Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Mining
2.2. Statistical Analysis
2.3. Signal Screening
3. Results
3.1. Population Characteristics
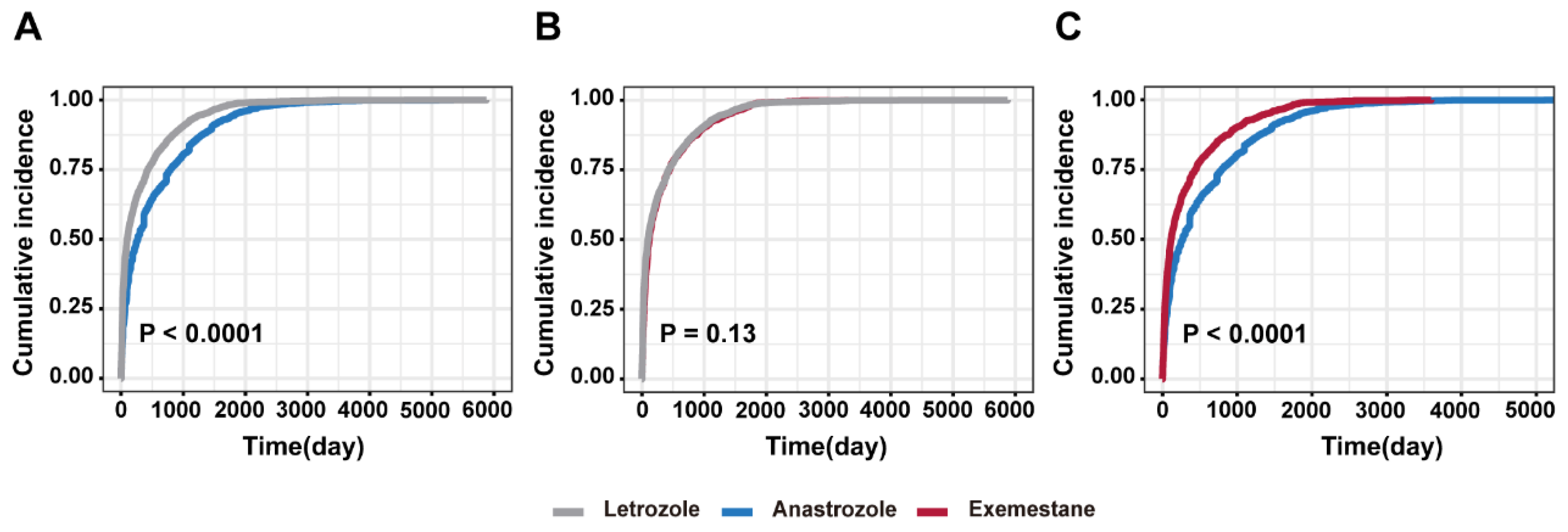
3.2. Time-to-Event Onset Analysis and Cumulative Incidence
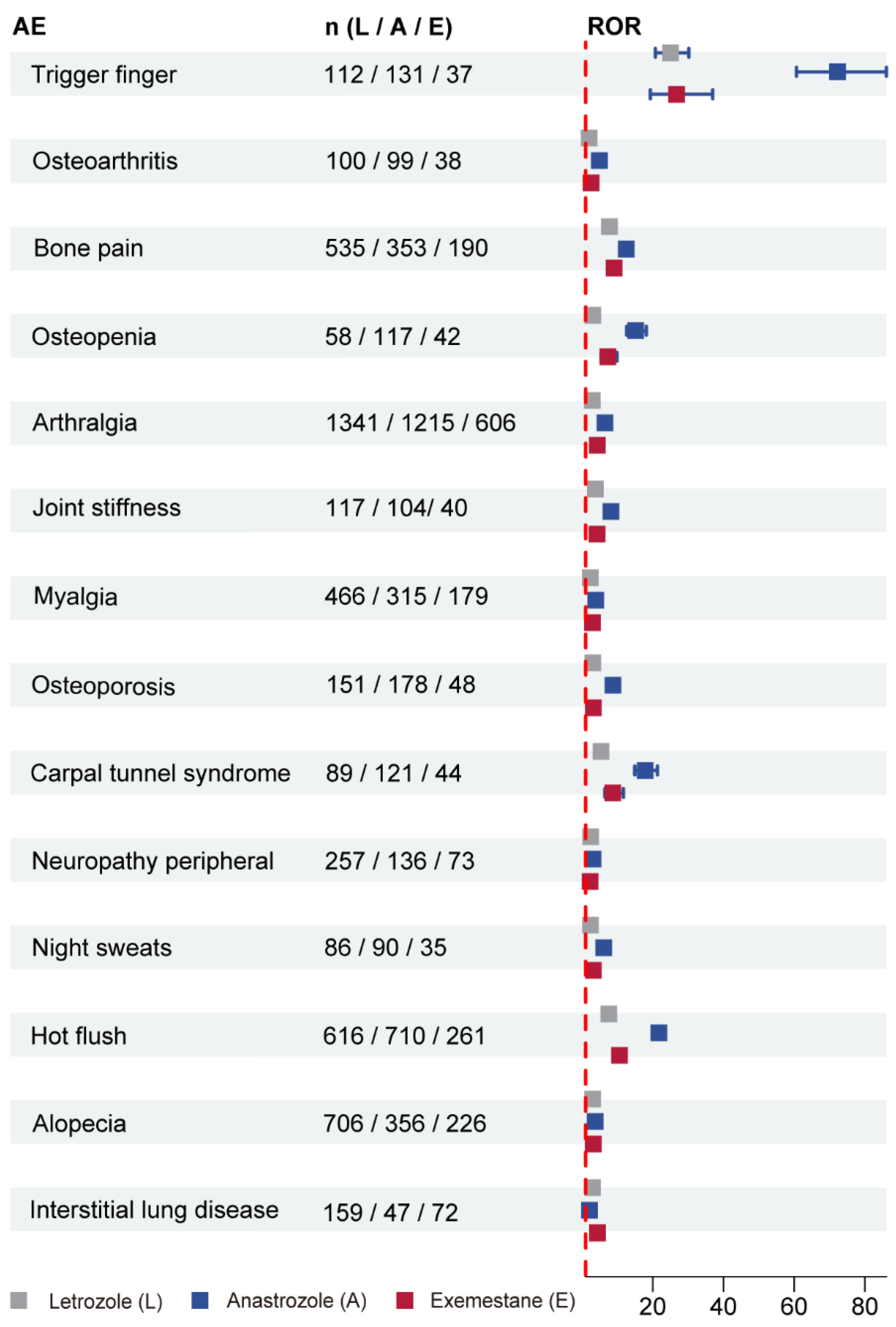
3.3. Disproportionality Analyses for Letrozole, Anastrozole, and Exemestane
3.4. SOC Analysis of AEs-Positive Signal Involvement for Three AIs
3.5. Subgroup Analyses Based on Medical and Non-Medical Professionals
4. Discussion
4.1. Joint and Musculoskeletal Toxicity
4.2. Hematologic Toxicity
4.3. Respiratory Toxicity
4.4. Hepatic Toxicity
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C. Tissue physiology and pathology of aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2017, 168, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rizner, T.L.; Romano, A. Targeting the formation of estrogens for treatment of hormone dependent diseases-current status. Front. Pharmacol. 2023, 14, 1155558. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.; Gohar, E.Y. Aromatase enzyme: Paving the way for exploring aromatization for cardio-renal protection. Biomed. Pharmacother. 2023, 168, 115832. [Google Scholar] [CrossRef]
- Hong, S.; Didwania, A.; Olopade, O.; Ganschow, P. The expanding use of third-generation aromatase inhibitors: What the general internist needs to know. J. Gen. Intern. Med. 2009, 24 (Suppl. S2), S383–S388. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, S. Aromatase inhibitors: Structural features and biochemical characterization. Ann. N. Y. Acad. Sci. 2006, 1089, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, L.; Gai, D.; He, S.; Jiang, X.; Zhang, N. Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Front. Pharmacol. 2022, 13, 851246. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef]
- Hammond, I.W.; Rich, D.S.; Gibbs, T.G. Effect of consumer reporting on signal detection: Using disproportionality analysis. Expert. Opin. Drug Saf. 2007, 6, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huo, X.C.; Wang, S.X.; Wang, F.; Zhao, Q. Data mining for adverse drug reaction signals of daptomycin based on real-world data: A disproportionality analysis of the US Food and Drug Administration adverse event reporting system. Int. J. Clin. Pharm. 2022, 44, 1351–1360. [Google Scholar] [CrossRef]
- Gu, S.Y.; Yu, S.D.; Zhou, Z.Y.; Wang, S.W.; Hu, S.S.; Shi, C.Y.; Qi, C.D.; Fan, G.R. Digestive Tract Cancer-Related Adverse Events Correlated with Proton Pump Inhibitors Use: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. J. Clin. Pharm. Ther. 2023, 2023, 6913722. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Umetsu, R.; Mataki, K.; Kato, Y.; Ueda, N.; Nakayama, Y.; Hane, Y.; Matsui, T.; Hatahira, H.; Sasaoka, S.; et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J. Pharm. Health Care Sci. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Umetsu, R.; Abe, J.; Matsui, T.; Ueda, N.; Kato, Y.; Sasaoka, S.; Tahara, K.; Takeuchi, H.; Kinosada, Y. Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J. Pharm. Health Care Sci. 2015, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Hosomi, K.; Yokoyama, S.; Takada, M. Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J. Clin. Pharm. Ther. 2020, 45, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sauzet, O.; Carvajal, A.; Escudero, A.; Molokhia, M.; Cornelius, V.R. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013, 36, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Jung, E.A.; Kim, Z.; Kim, B.Y. Risk of Cardiovascular Events and Lipid Profile Change in Patients with Breast Cancer Taking Aromatase Inhibitor: A Systematic Review and Meta-Analysis. Curr. Oncol. 2023, 30, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.B.; Bouganim, N.; Suissa, S.; Azoulay, L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women With Breast Cancer: A Population-Based Cohort Study. Circulation 2020, 141, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Hyder, T.; Marino, C.C.; Ahmad, S.; Nasrazadani, A.; Brufsky, A.M. Aromatase Inhibitor-Associated Musculoskeletal Syndrome: Understanding Mechanisms and Management. Front. Endocrinol. 2021, 12, 713700. [Google Scholar] [CrossRef]
- Perez, E.A.; Weilbaecher, K. Aromatase inhibitors and bone loss. Oncology 2006, 20, 1029–1039; discussion 1039–1040, 1042, 1048. [Google Scholar]
- Brufsky, A.M. Managing bone loss in women with early-stage breast cancer receiving aromatase inhibitors. Clin. Breast Cancer 2007, 8 (Suppl. S1), S22–S34. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Bryce, J.; Hadji, P. Aromatase inhibitor-associated bone loss and its management with bisphosphonates in patients with breast cancer. Breast Cancer 2012, 4, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Niravath, P. Aromatase inhibitor-induced arthralgia: A review. Ann. Oncol. 2013, 24, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. [Google Scholar] [CrossRef] [PubMed]
- Beckwee, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Henry, N.L.; Loprinzi, C.L. Management of Aromatase Inhibitor-Induced Musculoskeletal Symptoms. JCO Oncol. Pract. 2020, 16, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, J.; Liagre, B.; Vergne, P.; Bertin, P.; Beneytout, J.; Habrioux, G. Aromatase in synovial cells from postmenopausal women. Steroids 2001, 66, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Schicht, M.; Ernst, J.; Nielitz, A.; Fester, L.; Tsokos, M.; Guddat, S.S.; Brauer, L.; Bechmann, J.; Delank, K.S.; Wohlrab, D.; et al. Articular cartilage chondrocytes express aromatase and use enzymes involved in estrogen metabolism. Arthritis Res. Ther. 2014, 16, R93. [Google Scholar] [CrossRef]
- Fusi, C.; Materazzi, S.; Benemei, S.; Coppi, E.; Trevisan, G.; Marone, I.M.; Minocci, D.; De Logu, F.; Tuccinardi, T.; Di Tommaso, M.R.; et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat. Commun. 2014, 5, 5736. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Becker, G.; Palma, T.V.; Pillat, M.M.; Scussel, R.; Steiner, B.T.; Sampaio, T.B.; Ardisson-Araujo, D.M.P.; de Andrade, C.M.; Oliveira, M.S.; et al. Kinin B(1) and B(2) receptors mediate cancer pain associated with both the tumor and oncology therapy using aromatase inhibitors. Sci. Rep. 2023, 13, 4418. [Google Scholar] [CrossRef] [PubMed]
- Lintermans, A.; Van Asten, K.; Jongen, L.; Van Brussel, T.; Laenen, A.; Verhaeghe, J.; Vanderschueren, D.; Lambrechts, D.; Neven, P. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur. J. Cancer 2016, 56, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Giralt, N.; Rodriguez-Sanz, M.; Prieto-Alhambra, D.; Servitja, S.; Torres-Del Pliego, E.; Balcells, S.; Albanell, J.; Grinberg, D.; Diez-Perez, A.; Tusquets, I.; et al. Genetic determinants of aromatase inhibitor-related arthralgia: The B-ABLE cohort study. Breast Cancer Res. Treat. 2013, 140, 385–395. [Google Scholar] [CrossRef]
- Henry, N.L.; Skaar, T.C.; Dantzer, J.; Li, L.; Kidwell, K.; Gersch, C.; Nguyen, A.T.; Rae, J.M.; Desta, Z.; Oesterreich, S.; et al. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res. Treat. 2013, 138, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.D.; Su, H.I.; Satagopan, J.; Li, Q.S.; Seluzicki, C.M.; Dries, A.; DeMichele, A.M.; Mao, J.J. Clinical and genetic risk factors for aromatase inhibitor-associated arthralgia in breast cancer survivors. Breast 2020, 49, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Giles, J.T.; Stearns, V. Aromatase inhibitor-associated musculoskeletal symptoms: Etiology and strategies for management. Oncology 2008, 22, 1401–1408. [Google Scholar] [PubMed]
- Hollins, A.W.; Hein, R.E.; Atia, A.N.; Zhang, G.X.; Sergesketter, A.R.; Darner, G.; Morris, M.; Mithani, S.K. Variations in Incidence of Trigger Finger and Response to Corticosteroid Injection after Aromatase Inhibitor Therapy for Breast Cancer. Plast. Reconstr. Surg. 2023, 151, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.Y.; Kim, C.H. A Case of Bilateral Trigger Thumbs Secondary to Aromatase Inhibitor. Yonsei Med. J. 2015, 56, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Regent-Smith, A.J.; Childers, E.J.; Dzwierzynski, W.W.; Morgan, A.L. Incidence and Treatment Efficacy of Trigger Finger in the Breast Cancer Population on Aromatase Inhibitors. Hand 2023, 18, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Pans, S.; Paridaens, R.; Westhovens, R.; Timmerman, D.; Verhaeghe, J.; Wildiers, H.; Leunen, K.; Amant, F.; Berteloot, P.; et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: Associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res. Treat. 2007, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Esen, A.; Akkulah, S. Management of Large Oroantral Fistulas Caused by Medication-Related Osteonecrosis with the Combined Sequestrectomy, Buccal Fat Pad Flap and Platelet-Rich Fibrin. J. Maxillofac. Oral. Surg. 2021, 20, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, Y.; Kawashima, Y.; Shimonishi, T.; Koga, Y.; Miyake, S.; Hotta, C.; Yasushi, K. A Case of Recurrent Breast Cancer with Drug-Induced Interstitial Pneumonia Triggered by the Switch from an Original to a Generic Aromatase Inhibitor. Gan To Kagaku Ryoho 2020, 47, 1707–1709. [Google Scholar]
- Alsamman, M.; Pothen, J.; Inoyatov, M.; Cruz Salcedo, E.M.; Ramesh, C. Aortic Thrombus Extending to Left Subclavian in a Patient With Diffuse Venous Thromboembolism on Aromatase Inhibitor Therapy. Cureus 2021, 13, e16698. [Google Scholar] [CrossRef] [PubMed]
- Oyan, B.; Altundag, K.; Ozisik, Y. Does letrozole have any place in adjuvant setting in breast cancer patients with documented hypercoagulability? Am. J. Clin. Oncol. 2004, 27, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Lycette, J.L.; Luoh, S.W.; Beer, T.M.; Deloughery, T.G. Acute bilateral pulmonary emboli occurring while on adjuvant aromatase inhibitor therapy with anastrozole: Case report and review of the literature. Breast Cancer Res. Treat. 2006, 99, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gotzinger, F.; Lauder, L.; Sharp, A.S.P.; Lang, I.M.; Rosenkranz, S.; Konstantinides, S.; Edelman, E.R.; Bohm, M.; Jaber, W.; Mahfoud, F. Interventional therapies for pulmonary embolism. Nat. Rev. Cardiol. 2023, 20, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Gharia, B.; Seegobin, K.; Maharaj, S.; Marji, N.; Deutch, A.; Zuberi, L. Letrozole-induced hepatitis with autoimmune features: A rare adverse drug reaction with review of the relevant literature. Oxf. Med. Case Rep. 2017, 2017, omx074. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Oktar, S.; Ozkan, O.V.; Alcin, E.; Ozturk, O.H.; Nacar, A. Letrozole induces hepatotoxicity without causing oxidative stress: The protective effect of melatonin. Gynecol. Endocrinol. 2011, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Fetting, J.; Mumford, L.; Zorzi, J.; Shahverdi, K.; Jeter, S.; Herlong, F.; Stearns, V.; Lee, L. Severe prolonged cholestatic hepatitis caused by exemestane. Breast Cancer Res. Treat. 2010, 121, 789–791. [Google Scholar] [CrossRef] [PubMed]
- The Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group; Buzdar, A.; Howell, A.; Cuzick, J.; Wale, C.; Distler, W.; Hoctin-Boes, G.; Houghton, J.; Locker, G.Y.; Nabholtz, J.M. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: Long-term safety analysis of the ATAC trial. Lancet Oncol. 2006, 7, 633–643. [Google Scholar] [CrossRef]
- Zapata, E.; Zubiaurre, L.; Bujanda, L.; Pierola, A. Anastrozole-induced hepatotoxicity. Eur. J. Gastroenterol. Hepatol. 2006, 18, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, L.; Romero-Vazquez, J.; Jimenez-Saenz, M.; Padron, J.R.; Herrerias-Gutierrez, J.M. Severe acute hepatitis in a patient treated with anastrozole. Lancet 2007, 369, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Basso, M.; Vecchio, F.M.; Marsico, V.A.; Cerchiaro, E.; D’Argento, E.; Bagala, C.; Barone, C. Anastrozole-related acute hepatitis with autoimmune features: A case report. BMC Gastroenterol. 2011, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, S.; Chen, Y.; Peng, Y.; Zheng, J. Anastrozole and related glucuronic acid conjugate are electrophilic species. Xenobiotica 2022, 52, 380–388. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients, N (%) | |||
|---|---|---|---|---|
| Letrozole | Anastrozole | Exemestane | ||
| Gender | Male | 208 (1.15%) | 126 (1.53%) | 49 (0.70%) |
| Female | 16,423 (91.06%) | 7911 (95.98%) | 6467 (92.24%) | |
| Unknown | 1404 (7.78%) | 205 (2.49%) | 495 (7.06%) | |
| Age (year) | <18 | 48 (0.27%) | 22 (0.27%) | 2 (0.03%) |
| 18–64 | 5181 (28.73%) | 2316 (28.10%) | 2034 (29.01%) | |
| 65–85 | 4536 (25.15%) | 2761 (33.50%) | 2743 (39.12%) | |
| >85 | 321 (1.78%) | 209 (2.54%) | 226 (3.22%) | |
| Unknown | 7949 (44.08%) | 2934 (35.60%) | 2006 (28.61%) | |
| Weight (kg) | <50 | 364 (2.02%) | 157 (1.90%) | 88 (1.26%) |
| 50–100 | 4730 (26.23%) | 2734 (33.17%) | 2064 (29.44%) | |
| >100 | 335 (1.86%) | 193 (2.34%) | 166 (2.37%) | |
| Unknown | 12,606 (69.90%) | 5158 (62.58%) | 4693 (66.94%) | |
| Reporter | Medical professional | 11,716 (64.96%) | 2604 (31.59%) | 3590 (51.21%) |
| Non-medical professional | 5509 (30.55%) | 3846 (46.66%) | 3158 (45.04%) | |
| Unknown | 810 (4.49%) | 1792 (21.74%) | 263 (3.75%) | |
| Reporter’s country | USA | 4617 (25.60%) | 5997 (72.76%) | 4029 (57.47%) |
| Others | 13,418 (74.40%) | 2245 (27.24%) | 2982 (42.53%) | |
| Outcome of AEs | Hospitalization | 4519 (21.00%) | 1294 (14.55%) | 1389 (17.53%) |
| Disability | 552 (2.57%) | 324 (3.64%) | 168 (2.12%) | |
| Life-threatening | 658 (3.06%) | 136 (1.53%) | 169 (2.13%) | |
| Death | 1484 (6.90%) | 369 (4.15%) | 685 (8.65%) | |
| Congenital anomaly | 108 (0.50%) | 3 (0.03%) | 2 (0.03%) | |
| Permanent impairment/damage | 26 (0.12%) | 128 (1.44%) | 13 (0.16%) | |
| Others | 14,171 (65.86%) | 6641 (74.66%) | 5496 (69.38%) | |
| Drug | Patients (N) | Time-to-Onset (Median, IQR) | Scale Parameter: α (95% CI) | Shape Parameter: β (95% CI) | Type |
|---|---|---|---|---|---|
| Letrozole | 5228 | 99.50 (IQR 25.00–431.00) | 227.57 (217.32–237.81) | 0.64 (0.62–0.65) | Early failure |
| Anastrozole | 1999 | 268.00 (IQR 61.00–791.00) a,b | 433.43 (405.21–461.65) | 0.71 (0.68–0.73) | Early failure |
| Exemestane | 1533 | 116.00 (IQR 31.00–437.00) c | 250.66 (231.07–270.25) | 0.68 (0.65–0.70) | Early failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, L.; Liu, Y.; Zhang, J.; Zheng, L.; Zheng, M. Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Biomedicines 2024, 12, 1708. https://doi.org/10.3390/biomedicines12081708
Zhang Y, Zhao L, Liu Y, Zhang J, Zheng L, Zheng M. Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Biomedicines. 2024; 12(8):1708. https://doi.org/10.3390/biomedicines12081708
Chicago/Turabian StyleZhang, Yina, Lingzhu Zhao, Yanning Liu, Jingkang Zhang, Luyan Zheng, and Min Zheng. 2024. "Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS" Biomedicines 12, no. 8: 1708. https://doi.org/10.3390/biomedicines12081708





