Traumatic Neuroma of the Hard Palate Mimicking a Small Salivary Gland Tumor—A Case Report
Abstract
1. Introduction
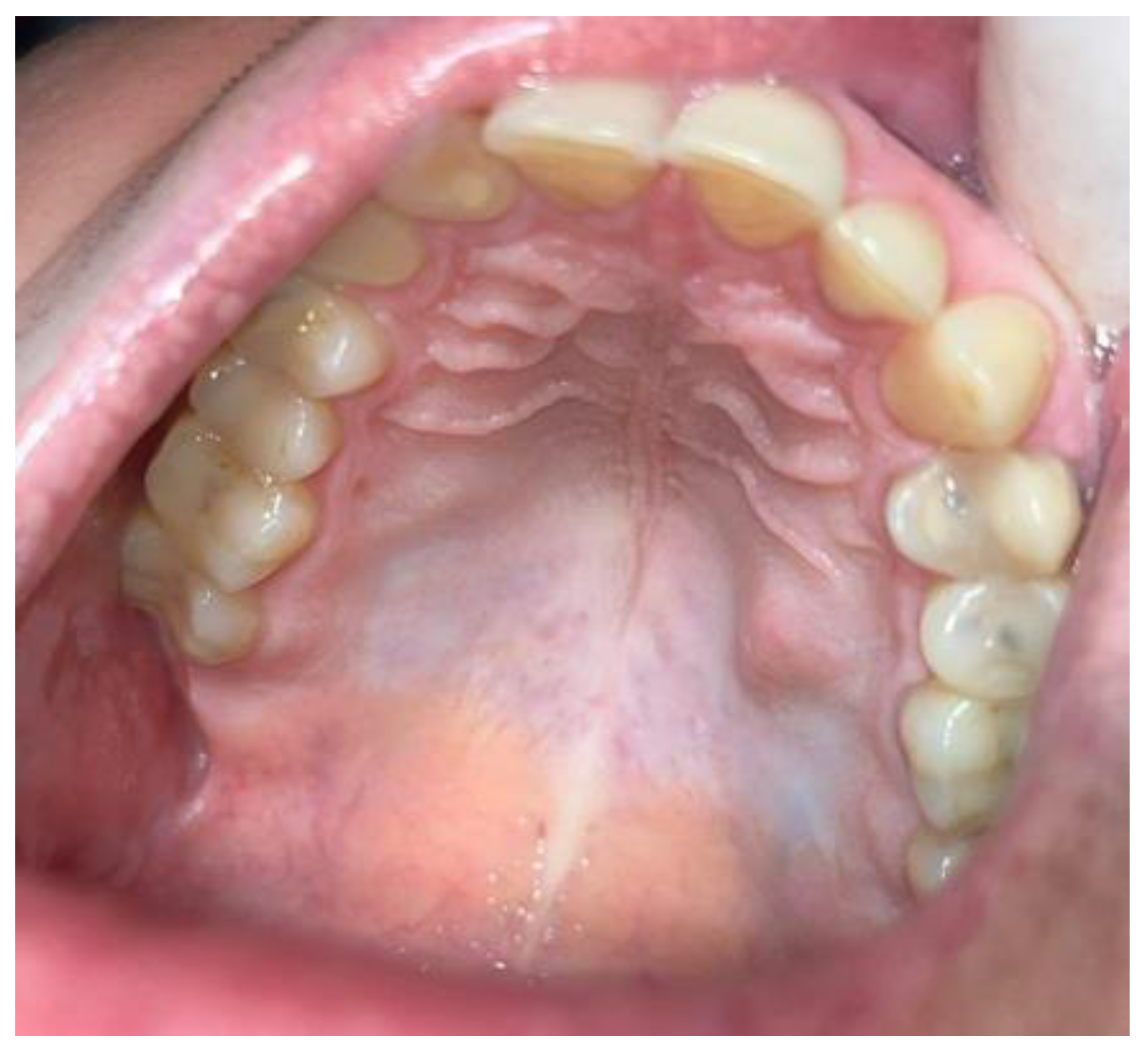
2. Case Description
Histological Findings




3. Discussion
- A close CBCT evaluation should exclude any odontogenic or teeth-related pathologies, cysts, and tumors in the oral cavity.
- A differential diagnosis of nasal, sinus, and small salivary gland tumors can be improved in any MRI studies.
- Excisional biopsy and gathering a good amount of studied tissue is the most effective way to investigate the nature of any oral neural tumor.
- Each case of a hard nodule in the oral cavity found accidentally and to be symptomless, and persisting for more than 14 days, should be scheduled for a biopsy to investigate it histopathologically.
- Oral neural tumors tend to mimic all other cysts, tumors, and lesions in the oral cavity.
- In cases of atypical lesions in the oral cavity, an improved histopathological specimen evaluation should be scheduled in order to improve the final results.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.W.; Tempski, J.; Surma, J.; Ratusznik, J.; Raputa, W.; Świerczek, I.; Pękala, J.R.; Tomaszewska, I.M. Anatomy of the greater palatine foramen and canal and their clinical significance in relation to the greater palatine artery: A systematic review and meta-analysis. Surg. Radiol. Anat. 2023, 45, 101–119. [Google Scholar] [CrossRef]
- Patil, M.S.; Patil, S.B.; Acharya, A.B. Palatine rugae and their significance in clinical dentistry: A review of the literature. J. Am. Dent. Assoc. 2008, 139, 1471–1478. [Google Scholar] [CrossRef]
- Lopes, M.A.; Vargas, P.A.; Almeida, O.P.; Takahama, A.; León, J.E. Oral traumatic neuroma with mature ganglion cells: A case report and review of the literature. J. Oral Maxillofac. Pathol. 2009, 13, 67–69. [Google Scholar]
- Jham, B.C.; Costa, N.L.; Batista, A.C.; Mendonça, E.F. Traumatic neuroma of the mandible: A case report with spontaneous remission. J. Clin. Exp. Dent. 2014, 6, 317–320. [Google Scholar] [CrossRef]
- Tokuc, B.; Altındis, S.; Coskunses, F.M.; Sinanoglu, A. Excision of Rare Intraosseous Traumatic Neuroma of the Mandible. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Ishida, R.; Ara, H.; Hamada, Y.; Kanai, I. A diffuse traumatic neuroma in the palate: A case report. J. Med. Case Rep. 2016, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Vedantham, R.; Annamalai, N.; Pitchumani, P.K. Bilateral intraoral traumatic neuroma: Case report of a diagnostic challenge. J. Indian Prosthodont. Soc. 2021, 21, 430–433. [Google Scholar] [PubMed]
- Koutlas, I.G.; Scheithauer, B.W. Palisaded encapsulated (“solitary circumscribed”) neuroma of the oral cavity: A review of 55 cases. Head Neck Pathol. 2010, 4, 15–26. [Google Scholar] [CrossRef]
- Young, A.; Okuyemi, O.T. Malignant Tumors of the Palate. [Updated 2022 Oct 17]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564515 (accessed on 18 March 2023).
- Hammouda, Y.; Halily, S.; Oukessou, Y.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Malignant tumors of the hard palate: Report of 4 cases and review of the literature. Int. J. Surg. Case Rep. 2021, 78, 228–234. [Google Scholar] [CrossRef]
- Bondi, S.; Vinciguerra, A.; Lissoni, A.; Rizzo, N.; Barbieri, D.; Indelicato, P.; Abati, S. Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction. Int. J. Environ. Res. Public Health 2021, 18, 3341. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K. A Current Review of Machine Learning and Deep Learning Models in Oral Cancer Diagnosis: Recent Technologies, Open Challenges, and Future Research Directions. Diagnostics 2023, 13, 1353. [Google Scholar] [CrossRef]
- Abu Rass, N.; Surougi, E.; Baheydarah, S.; Baroom, A.; ALGhamdi, H.; AlTuwayjiri, H.; AlMansour, N. Neoplasms of the Palate: A Review. Egypt. J. Hosp. Med. 2018, 70, 1393–1400. [Google Scholar] [CrossRef]
- The CARE Guidelines (for CAseREports) Were Developed. Available online: https://www.care-statement.org/checklist (accessed on 10 January 2024).
- Sarmento, D.J.; Morais, M.L.; Costa, A.L.; Silveira, É.J. Minor intraoral salivary gland tumors: A clinical-pathological study. Einstein (Sao Paulo) 2016, 14, 508–512. [Google Scholar] [CrossRef]
- Błochowiak, K.; Farynowska, J.; Sokalski, J.; Wyganowska-Świątkowska, M.; Witmanowski, H. Benign tumours and tumour-like lesions in the oral cavity: A retrospective analysis. Postep. Dermatol. Alergol. 2019, 36, 744–751. [Google Scholar] [CrossRef]
- Hunter, K.D.; Niklander, S. Pitfalls in odontogenic lesions and tumours: A practical guide. Diagn. Histopathol. 2020, 26, 173–180. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, Z.; Zhang, H.; She, D.; Lin, X.; Lin, Y.; Cao, D. Differentiation between benign and malignant palatal tumors using conventional MRI: A retrospective analysis of 130 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 343–350. [Google Scholar] [CrossRef]
- Thomas, D.C.; Mallareddy, S.D.; Okeson, J.P.; Thankachan, J.; Pitchumani, P.K.; Pichammal, R.C. Trigeminal traumatic neuroma: A comprehensive review of the literature based on a rare case. Curr. Pain Headache Rep. 2022, 26, 219–233. [Google Scholar] [CrossRef]
- Ural, A.; Livaoğlu, M.; Bektaş, D.; Bahadır, O.; Hesapçıoğlu, A.; Imamoğlu, M.; Işık, A.U. Approach to benign tumors of the palate: Analysis of 28 cases. Ear Nose Throat J. 2011, 90, 382–385. [Google Scholar] [CrossRef]
- López-Carriches, C.; Baca-Pérez-Bryan, R.; Montalvo-Montero, S. Schwannoma located in the palate: Clinical case and literature review. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, 465–468. [Google Scholar]
- Atarbashi-Moghadam, S.; Lotfi, A.; Salehi Zalani, S.; Mokhtari, S. Palisaded Encapsulated (Solitary Circumscribed) Neuroma of the Buccal Mucosa: A Rare Case. J. Dent. 2017, 18, 314–317. [Google Scholar]
- Tamiolakis, P.; Chrysomali, E.; Sklavounou-Andrikopoulou, A.; Nikitakis, N.G. Oral neural tumors: Clinicopathologic analysis of 157 cases and review of the literature. J. Clin. Exp. Dent. 2019, 11, 721–731. [Google Scholar] [CrossRef]
- Mittal, A.; Sagi, V.; Gupta, M.; Gupta, K. Mast Cell Neural Interactions in Health and Disease. Front. Cell. Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotulska, K.; Larysz-Brysz, M.; Marcol, W.; Grajkowska, W.; Jóźwiak, S.; Lewin-Kowalik, J. Original paperThe role of trkB receptor in the formation of post-traumatic neuroma. Folia Neuropathol. 2006, 44, 221–227. [Google Scholar] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelke, K.; Janeczek, M.; Pasicka, E.; Żak, K.; Łukaszewski, M.; Nienartowicz, J.; Gogolewski, G.; Maag, I.; Kuropka, P.; Dobrzyński, M. Traumatic Neuroma of the Hard Palate Mimicking a Small Salivary Gland Tumor—A Case Report. Biomedicines 2024, 12, 1688. https://doi.org/10.3390/biomedicines12081688
Nelke K, Janeczek M, Pasicka E, Żak K, Łukaszewski M, Nienartowicz J, Gogolewski G, Maag I, Kuropka P, Dobrzyński M. Traumatic Neuroma of the Hard Palate Mimicking a Small Salivary Gland Tumor—A Case Report. Biomedicines. 2024; 12(8):1688. https://doi.org/10.3390/biomedicines12081688
Chicago/Turabian StyleNelke, Kamil, Maciej Janeczek, Edyta Pasicka, Krzysztof Żak, Marceli Łukaszewski, Jan Nienartowicz, Grzegorz Gogolewski, Irma Maag, Piotr Kuropka, and Maciej Dobrzyński. 2024. "Traumatic Neuroma of the Hard Palate Mimicking a Small Salivary Gland Tumor—A Case Report" Biomedicines 12, no. 8: 1688. https://doi.org/10.3390/biomedicines12081688
APA StyleNelke, K., Janeczek, M., Pasicka, E., Żak, K., Łukaszewski, M., Nienartowicz, J., Gogolewski, G., Maag, I., Kuropka, P., & Dobrzyński, M. (2024). Traumatic Neuroma of the Hard Palate Mimicking a Small Salivary Gland Tumor—A Case Report. Biomedicines, 12(8), 1688. https://doi.org/10.3390/biomedicines12081688