The Predictive Performance of Risk Scores for the Outcome of COVID-19 in a 2-Year Swiss Cohort
Abstract
:1. Introduction
Objectives
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Outcomes and Scores
2.4. Data Collection and Management
2.5. Statistical Analysis
2.6. Ethical Considerations
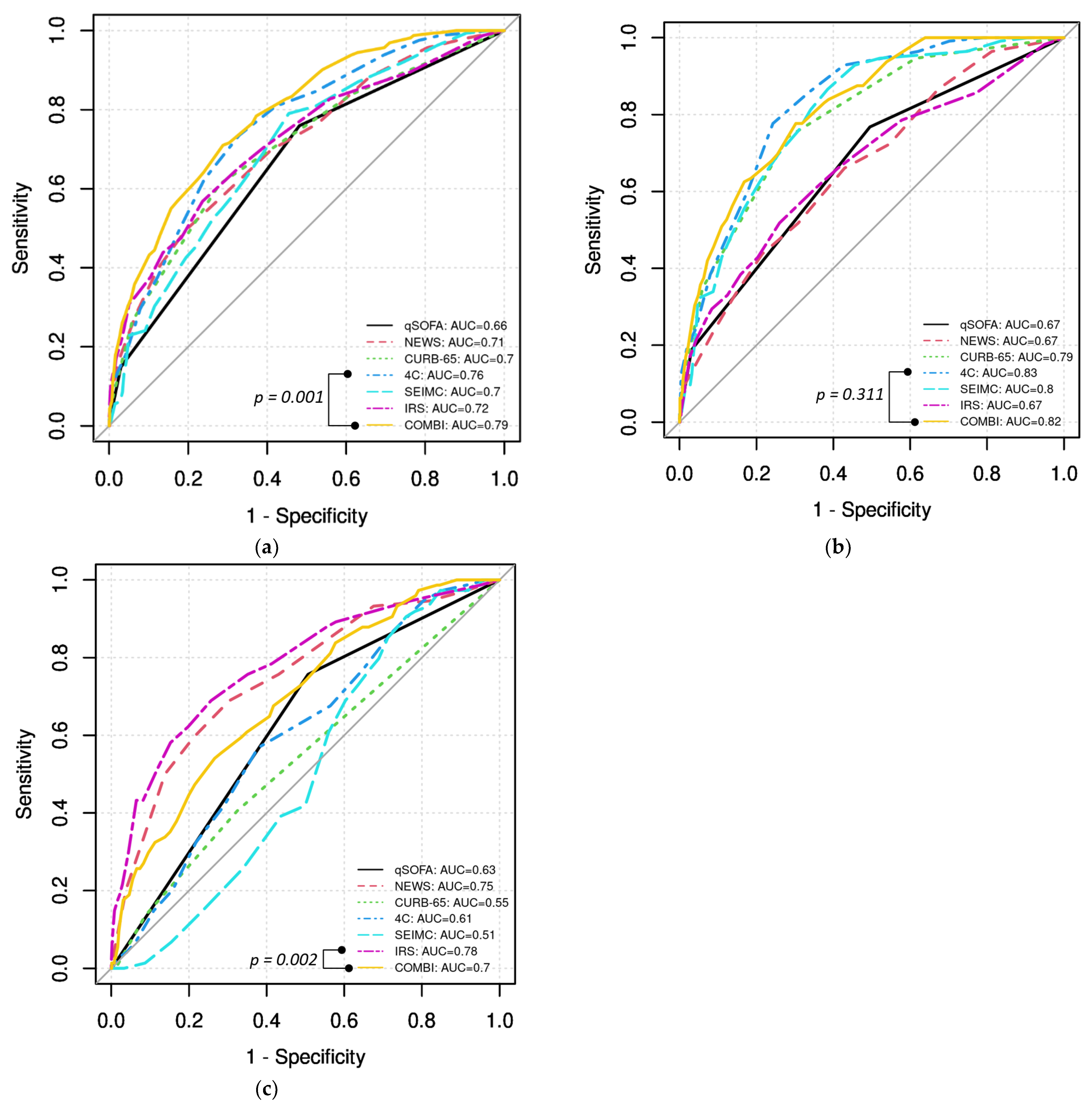
3. Results
3.1. Patient Characteristics and Outcomes
3.2. Prediction of Severe Course, In-Hospital Death, and Invasive Mechanical Ventilation
4. Discussion
- The 4C and COVID-IRS both showed good accuracy for the prediction of severe course.
- The new COVID-COMBI score showed significantly better performance than all other established scores in predicting severe course.
- The new COVID-COMBI score showed good accuracy for the prediction of in-hospital death and invasive mechanical ventilation.
4.1. Predictive Accuracy of Established Scores
4.2. Predictive Accuracy of COVID-COMBI
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | qSOFA Points | |
|---|---|---|
| Altered mental status (GCS < 15) | No | 0 |
| Yes | 1 | |
| Respiratory rate (brpm) | <22 | 0 |
| ≥22 | 1 | |
| Systolic blood pressure (mmHg) | ≤100 | 1 |
| >100 | 0 |
| Parameter | NEWS Points | |
|---|---|---|
| Respiratory rate (brpm) | ≤8 | 3 |
| 9–11 | 1 | |
| 12–20 | 0 | |
| 21–24 | 2 | |
| ≥25 | 3 | |
| Peripheral oxygen saturation (%) | ≤91 | 3 |
| 92–93 | 2 | |
| 94–95 | 1 | |
| ≥96 | 0 | |
| Any supplemental oxygen | No | 0 |
| Yes | 2 | |
| Temperature (°C) | ≤35.0 | 3 |
| 35.1–36.0 | 1 | |
| 36.1–38.0 | 0 | |
| 38.1–39.0 | 1 | |
| ≥39.1 | 2 | |
| Systolic blood pressure (mmHg) | ≤90 | 3 |
| 91–100 | 2 | |
| 101–110 | 1 | |
| 111–219 | 0 | |
| ≥220 | 3 | |
| Heart rate (bpm) | ≤40 | 3 |
| 41–50 | 1 | |
| 51–90 | 0 | |
| 91–110 | 1 | |
| 111–130 | 2 | |
| ≥131 | 3 | |
| AVPU score | A | 0 |
| (Alert, Voice, Pain, Unresponsive) | V, P, or U | 3 |
| Parameter | CURB-65 Points | |
|---|---|---|
| Confusion | No | 0 |
| Yes | 1 | |
| BUN (mg/dL)/urea (mmol/L) | ≤19/≤7 | 0 |
| >19/>7 | 1 | |
| Respiratory rate (brpm) | <30 | 0 |
| ≥30 | 1 | |
| Blood pressure (mmHg) | systolic ≥ 90 and diastolic > 60 | 0 |
| systolic < 90 or diastolic ≤ 60 | 1 | |
| Age (years) | <65 | 0 |
| ≥65 | 1 |
| Parameter | 4C Points | |
|---|---|---|
| Age (years) | <50 | 0 |
| 50–59 | 2 | |
| 60–69 | 4 | |
| 70–79 | 6 | |
| ≥80 | 7 | |
| Sex at birth | Female | 0 |
| Male | 1 | |
| Number of comorbidities a | 0 | 0 |
| 1 | 1 | |
| ≥2 | 2 | |
| Respiratory rate (brpm) | <20 | 0 |
| 20–29 | 1 | |
| ≥ 30 | 2 | |
| Peripheral oxygen saturation | ≥92 | 0 |
| on room air (%) | <92 | 2 |
| GCS | 15 | 0 |
| <15 | 2 | |
| BUN (mg/dL)/urea (mmol/L) | <19.6/<7 | 0 |
| ≥19.6–39.2/7–14 | 1 | |
| >39.2/>14 | 3 | |
| C-reactive protein (mg/L) | <50 mg/L | 0 |
| 50–99 | 1 | |
| ≥100 | 2 |
| Parameter | COVID-SEIMC Points | |
|---|---|---|
| Age (years) | <40 | 0 |
| 40–54 | 1 | |
| 55–64 | 3 | |
| 65–74 | 5 | |
| 75–79 | 9 | |
| 80–84 | 14 | |
| 85–89 | 15 | |
| ≥80 | 21 | |
| Low age-adjusted peripheral | No | 0 |
| oxygen saturation a | Yes | 2 |
| Neutrophil–lymphocyte ratio | <3.22 | 0 |
| 3.22–6.33 | 1 | |
| >6.33 | 2 | |
| eGFR mL/min/1.73 m2 | ≥60 | 0 |
| 30–59 | 2 | |
| <30 | 3 | |
| Dyspnea | No | 0 |
| Yes | 1 | |
| Sex | Female | 0 |
| Male | 1 |
| Parameter | COVID-IRS Points | |
|---|---|---|
| Respiratory rate (brpm) | <22 | 0 |
| 22–29 | 1 | |
| 30–33 | 2.5 | |
| ≥34 | 3 | |
| SpO2/FiO2 ratio | >200 | 0 |
| 101–200 | 2 | |
| ≤100 | 3.5 | |
| Lactate dehydrogenase (U/L) | ≤200 | 0 |
| 200–299 | 1 | |
| 300–399 | 2 | |
| 400–499 | 2.5 | |
| ≥500 | 4 | |
| Neutrophil–lymphocyte ratio | <4 | 0 |
| 4–7.9 | 1 | |
| 8–13.9 | 2 | |
| ≥14 | 2.5 |
References
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- Zarocostas, J. What next for the coronavirus response? Lancet 2020, 395, 401. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 2020, 368, m1036. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef]
- Tsonas, A.M.; Botta, M.; Serpa Neto, A.; Horn, J.; Paulus, F.; Schultz, M.J. Ventilation management in acute respiratory failure related to COVID-19 versus ARDS from another origin—A descriptive narrative review. Expert Rev. Respir. Med. 2021, 15, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: COVID-19 Epidemiological Update—19 January 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---19-january-2024 (accessed on 29 March 2022).
- Hedberg, P.; Parczewski, M.; Serwin, K.; Marchetti, G.; Bai, F.; Ole Jensen, B.E.; Pereira, J.P.V.; Drobniewski, F.; Reschreiter, H.; Naumovas, D.; et al. In-hospital mortality during the wild-type, alpha, delta, and omicron SARS-CoV-2 waves: A multinational cohort study in the EuCARE project. Lancet Reg. Health Eur. 2024, 38, 100855. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Xu, W.; Sun, N.-N.; Gao, H.-N.; Chen, Z.-Y.; Yang, Y.; Ju, B.; Tang, L.-L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci. Rep. 2021, 11, 2933. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.H.; Lee, K.S. Current and Emerging Knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Homayounieh, F.; Zhang, E.W.; Babaei, R.; Mobin, H.K.; Sharifian, M.; Mohseni, I.; Kuo, A.; Arru, C.; Kalra, M.K.; Digumarthy, S.R. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PLoS ONE 2020, 15, e0239519. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.; Smith, G.B.; Prytherch, D.; Meredith, P.; Price, C.; Chauhan, A.; Scott, P. The performance of the National Early Warning Score and National Early Warning Score 2 in hospitalised patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Resuscitation 2021, 159, 150–157. [Google Scholar] [CrossRef]
- Fan, G.; Tu, C.; Zhou, F.; Liu, Z.; Wang, Y.; Song, B.; Gu, X.; Wang, Y.; Wei, Y.; Li, H.; et al. Comparison of severity scores for COVID-19 patients with pneumonia: A retrospective study. Eur. Respir. J. 2020, 56, 2002113. [Google Scholar] [CrossRef]
- Citu, C.; Citu, I.M.; Motoc, A.; Forga, M.; Gorun, O.M.; Gorun, F. Predictive Value of SOFA and qSOFA for In-Hospital Mortality in COVID-19 Patients: A Single-Center Study in Romania. J. Pers. Med. 2022, 12, 878. [Google Scholar] [CrossRef]
- Villar, S.S.; Machiwenyika, J.M.T.; Zhu, Y.; Mackay, J.H. The performance of the National Early Warning Score in hospitalised patients infected by Covid-19. Resuscitation 2021, 162, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Madrazo, M.; Fernandez-Garces, M.; Miguez, A.M.; Garcia, A.G.; Vieitez, A.C.; Guijarro, E.G.; Aizpuru, E.M.F.; Gomez, M.G.; Manrique, M.A.; et al. Severity Scores in COVID-19 Pneumonia: A Multicenter, Retrospective, Cohort Study. J. Gen. Intern. Med. 2021, 36, 1338–1345. [Google Scholar] [CrossRef]
- Bradley, J.; Sbaih, N.; Chandler, T.R.; Furmanek, S.; Ramirez, J.A.; Cavallazzi, R. Pneumonia Severity Index and CURB-65 Score Are Good Predictors of Mortality in Hospitalized Patients With SARS-CoV-2 Community-Acquired Pneumonia. Chest 2022, 161, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gordillo, J.A.; Camiro-Zúñiga, A.; Aguilar-Soto, M.; Cuenca, D.; Cadena-Fernández, A.; Khouri, L.S.; Rayek, J.N.; Mercado, M.; The ARMII Study Group. COVID-IRS: A novel predictive score for risk of invasive mechanical ventilation in patients with COVID-19. PLoS ONE 2021, 16, e0248357. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Borobia, A.M.; Ryan, P.; Rodríguez-Baño, J.; Bellón, J.M.; Jarrín, I.; Carratalà, J.; Pachón, J.; Carcas, A.J.; Yllescas, M.; et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: The COVID-19 SEIMC score. Thorax 2021, 76, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Harrison, E.M. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.; A Lewis, S.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef]
- Wilson, M.E.; Mittal, A.; Karki, B.; Dobler, C.C.; Wahab, A.; Curtis, J.R.; Erwin, P.J.; Majzoub, A.M.; Montori, V.M.; Gajic, O.; et al. Do-not-intubate orders in patients with acute respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Mashayekhi, S.; Cuvelier, G.; Legrand, A.; Reychler, G. A New Formula for Predicting the Fraction of Delivered Oxygen During Low-Flow Oxygen Therapy. Respir. Care 2018, 63, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L. Le Manuel de Réanimation, Soins Intensifs et Médecine D’urgence; Springer: Paris, France, 2013. [Google Scholar]
- Mucherino, A.; Papajorgji, P.J.; Pardalos, P.M.; Mucherino, A.; Papajorgji, P.J.; Pardalos, P.M. K-nearest neighbor classification. In Data Mining in Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–106. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 29 March 2022).
- Lombardi, Y.; Azoyan, L.; Szychowiak, P.; Bellamine, A.; Lemaitre, G.; Bernaux, M.; Daniel, C.; Leblanc, J.; Riller, Q.; Steichen, O. External validation of prognostic scores for COVID-19: A multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. Intensive Care Med. 2021, 47, 1426–1439. [Google Scholar] [CrossRef]
- Wirth, A.; Goetschi, A.; Held, U.; Sendoel, A.; Stuessi-Helbling, M.; Huber, L.C. External Validation of the Modified 4C Deterioration Model and 4C Mortality Score for COVID-19 Patients in a Swiss Tertiary Hospital. Diagnostics 2022, 12, 1129. [Google Scholar] [CrossRef]
- Chung, H.-P.; Tang, Y.-H.; Chen, C.-Y.; Chen, C.-H.; Chang, W.-K.; Kuo, K.-C.; Chen, Y.-T.; Wu, J.-C.; Lin, C.-Y.; Wang, C.-J. Outcome prediction in hospitalized COVID-19 patients: Comparison of the performance of five severity scores. Front. Med. 2023, 10, 1121465. [Google Scholar] [CrossRef]
- Cena, T.; Cammarota, G.; Azzolina, D.; Barini, M.; Bazzano, S.; Zagaria, D.; Negroni, D.; Castello, L.; Carriero, A.; Della Corte, F.; et al. Predictors of intubation and mortality in COVID-19 patients: A retrospective study. J. Anesth. Analg. Crit. Care 2021, 1, 19. [Google Scholar] [CrossRef]
- De Vita, N.; Scotti, L.; Cammarota, G.; Racca, F.; Pissaia, C.; Maestrone, C.; Colombo, D.; Olivieri, C.; Della Corte, F.; Barone-Adesi, F.; et al. Predictors of intubation in COVID-19 patients treated with out-of-ICU continuous positive airway pressure. Pulmonology 2022, 28, 173–180. [Google Scholar] [CrossRef]
- Varzaneh, Z.A.; Orooji, A.; Erfannia, L.; Shanbehzadeh, M. A new COVID-19 intubation prediction strategy using an intelligent feature selection and K-NN method. Inform. Med. Unlocked 2022, 28, 100825. [Google Scholar] [CrossRef]
- Li, W.; Lin, F.; Dai, M.; Chen, L.; Han, D.; Cui, Y.; Pan, P. Early predictors for mechanical ventilation in COVID-19 patients. Ther. Adv. Respir. Dis. 2020, 14, 1753466620963017. [Google Scholar] [CrossRef]
- Nair, P.R.; Maitra, S.; Ray, B.R.; Anand, R.K.; Baidya, D.K.; Subramaniam, R. Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Predictors of the Early Requirement of Mechanical Ventilation in COVID-19 Patients. Indian J. Crit. Care Med. 2020, 24, 1143–1144. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; He, Y.; Liu, S. Comparison of Prognostic Scores for Patients with COVID-19 Presenting with Dyspnea in the Emergency Department. J. Emerg. Med. 2023, 65, e487–e494. [Google Scholar] [CrossRef]
- Heldt, S.; Neubock, M.; Kainzbauer, N.; Shao, G.; Tschoellitsch, T.; Duenser, M.; Bernhard, K.; Markus, W.; Christian, P.; Jens, M. qSOFA score poorly predicts critical progression in COVID-19 patients. Wien. Med. Wochenschr. 2022, 172, 211–219. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef]
- Lee, S.I.; Ju, Y.R.; Kang, D.H.; Lee, J.E. Characteristics and outcomes of patients with do-not-resuscitate and physician orders for life-sustaining treatment in a medical intensive care unit: A retrospective cohort study. BMC Palliat. Care 2024, 23, 42. [Google Scholar] [CrossRef]
- Jelodar, M.G.; Mirzaei, S.; Saghafi, F.; Rafieian, S.; Rezaei, S.; Saatchi, A.; Avare, Z.D.; Niri, M.D. Impact of vaccination status on clinical outcomes of hospitalized COVID-19 patients. BMC Infect. Dis. 2024, 24, 254. [Google Scholar] [CrossRef]
- Baek, M.S.; Lee, M.T.; Kim, W.Y.; Choi, J.C.; Jung, S.Y. COVID-19-related outcomes in immunocompromised patients: A nationwide study in Korea. PLoS ONE 2021, 16, e0257641. [Google Scholar] [CrossRef]
- Russo, A.; Pisaturo, M.; Zollo, V.; Martini, S.; Maggi, P.; Numis, F.G.; Gentile, I.; Sangiovanni, N.; Rossomando, A.M.; Bianco, V.; et al. Obesity as a Risk Factor of Severe Outcome of COVID-19: A Pair-Matched 1:2 Case-Control Study. J. Clin. Med. 2023, 12, 4055. [Google Scholar] [CrossRef] [PubMed]
- Marano, D.; Amaral, Y.; Rebelo, F.; Abranches, A.; Vilarim, M.; Moreira, M.E.L. The effect of obesity on the mortality of hospitalized adults with COVID-19 considering the human development index: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13591. [Google Scholar] [CrossRef] [PubMed]
| Tool | Original Purpose | Parameters Included |
|---|---|---|
| qSOFA | Identify patients with infection at high risk of in-hospital mortality | GCS, respiratory rate, systolic blood pressure |
| NEWS | Early detection of clinical deterioration (general) | Respiratory rate, SpO2, O2 supplementation, body temperature, systolic blood pressure, heart rate, AVPU |
| CURB-65 | Predict 30-day mortality in community-acquired pneumonia | Age, confusion, BUN or urea, respiratory rate, systolic blood pressure |
| 4C | Predict in-hospital mortality in hospitalized COVID-19 patients | Age, sex at birth, number of comorbidities a, respiratory rate, SpO2 at room air, GCS, urea, CRP |
| COVID-SEIMC | Predict 30-day mortality in hospitalized COVID-19 patients | Age, sex, age-adjusted SpO2, NLR, eGFR, dyspnea |
| COVID-IRS (NLR) | Predict the need for mechanical ventilation in hospitalized COVID-19 patients | Respiratory rate, SpO2/FiO2 ratio, LDH, NLR |
| Parameter | COVID-COMBI Points | |
|---|---|---|
| Age (years) | <50 | 0 |
| 50–59 | 2 | |
| 60–69 | 4 | |
| 70–79 | 6 | |
| ≥80 | 7 | |
| Sex at birth | Female | 0 |
| Male | 1 | |
| No. of comorbidities a | 0 | 0 |
| 1 | 1 | |
| ≥2 | 2 | |
| SpO2 at room air (%) | ≥92 | 0 |
| <92 | 2 | |
| GCS | 15 | 0 |
| <15 | 2 | |
| Urea (mmol/L) | <7 | 0 |
| 7–14 | 1 | |
| >14 | 3 | |
| C-reactive protein (mg/L) | <50 | 0 |
| 50–99 | 1 | |
| ≥100 | 2 | |
| Respiratory rate (breaths/min) | <22 | 0 |
| 22–29 | 1 | |
| 30–33 | 2.5 | |
| ≥34 | 3 | |
| SpO2/FiO2 ratio | >200 | 0 |
| 101–200 | 2 | |
| ≤100 | 3.5 | |
| Lactate dehydrogenase (U/L) | ≤200 | 0 |
| 201–299 | 1 | |
| 300–399 | 2 | |
| 400–499 | 2.5 | |
| ≥500 | 4 | |
| Neutrophil–lymphocyte ratio | <4 | 0 |
| 4–7.9 | 1 | |
| 8–13.9 | 2 | |
| ≥14 | 2.5 |
| Overall (n = 1051) | Missing (%) | |
|---|---|---|
| Demographics | ||
| age in years, median (IQR) (range) | 65 (54, 79) (19–99) | 0 |
| male, n (%) | 627 (59.7) | 0 |
| Comorbidities | ||
| arterial hypertension (%) | 481 (45.8) | 0 |
| diabetes, n (%) | 242 (23.0) | 0 |
| obesity, n (%) | 286 (31.0) | 12.3 |
| chronic kidney disease, n (%) | 205 (19.5) | 0 |
| chronic liver disease, n (%) | 59 (5.6) | 0 |
| chronic respiratory disease, n (%) | 201 (19.1) | 0 |
| active cancer, n (%) | 55 (5.2) | 0 |
| immunosuppression, n (%) | 71 (6.8) | 0 |
| COVID-19 vaccination status | 15.2 | |
| not vaccinated, n (%) | 178 (19.9) | |
| vaccinated, n (%) | 101 (11.3) | |
| no vaccination available, n (%) | 615 (68.5) | |
| Admission symptoms | ||
| dyspnea, n (%) | 483 (46.2) | 0.5 |
| cough, n (%) | 715 (68.5) | 0.7 |
| new confusion, n (%) | 37 (3.5) | 0.6 |
| Admission vital signs | ||
| heart rate (bpm), median (IQR) | 84 (74, 94) | 1.3 |
| systolic blood pressure (mmHg), median (IQR) | 134 (120, 149) | 0 |
| body temperature (°C), median (IQR) | 37.4 (37.0, 38.3) | 1.3 |
| fever (body temperature ≥ 38 °C), n (%) | 397 (38.4) | 1.5 |
| respiratory rate (brpm), median (IQR) | 21 (18, 25) | 0.8 |
| O2 saturation at room air (%), median (IQR) | 94 (90, 96) | 12.0 |
| O2 supplementation, n (%) | 250 (23.8) | 0 |
| GCS, median (IQR) | 15 (15, 15) | 0.3 |
| Admission biomarkers | ||
| leucocytes (×109), median (IQR) a | 6.1 (4.6, 8.0) | 0.3 |
| neutrophil–lymphocyte ratio, median (IQR) b | 5.1 (3.1, 8.5) | 5.7 |
| C-reactive protein (mg/L), median (IQR) c | 64.5 (28.3, 117.0) | 2.0 |
| urea (mmol/L), median (IQR) d | 5.6 (4.0, 8.3) | 2.4 |
| eGFR (mL/min/1.73m2), median (IQR) e | 75 (54, 93) | 2.1 |
| lactate dehydrogenase (U/L), median (IQR) f | 296 (226, 391) | 12.8 |
| Score | Overall n = 1051 | Severe Course n = 162 | In-Hospital Death n = 112 | Mechanical Ventilation n = 74 | Missing (%) |
|---|---|---|---|---|---|
| qSOFA | 1 (0, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.9 |
| NEWS | 4 (2, 6) | 6 (4, 9) | 6 (3, 8) | 7.5 (5, 9) | 3.4 |
| CURB-65 | 1 (0, 2) | 2 (1, 3) | 2 (2, 3) | 1 (0, 2) | 3.7 |
| 4C | 8 (5, 11) | 11 (9, 14) | 13 (11, 15) | 10 (7, 12) | 4.9 |
| COVID-SEIMC | 8 (5, 15) | 13 (8, 19) | 17 (11, 21) | 8 (5, 11) | 7.8 |
| COVID-IRS (NLR) | 3 (2, 5) | 5 (3, 7) | 5 (3, 7) | 6 (4, 8) | 15.4 |
| COVID-COMBI | 11 (8, 14) | 16 (12, 20) | 17 (14, 20) | 14 (11, 19) | 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boesing, M.; Lüthi-Corridori, G.; Büttiker, D.; Hunziker, M.; Jaun, F.; Vaskyte, U.; Brändle, M.; Leuppi, J.D. The Predictive Performance of Risk Scores for the Outcome of COVID-19 in a 2-Year Swiss Cohort. Biomedicines 2024, 12, 1702. https://doi.org/10.3390/biomedicines12081702
Boesing M, Lüthi-Corridori G, Büttiker D, Hunziker M, Jaun F, Vaskyte U, Brändle M, Leuppi JD. The Predictive Performance of Risk Scores for the Outcome of COVID-19 in a 2-Year Swiss Cohort. Biomedicines. 2024; 12(8):1702. https://doi.org/10.3390/biomedicines12081702
Chicago/Turabian StyleBoesing, Maria, Giorgia Lüthi-Corridori, David Büttiker, Mireille Hunziker, Fabienne Jaun, Ugne Vaskyte, Michael Brändle, and Jörg D. Leuppi. 2024. "The Predictive Performance of Risk Scores for the Outcome of COVID-19 in a 2-Year Swiss Cohort" Biomedicines 12, no. 8: 1702. https://doi.org/10.3390/biomedicines12081702






