C.; Witschey, W.R.T. Assessment of Myocardial Injury after Reperfused Infarction by T1ρ Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 17. [Google Scholar] [CrossRef]
- Jackowski, C.; Christe, A.; Sonnenschein, M.; Aghayev, E.; Thali, M.J. Postmortem Unenhanced Magnetic Resonance Imaging of Myocardial Infarction in Correlation to Histological Infarction Age Characterization. Eur. Heart J. 2006, 27, 2459–2467. [Google Scholar] [CrossRef]
- Emrich, T.; Halfmann, M.; Schoepf, U.J.; Kreitner, K.F. CMR for Myocardial Characterization in Ischemic Heart Disease: State-of-the-Art and Future Developments. Eur. Radiol. Exp. 2021, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Carberry, J.; Teng, V.; Carrick, D.; Berry, C. Risk Assessment in Patients with an Acute ST-Elevation Myocardial Infarction. J. Comp. Eff. Res. 2016, 5, 581–593. [Google Scholar] [CrossRef] [PubMed]
- López, K.; Neji, R.; Mukherjee, R.K.; Whitaker, J.; Phinikaridou, A.; Razavi, R.; Prieto, C.; Roujol, S.; Botnar, R. Contrast-Free High-Resolution 3D Magnetization Transfer Imaging for Simultaneous Myocardial Scar and Cardiac Vein Visualization. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Kociemba, A.; Pyda, M.; Katulska, K.; Łanocha, M.; Siniawski, A.; Janus, M.; Grajek, S. Comparison of Diffusion-Weighted with T2-Weighted Imaging for Detection of Edema in Acute Myocardial Infarction. J. Cardiovasc. Magn. Reson. 2013, 15, 90. [Google Scholar] [CrossRef]
- Larson, P.E.Z.; Tang, S.; Liu, X.; Sinha, A.; Dwork, N.; Sivalokanathan, S.; Liu, J.; Bok, R.; Ordovas, K.G.; Slater, J.; et al. Regional Quantification of Cardiac Metabolism with Hyperpolarized [1-13C]-Pyruvate CMR Evaluated in an Oral Glucose Challenge. J. Cardiovasc. Magn. Reson. 2023, 25, 77. [Google Scholar] [CrossRef]
- Sepponen, R.E.; Pohjonen, J.A.; Sipponen, J.T.; Tanttu, J.I. A Method for Tlp Imaging. J. Comput. Assist. Tomogr. 1985, 9, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Sorce, D.J.; Springer, C.S.; Ugurbil, K.; Garwood, M. T1ρ MRI Contrast in the Human Brain: Modulation of the Longitudinal Rotating Frame Relaxation Shutter-Speed during an Adiabatic RF Pulse. J. Magn. Reson. 2006, 181, 135–147. [Google Scholar] [CrossRef]
- Witschey, W.R.T.; Pilla, J.J.; Ferrari, G.; Koomalsingh, K.; Haris, M.; Hinmon, R.; Zsido, G.; Gorman, J.H.; Gorman, R.C.; Reddy, R. Rotating Frame Spin Lattice Relaxation in a Swine Model of Chronic, Left Ventricular Myocardial Infarction. Magn. Reson. Med. 2010, 64, 1453–1460. [Google Scholar] [CrossRef]
- Musthafa, H.S.N.; Dragneva, G.; Lottonen, L.; Merentie, M.; Petrov, L.; Heikura, T.; Ylä-Herttuala, E.; Ylä-Herttuala, S.; Gröhn, O.; Liimatainen, T. Longitudinal Rotating Frame Relaxation Time Measurements in Infarcted Mouse Myocardium in Vivo. Magn. Reson. Med. 2013, 69, 1389–1395. [Google Scholar] [CrossRef]
- van Oorschot, J.W.; El Aidi, H.; Jansen of Lorkeers, S.J.; Gho, J.M.; Froeling, M.; Visser, F.; Chamuleau, S.A.; Doevendans, P.A.; Luijten, P.R.; Leiner, T.; et al. Endogenous Assessment of Chronic Myocardial Infarction with T1ρ-Mapping in Patients. J. Cardiovasc. Magn. Reson. 2014, 16, 104. [Google Scholar] [CrossRef]
- Liimatainen, T.; Sorce, D.J.; O’Connell, R.; Garwood, M.; Michaeli, S. MRI Contrast from Relaxation along a Fictitious Field (RAFF). Magn. Reson. Med. 2010, 64, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Liimatainen, T.; Hakkarainen, H.; Mangia, S.; Huttunen, J.M.J.; Storino, C.; Idiyatullin, D.; Sorce, D.; Garwood, M.; Michaeli, S. MRI Contrasts in High Rank Rotating Frames; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ylä-Herttuala, E.; Vuorio, T.; Kettunen, S.; Laidinen, S.; Ylä-Herttuala, S.; Liimatainen, T. Lymphatic Insufficiency Leads to Distinct Myocardial Infarct Content Assessed by Magnetic Resonance TRAFFn, T1ρ and T2 Relaxation Times. Sci. Rep. 2023, 13, 1579. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhu, Y.; Jang, J.; Rodriguez, J.; Neisius, U.; Fahmy, A.S.; Nezafat, R. Non-Contrast Myocardial Infarct Scar Assessment Using a Hybrid Native T1 and Magnetization Transfer Imaging Sequence at 1.5T. Magn. Reson. Med. 2019, 81, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.R.; Aletras, A.H.; London, J.F.; Taylor, J.L.; Epstein, F.H.; Wassmuth, R.; Balaban, R.S.; Arai, A.E. Stunned, Infarcted, and Normal Myocardium in Dogs: Simultaneous Differentiation by Using Gadolinium-Enhanced Cine MR Imaging with Magnetization Transfer Contrast. Radiology 2003, 226, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, C.T.; Hu, P.; Peters, D.C.; Kissinger, K.V.; Goddu, B.; Goepfert, L.; Ngo, L.; Manning, W.J.; Kozerke, S.; Nezafat, R. Optimization of On-Resonant Magnetization Transfer Contrast in Coronary Vein MRI. Magn. Reson. Med. 2010, 64, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Weber, O.M.; Speier, P.; Scheffler, K.; Bieri, O. Assessment of Magnetization Transfer Effects in Myocardial Tissue Using Balanced Steady-State Free Precession (BSSFP) Cine MRI. Magn. Reson. Med. 2009, 62, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; El Ghannudi, S.; Labani, A.; Jeung, M.Y.; Gangi, A.; Ohlmann, P.; Roy, C. A Dual Flip Angle 3D BSSFP Magnetization Transfer-like Method to Differentiate between Recent and Old Myocardial Infarction. J. Magn. Reson. Imaging 2018, 47, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Fan, Z.; Xie, Y.; Dawkins, J.; Tseliou, E.; Bi, X.; Sharif, B.; Dharmakumar, R.; Marbán, E.; Li, D. In Vivo Contrast Free Chronic Myocardial Infarction Characterization Using Diffusion-Weighted Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 68. [Google Scholar] [CrossRef]
- Apps, A.; Lau, J.; Peterzan, M.; Neubauer, S.; Tyler, D.; Rider, O. Hyperpolarised Magnetic Resonance for in Vivo Real-Time Metabolic Imaging. Heart 2018, 104, 1484. [Google Scholar] [CrossRef]
- Shuvaev, S.; Akam, E.; Caravan, P. Molecular MR Contrast Agents. Investig. Radiol. 2021, 56, 20–34. [Google Scholar] [CrossRef]
- Uhlig, J.; Lücke, C.; Vliegenthart, R.; Loewe, C.; Grothoff, M.; Schuster, A.; Lurz, P.; Jacquier, A.; Francone, M.; Zapf, A.; et al. Acute Adverse Events in Cardiac MR Imaging with Gadolinium-Based Contrast Agents: Results from the European Society of Cardiovascular Radiology (ESCR) MRCT Registry in 72,839 Patients. Eur. Radiol. 2019, 29, 3686–3695. [Google Scholar] [CrossRef]
- Woolen, S.A.; Shankar, P.R.; Gagnier, J.J.; MacEachern, M.P.; Singer, L.; Davenport, M.S. Risk of Nephrogenic Systemic Fibrosis in Patients with Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2020, 180, 223–230. [Google Scholar] [CrossRef]
- Sosnovik, D.E. Molecular Imaging in Cardiovascular Magnetic Resonance Imaging: Current Perspective and Future Potential. Top. Magn. Reson. Imaging 2008, 19, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.H.; Helm, P.A.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.-C.; et al. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angew. Chem. Int. Ed. 2007, 46, 8171–8173. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial Aνβ3 Integrin-Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bengel, F.M. Molecular Imaging of Myocardial Inflammation with Positron Emission Tomography Post-Ischemia: A Determinant of Subsequent Remodeling or Recovery. JACC Cardiovasc. Imaging 2018, 11, 1340–1355. [Google Scholar] [CrossRef]
- Lau, A.Z.; Miller, J.J.; Robson, M.D.; Tyler, D.J. Simultaneous Assessment of Cardiac Metabolism and Perfusion Using Copolarized [1-13C]Pyruvate and 13C-Urea. Magn. Reson. Med. 2017, 77, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rider, O.J.; Tyler, D.J. Clinical Implications of Cardiac Hyperpolarized Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. 2013, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.H.; Lau, J.Y.C.; Chen, A.P.; Geraghty, B.J.; Perks, W.J.; Roifman, I.; Wright, G.A.; Connelly, K.A. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. 2016, 119, 1177–1182. [Google Scholar] [CrossRef]
- Merritt, M.E.; Harrison, C.; Storey, C.; Mark Jeffrey, F.; Dean Sherry, A.; Malloy, C.R. Hyperpolarized13C Allows a Direct Measure of Fluxthrough a Single Enzyme-Catalyzed Step by NMR. Proc. Natl. Acad. Sci. USA 2007, 104, 19773–19777. [Google Scholar] [CrossRef]
- Lau, A.Z.; Chen, A.P.; Barry, J.; Graham, J.J.; Dominguez-Viqueira, W.; Ghugre, N.R.; Wright, G.A.; Cunningham, C.H. Reproducibility Study for Free-Breathing Measurements of Pyruvate Metabolism Using Hyperpolarized 13C in the Heart. Magn. Reson. Med. 2013, 69, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Petersson, J.S.; Magnusson, P.; Johansson, E.; Åkeson, P.; Chai, C.M.; Hansson, G.; Månsson, S. Cardiac Metabolism Measured Noninvasively by Hyperpolarized 13C MRI. Magn. Reson. Med. 2008, 59, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Frijia, F.; Positano, V.; Menichetti, L.; Santarelli, M.F.; Lionetti, V.; Giovannetti, G.; Recchia, F.A.; Landini, L. Cardiac Metabolism in a Pig Model of Ischemia-Reperfusion by Cardiac Magnetic Resonance with Hyperpolarized 13C-Pyruvate. IJC Metab. Endocr. 2015, 6, 17–23. [Google Scholar] [CrossRef]
- Ball, D.R.; Cruickshank, R.; Carr, C.A.; Stuckey, D.J.; Lee, P.; Clarke, K.; Tyler, D.J. Metabolic Imaging of Acute and Chronic Infarction in the Perfused Rat Heart Using Hyperpolarised [1-13C]Pyruvate. NMR Biomed. 2013, 26, 1441–1450. [Google Scholar] [CrossRef]
- Oh-Ici, D.; Wespi, P.; Busch, J.; Wissmann, L.; Krajewski, M.; Weiss, K.; Sigfridsson, A.; Messroghli, D.; Kozerke, S. Hyperpolarized Metabolic MR Imaging of Acute Myocardial Changes and Recovery after Ischemia-Reperfusion in a Small-Animal Model. Radiology 2015, 278, 742–751. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New Type of Cardiomyopathy Associated with Diabetic Glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Swietach, P.; Atherton, H.J.; Gallagher, F.A.; Lee, P.; Radda, G.K.; Clarke, K.; Tyler, D.J. Measuring Intracellular PH in the Heart Using Hyperpolarized Carbon Dioxide and Bicarbonate: A 13C and 31P Magnetic Resonance Spectroscopy Study. Cardiovasc. Res. 2010, 86, 82–91. [Google Scholar] [CrossRef]
- Lau, A.Z.; Miller, J.J.; Tyler, D.J. Mapping of Intracellular PH in the in Vivo Rodent Heart Using Hyperpolarized [1-13C]Pyruvate. Magn. Reson. Med. 2017, 77, 1810–1817. [Google Scholar] [CrossRef]
- Lavin, B.; Protti, A.; Lorrio, S.; Dong, X.; Phinikaridou, A.; Botnar, R.M.; Shah, A. MRI with Gadofosveset: A Potential Marker for Permeability in Myocardial Infarction. Atherosclerosis 2018, 275, 400–408. [Google Scholar] [CrossRef]
- Engel, L.C.; Landmesser, U.; Abdelwahed, Y.S.; Gigengack, K.; Wurster, T.; Manes, C.; Skurk, C.; Lauten, A.; Schuster, A.; Noutsias, M.; et al. In Vivo Assessment of Endothelial Permeability of Coronary Lesions with Variable Degree of Stenosis Using an Albumin-Binding MR Probe. Int. J. Cardiovasc. Imaging 2021, 37, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.A.; Caravan, P.; French, B.A.; Jacques, V.; Shen, L.; Xu, Y.; Beyers, R.J.; Roy, R.J.; Kramer, C.M.; Epstein, F.H. Postinfarction Myocardial Scarring in Mice: Molecular MR Imaging with Use of a Collagen-Targeting Contrast Agent. Radiology 2008, 247, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Alessio, A. What Are the Basic Concepts of Temporal, Contrast, and Spatial Resolution in Cardiac CT? J. Cardiovasc. Comput. Tomogr. 2009, 3, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Beckett, A.J.S.; Vu, A.T.; Stockmann, J.; Huber, L.; Ma, S.; Ahn, S.; Setsompop, K.; Cao, X.; Park, S.; et al. Next-Generation MRI Scanner Designed for Ultra-High-Resolution Human Brain Imaging at 7 Tesla. Nat. Methods 2023, 20, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.S.; Tawakol, A. Imaging of Coronary Inflammation with FDG-PET: Feasibility and Clinical Hurdles. Curr. Cardiol. Rep. 2011, 13, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerwin, W.S.; Caldwell, J.H.; Ferguson, M.S.; Hippe, D.S.; Alessio, A.M.; Martinez-Malo, V.; Pimentel, K.; Miyaoka, R.S.; Kohler, T.R.; et al. High Resolution FDG-MicroPET of Carotid Atherosclerosis: Plaque Components Underlying Enhanced FDG Uptake. Int. J. Cardiovasc. Imaging 2016, 32, 145–152. [Google Scholar] [CrossRef]
- Salarian, M.; Sadeghi, M.M. Hype or Hope: 18f-Naf Positron Emission Tomography for Vulnerable Coronary Plaque Imaging. Circ. Cardiovasc. Imaging 2019, 12, e009591. [Google Scholar] [CrossRef]
- Papadakis, G.Z.; Kochiadakis, G.; Lazopoulos, G.; Marias, K.; Klapsinos, N.; Hannah-Shmouni, F.; Igoumenaki, G.G.; Nikolouzakis, T.K.; Kteniadakis, S.; Spandidos, D.A.; et al. Targeting Vulnerable Atherosclerotic Plaque via PET-Tracers Aiming at Cell-Surface Overexpression of Somatostatin Receptors. Biomed. Rep. 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Silvola, J.M.U.; Li, X.G.; Virta, J.; Marjamäki, P.; Liljenbäck, H.; Hytönen, J.P.; Tarkia, M.; Saunavaara, V.; Hurme, S.; Palani, S.; et al. Aluminum Fluoride-18 Labeled Folate Enables in Vivo Detection of Atherosclerotic Plaque Inflammation by Positron Emission Tomography. Sci. Rep. 2018, 8, 9720. [Google Scholar] [CrossRef]
- Cerqueira, M.; Jacobson, A.F. Assessment of Myocardial Viability with SPECT and PET Imaging. Am. J. Roentgenol. 1989, 153, 477–483. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Bax, J.J.; Poldermans, D.; Elhendy, A.; Ferrari, R.; Rahimtoola, S.H. Hibernating Myocardium: Diagnosis and Patient Outcomes. Curr. Probl. Cardiol. 2007, 32, 375–410. [Google Scholar] [CrossRef]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction and Suspected Coronary Disease. A Randomized, Controlled Trial (PARR-2). J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Coronary Revascularization Myocardial Viability Testing and Impact of Revascularization on Prognosis in Patients with Coronary Artery Disease and Left Ventricular Dysfunction: A Meta-Analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity after Myocardial Infarction Using a 68Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Wang, Y.; Zhu, K.; Zheng, D.; Song, Y.; Jiang, D.; Qin, C.; Lan, X. Noninvasive Monitoring of Reparative Fibrosis after Myocardial Infarction in Rats Using 68Ga-FAPI-04 PET/CT. Mol. Pharm. 2022, 19, 4171–4178. [Google Scholar] [CrossRef]
- Diekmann, J.; Koenig, T.; Thackeray, J.T.; Derlin, T.; Czerner, C.; Neuser, J.; Ross, T.L.; Schcafer, A.; Tillmanns, J.; Bauersachs, J.; et al. Cardiac Fibroblast Activation in Patients Early After Acute Myocardial Infarction: Integration with MR Tissue Characterization and Subsequent Functional Outcome. J. Nucl. Med. 2022, 63, 1415–1423. [Google Scholar] [CrossRef]
- Zhang, M.; Quan, W.; Zhu, T.; Feng, S.; Huang, X.; Meng, H.; Du, R.; Zhu, Z.; Qu, X.; Li, P.; et al. [68Ga]Ga-DOTA-FAPI-04 PET/MR in Patients with Acute Myocardial Infarction: Potential Role of Predicting Left Ventricular Remodeling. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 839–848. [Google Scholar] [CrossRef]

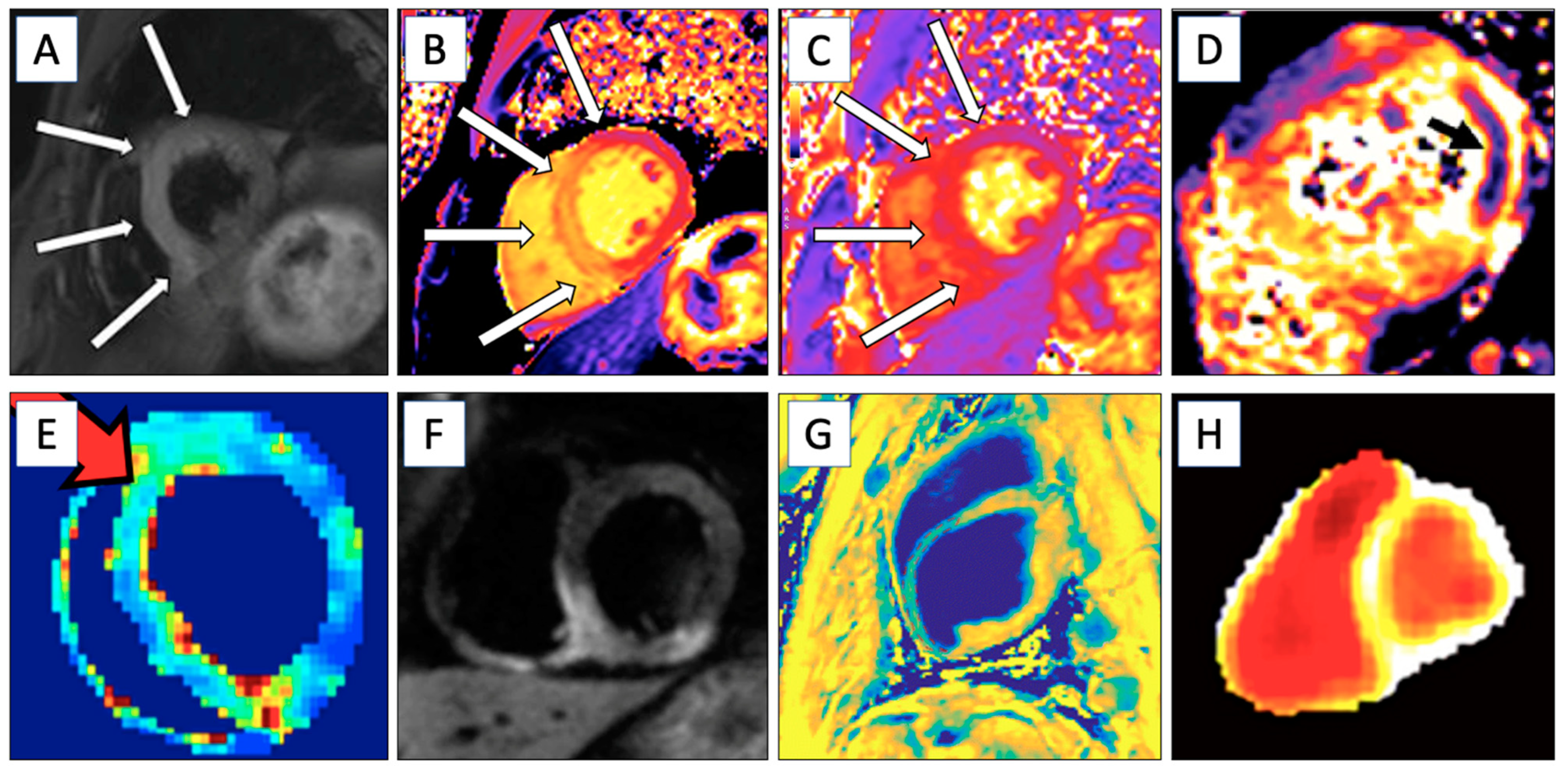
| CMR Technique | Information Obtained | LGE | Examples of Diseases Studied | Figure |
|---|---|---|---|---|
| T1 Mapping | Identifies myocardial tissue properties like fibrosis. Extracellular volume can be measured if LGE is used. | No/Yes | Myocardial infarction, myocarditis, amyloidosis, etc. | Figure 2B |
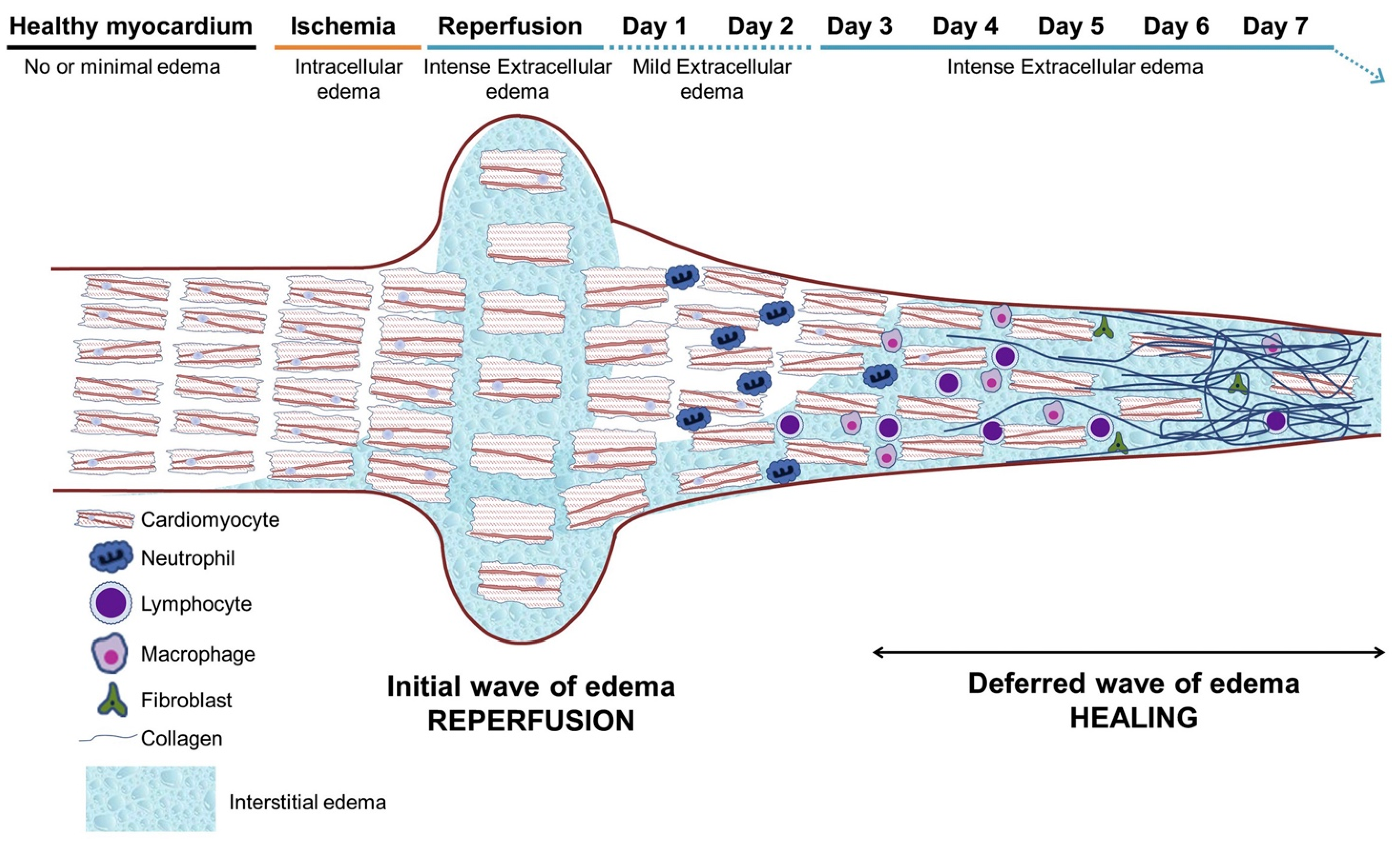
| T2 Mapping | Evaluates edema, suitable for detection of acute ischemic areas. | No | Acute ischemia, myocarditis, etc. | Figure 2C and Figure 3 |
| T2* Relaxation | Myocardial hemorrhage in the acute phase. | No | Ischemic hemorrhage and iron overload | Figure 2D |
| Late Gadolinium Enhancement | Determines ischemic and scar areas and visualizes damage from healthy myocardial tissue. Perfusion imaging. | Yes | Ischemic heart disease (fibrosis), inflammation, etc. | Figure 2A and Figure 3 |
| T1ρ Imaging | Detects subtle changes in tissue composition, with enhanced contrast and sensitivity for ischemia and scarring. | No | Fibrosis | Figure 2E |
| RAFFn Imaging | Analyzes molecular levels of ischemic and scar tissue with low SAR values, suitable for fibrotic areas. | No | Fibrosis | Figure 3 |
| Magnetization Transfer Imaging | Determines water–macromolecular proton interactions and enhances contrast between myocardial infarction and remote myocardium. | No | Myocardial infarction | Figure 2F |
| Diffusion-Weighted Imaging | Detects areas of myocardial infarction by identifying regions with restricted water diffusion, which correspond to ischemic injury. Also detects myocardial fibrosis with altered diffusion properties. | No | Ischemic heart disease | Figure 2G |
| Hyperpolarized MRI (hMRI) | Determines real-time cardiac energy metabolism with hyperpolarized [1-13C]pyruvate. | No | Ischemic heart disease | Figure 2H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillanmäki, S.; Hartikainen, S.; Ylä-Herttuala, E. Review of Myocardial Ischemia, Scar, and Viability Estimation with Molecular Magnetic Resonance Imaging. Biomedicines 2024, 12, 1681. https://doi.org/10.3390/biomedicines12081681
Sillanmäki S, Hartikainen S, Ylä-Herttuala E. Review of Myocardial Ischemia, Scar, and Viability Estimation with Molecular Magnetic Resonance Imaging. Biomedicines. 2024; 12(8):1681. https://doi.org/10.3390/biomedicines12081681
Chicago/Turabian StyleSillanmäki, Saara, Suvi Hartikainen, and Elias Ylä-Herttuala. 2024. "Review of Myocardial Ischemia, Scar, and Viability Estimation with Molecular Magnetic Resonance Imaging" Biomedicines 12, no. 8: 1681. https://doi.org/10.3390/biomedicines12081681






